Back to Journals » Cancer Management and Research » Volume 11
microRNA-877 inhibits malignant progression of colorectal cancer by directly targeting MTDH and regulating the PTEN/Akt pathway
Authors Zhang L, Li C, Cao L, Li H, Zou H, Li H, Pei H
Received 9 November 2018
Accepted for publication 15 February 2019
Published 8 April 2019 Volume 2019:11 Pages 2769—2781
DOI https://doi.org/10.2147/CMAR.S194073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
This paper has been retracted.
Lunqiang Zhang, 1 Chenglong Li, 1 Lijun Cao, 2 Hui Li, 2 Haiding Zou, 2 Hongqin Li, 2 Haiping Pei 1
1Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Hunan 410008, People’s Republic of China; 2Department of Anesthesiology, The Second Xiangya Hospital of Central South University, Hunan 410011, People’s Republic of China
Background: Recently, microRNA-877-5p (miR-877) was recognized as a cancer-associated miRNA in hepatocellular and renal cell carcinomas. However, little is known regarding its expression pattern and role in colorectal cancer (CRC) tumorigenesis.
Material and methods: In the present study, reverse-transcription quantitative polymerase chain reaction was performed to detect miR-877 expression in CRC tissues and cell lines. A series of functional experiments were used to determine the effects of miR-877 upregulation on CRC cell proliferation, colony formation, apoptosis, migration, and invasion. In addition, the regulatory role of miR-877 in tumor growth was examined in vivo using a xenograft experiment. More importantly, the mechanisms underlying the action of miR-877 in CRC were explored.
Results: A significant decrease in the expression of miR-877 was observed in CRC tissues and cell lines. Low miR-877 expression correlated with lymph node metastasis and TNM stage of CRC patients. Functional experiments revealed that ectopic expression of miR-877 suppressed CRC cell proliferation and colony formation ability, induced cell apoptosis, inhibited cell migration and invasion in vitro, and reduced tumor growth in vivo. Metadherin (MTDH) was recognized as a direct target of miR-877 in CRC cells. It was notably overexpressed in CRC tissues, and its expression was inversely correlated with that of miR-877 expression. Furthermore, MTDH knockdown simulated the tumor suppressor activity of miR-877 in CRC cells. MTDH restoration impaired the suppressive effects of miR-877 on malignant phenotypes of CRC cells. In addition, miR-877 inhibited the activation of the PTEN/Akt signaling pathway by regulating MTDH expression both in vitro and in vivo.
Conclusion: Collectively, these results demonstrate that miR-877 inhibits the progression of CRC, at least partly by the direct targeting of MTDH and regulation of the PTEN/Akt pathway. Thus, miR-877 may serve as a potential therapeutic target for the treatment of patients with CRC.
Keywords: microRNA-877, colorectal cancer, metadherin, PTEN/Akt pathway
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy and fourth most frequent cause of cancer-related deaths worldwide.1 Over one million new cases of CRC and 600,000 CRC-related deaths are reported globally each year.2 At present, surgical resection, systemic adjuvant chemotherapy, radiation therapy, immunotherapy, and targeted therapy are the primary strategies implemented for the treatment of patients with CRC.3 Despite tremendous progress in the diagnosis and treatment of cancer, the prognosis of patients with CRC remains dissatisfactory owing to the high rate of tumor recurrence and metastasis.4 Extensive evidence demonstrates the involvement of multiple factors, such as the inactivation of tumor suppressor genes, activation of oncogenes, and mutation of mismatch repair genes, in CRC pathogenesis; however, the detailed mechanisms are not yet completely understood.5–7 Hence, it is of considerable importance to improve our understanding of the molecular mechanisms underlying CRC occurrence and development to provide new strategies for cancer diagnosis, therapy, and prevention.
microRNAs (miRNAs) are single-stranded, noncoding, short RNA molecules comprising approximately 18–23 nucleotides.8 miRNAs negatively regulate gene expression by causing translational inhibition and/or promoting messenger RNA (mRNA) degradation via direct binding to seed regions in the 3ʹ-untranslated regions (3ʹ-UTRs) of their target mRNAs.9 Over half of known miRNA genes are located in cancer-related genomic regions, suggestive of their close association with cancer initiation and progression.10 Thus far, several miRNAs have been demonstrated to show aberrant expression in human malignancies, such as gastric cancer,11 lung cancer,12 prostate cancer,13 glioblastoma,14 and pancreatic cancer.15 In particular, a variety of miRNAs are dysregulated in CRC and play either tumor-suppressive or oncogenic roles depending on the biological behaviors of their target genes.16–18 Aberrantly expressed miRNAs contribute to the carcinogenesis and progression of CRC by affecting numerous biological processes, including cell proliferation, cell cycle arrest, apoptosis, invasion, metastasis, epithelial-mesenchymal transition, angiogenesis, and resistance to radiochemotherapy.19–21 Therefore, investigation of CRC-related miRNAs is useful for understanding their roles and providing effective therapeutic targets for the management of CRC.
miR-877-5p (miR-877) was recently found to be related to tumorigenesis in hepatocellular carcinoma22,23 and renal cell carcinoma.24 However, little is known regarding the expression patterns and biological functions of miR-877 in CRC tumorigenesis. In the present study, we detected the expression of miR-877 in CRC and examined its clinical significance in patients with CRC. In addition, we investigated the effects of miR-877 upregulation on CRC cell proliferation, colony formation, apoptosis, and metastasis in vitro and tumor growth in vivo. Furthermore, the mechanism underlying the tumor suppressor action of miR-877 in CRC cells was explored,
Material and methods
Patients and tissue specimens
This study was approved by the Ethical Committee of Xiangya Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients enrolled in the study. In total, 53 pairs of CRC tissues and adjacent non-tumor tissues were collected from patients who underwent surgical tumor resection at Xiangya Hospital between June 2015 and August 2017. None of the patients had received either chemotherapy or radiotherapy prior to surgical resection. Following surgical resection, all tissue samples were quickly frozen in liquid nitrogen and stored at −80 °C for further use.
Cell lines
A normal human colon epithelium cell line (FHC) and four CRC cell lines (SW480, SW620, HT29, and HCT116) were purchased from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin mixture (all from Gibco-Thermo Fisher Scientific Inc., Waltham, MA, USA) and were maintained at 37 °C in a humidified incubator supplied with 5% CO2.
Oligonucleotide and plasmid transfection
miR-877 mimics and negative control (miR-NC) were obtained from GenePharma Co. Ltd. (Shanghai, China). Small interfering RNA (siRNA) used to knock down endogenous MTDH (MTDH siRNA) and negative control siRNA (NC siRNA) were purchased from RiboBio Co. Ltd. (Guangzhou, China). The pcDNA3.1-MTDH (pc-MTDH) plasmid used for MTDH overexpression and the empty pcDNA3.1 plasmid were constructed by the Chinese Academy of Sciences (Changchun, China). Cells were seeded into 6-well plates, and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for all transfection experiments.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissue samples or cultured cells using TRIzol® (Invitrogen), and the concentration of total RNA was measured using a NanoDrop 2000/2000c (Thermo Fisher Scientific, Inc.). The integrity of total RNA was determined by agarose gel electrophoresis. To quantify miR-877 expression, total RNA was reverse-transcribed into complementary DNA (cDNA) using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The expression level of miR-877 was determined with a TaqMan MicroRNA PCR Kit (Applied Biosystems). For MTDH mRNA detection, cDNA was synthesized from total RNA using a PrimeScript™ RT Reagent Kit (Takara Biotechnology Co. Ltd., Dalian, China). Quantitative PCR was conducted using SYBR Premix Ex Taq master mix (Takara Biotechnology Co. Ltd., Dalian, China). The U6 small nuclear RNA and glyceraldehyde-3-phosphate (GAPDH) were used as internal controls to normalize the relative expression levels of miR-877 and MTDH mRNA, respectively. Each assay was performed in triplicate, and all data were analyzed using the 2−∆∆Ct method.25
Cell counting kit-8 (CCK-8) and colony formation assays
Transfected cells were collected after 24 h of incubation, suspended in DMEM, and inoculated in 96-well plates at a density of 3×103 cells/well. Cellular proliferation was measured at different time points (0, 24, 48, and 72 h) after inoculation. At these specified times, CCK-8 assays were performed by adding 10 μL of CCK-8 solution (Beyotime Institute of Biotechnology, Haimen, China) to each well. After 2 h of incubation at 37 °C, the optical density (OD) value was determined at a wavelength of 450 nm using a microplate reader (iMark™; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
For the colony formation assay, the transfected cells were harvested at 24 h post-transfection and seeded into 6-well plates at an initial density of 1×103 cells/well. Cells were grown at 37 °C in 5% CO2 for 14 days. On day 15, cells were fixed with 4% paraformaldehyde and stained with methyl violet. After extensive washing with phosphate-buffered saline (PBS), the number of colonies (>50 cells/colony) was counted under an inverted light microscope (Olympus Corporation, Tokyo, Japan).
Assessment of apoptosis using annexin v-fluorescein isothiocyanate (FITC)
The annexin V-FITC apoptosis detection kit (BioLegend, San Diego, CA, USA) was used for the detection of cell apoptosis. The transfected cells were trypsinized 48 h after transfection and then washed with ice-cold PBS. These cells were resuspended in 100 µL of binding buffer and treated with 5 µL of annexin V-FITC and 5 µL of propidium iodide. Following 20 min of incubation at room temperature in the dark, the cells were analyzed using flow cytometry (FACScan™; BD Biosciences, Franklin Lakes, NJ, USA).
Transwell migration and invasion assays
Single-cell suspensions of transfected cells were prepared by trypsinization at 48 h post-transfection. For migration assays, a total of 5×104 transfected cells suspended in FBS-free DMEM were seeded into the upper compartments of a 24-well Transwell chamber (Corning Costar, Corning, NY, USA) containing a polycarbonate membrane filter (pore size: 8 μM). The lower compartments were filled with 500 µL DMEM supplemented with 10% FBS to serve as a chemoattractant. After 24 h of incubation, cells that failed to pass through the membrane were removed using a cotton swab. The migrated cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. For each chamber insert, five visual fields were randomly selected, and the average number of migrated cells was counted under an inverted light microscope. The procedure for the invasion assay was similar to that for the migration assay, except that the Transwell chambers were precoated with Matrigel (BD Biosciences).
Xenograft experiment
A total of eight BALB/c nude mice (4 weeks old) were purchased from the Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, China). SW480 cells transfected with miR-877 mimics or miR-NC were collected after 24 h of incubation. In total, 5×106 transfected cells in 100 µL culture medium were subcutaneously injected into the dorsal flanks of nude mice. Tumor length and width were measured every 4 days, and tumor volume was calculated using the following formula: tumor volume (mm3) = width2 (mm2) × length (mm)/2.26 All mice were sacrificed 30 days after implantation, and the xenografts formed were excised and weighed. The xenograft experiments were approved by the Ethics Review Committee of Xiangya Hospital and performed in accordance with the “Animal Protection Law of the People’s Republic of China-2009” for experimental animals.
Bioinformatics analysis
The putative targets of miR-877 were predicted using TargetScan (
Luciferase reporter assay
The 3′-UTR of MTDH containing a wild-type (WT) or mutated (MUT) version of the putative binding region of miR-877 was amplified by Shanghai GenePharma Co. Ltd. and inserted into the pGL3-promoter vector (Promega, Madison, WI, USA). The chemically constructed luciferase reporter vectors were referred to as pGL3-WT-MTDH-3′-UTR and pGL3-MUT-MTDH-3′-UTR, respectively. For luciferase reporter assays, cells in 24-well plates were co-transfected with miR-877 mimics or miR-NC and pGL3-WT-MTDH-3′-UTR or pGL3-MUT-MTDH-3′-UTR using Lipofectamine 2000, according to the manufacturer’s instructions. After 6 h of incubation at 37 °C and 5% CO2, the culture medium was replaced with fresh DMEM containing 10% FBS. The luciferase activity was assessed at 48 h post-transfection using a dual-luciferase reporter assay system (Promega). The activity of firefly luciferase was normalized to that of Renilla luciferase.
Western blotting analysis
Total protein was isolated from tissue samples or cultured cells using a Total Protein Extraction kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), and the protein concentration was evaluated using a BCA kit (Pierce, Rockford, IL, USA). Equivalent amounts of proteins were loaded and separated on a 10% sodium dodecyl sulfate polyacrylamide gel, and the separated bands were transferred onto polyvinylidene difluoride membranes (Beyotime Institute of Biotechnology). The membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) and incubated overnight at 4 °C with primary antibodies. Following extensive washing with TBST, horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (ab6728; 1:5,000 dilution; Abcam, Cambridge, UK) were used to probe the membranes at room temperature for 2 h. The protein signals were visualized using an enhanced chemiluminescence (ECL) protein detection kit (Pierce). The primary antibodies used in this study were as follows: mouse anti-human monoclonal PTEN antibody (ab77161; 1:1,000 dilution; Abcam), mouse anti-human MTDH monoclonal antibody (sc-517220; 1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-human monoclonal AKT antibody (sc-81434; 1:1,000 dilution; Santa Cruz Biotechnology), mouse anti-human monoclonal p-AKT antibody (sc-514032; 1:1,000 dilution; Santa Cruz Biotechnology), and mouse anti-human monoclonal GADPH antibody (sc-51907; 1:1,000 dilution; Santa Cruz Biotechnology).
Statistical analysis
Data are expressed as mean ± standard deviation (mean ± SD) of at least three separate experiments. All data were analyzed using SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism V5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Associations between miR-877 and clinicopathological factors were examined using chi-square tests. Differences between multiple groups or two groups were analyzed with one-way analysis of variance followed by Tukey’s post-hoc test or Student’s t-test, respectively. Spearman’s correlation analysis was performed to determine the relationship between miR-877 and MTDH mRNA levels in CRC tissues. Results with values of P<0.05 were considered statistically significant.
Results
miR-877 is downregulated in CRC tissues and cell lines
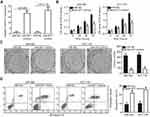
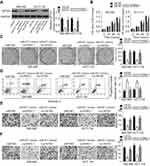
The expression levels of miR-877 were evaluated in 53 pairs of CRC tissues and adjacent non-tumor tissues using RT-qPCR. miR-877 expression was notably lower in CRC tissues that in the adjacent non-tumor tissues (Figure 1A, P<0.05). To evaluate the clinical significance of miR-877 in CRC, all patients were divided into groups with low and high miR-877 expression according to the median value of miR-877. Low miR-877 expression was significantly correlated with lymph node metastasis (P=0.039) and TNM stage (P=0.018). However, no obvious association was identified between the expression of miR-877 and other clinicopathological factors (all P>0.05; Table 1). Furthermore, the expression level of miR-877 was evaluated in four CRC cell lines (SW480, SW620, HT29, and HCT116) and a normal human colon epithelium cell line (FHC). All four CRC cell lines showed significant downregulation of miR-877 as compared with expression in FHC cells (Figure 1B, P<0.05). These results suggest that miR-877 may be involved in colorectal carcinogenesis.
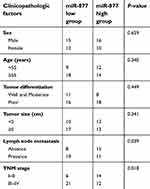 | Table 1 The association between miR-877 expression levels and clinicopathological factors of patients with colorectal cancer |
miR-877 upregulation inhibits cell proliferation and colony formation and induces cell apoptosis in CRC
To reveal the specific role of miR-877 in CRC, the SW480 and HCT116 cell lines, which showed the lowest miR-877 level among the four CRC cell lines, were chosen for functional assays. We transfected the two cell lines with miR-877 mimics or miR-NC. After transfection, RT-qPCR analysis demonstrated that miR-877 expression was notably upregulated in SW480 and HCT116 cells treated with miR-877 mimics (Figure 2A, P<0.05). CCK-8 assay was used to determine the effect of miR-877 overexpression on the proliferation of CRC cells and showed that ectopic miR-877 expression significantly suppressed the proliferative ability of SW480 and HCT116 cells (Figure 2B, P<0.05). Furthermore, colony formation assay results revealed that fewer colonies were formed by SW480 and HCT116 cells transfected with miR-877 mimics than by cells transfected with miR-NC (Figure 2C, P<0.05). Next, the influence of miR-877 upregulation on CRC cell apoptosis was examined, and a significant increase in the apoptosis rate was observed in SW480 and HCT116 cells transfected with miR-877 mimics compared to that in cells transfected with miR-NC (Figure 2D, P<0.05). These results suggest that miR-877 may inhibit the growth of CRC cells in vitro.
miR-877 overexpression attenuates the migration and invasion of CRC cells
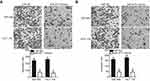
Transwell migration and invasion assays were performed to investigate the functional role of miR-877 in the migration and invasion of CRC cells. Restoration of miR-877 expression resulted in significantly reduced migration (Figure 3A, P<0.05) and invasion (Figure 3B, P<0.05) capacities of SW480 and HCT116 cells. These results suggest that miR-877 may inhibit the metastasis of CRC cells in vitro.
MTDH is a direct target of miR-877 in CRC cells
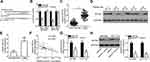
To understand the mechanism underlying the tumor suppressive role of miR-877 in CRC, the putative targets of miR-877 were predicted using two different miRNA target prediction programs: TargetScan and miRDB. MTDH was predicted to be a potential target of miR-877 by both target prediction programs. The 3′-UTR of MTDH carries a sequence complementary to that of miR-877 (Figure 4A). Thus, MTDH, which is abnormally upregulated in CRC and is known to play crucial roles in the development of CRC,27–34 was chosen for further analysis. To confirm that MTDH is a target of miR-877, luciferase reporter assays were performed in SW480 and HCT116 cells co-transfected with miR-877 mimics or miR-NC and a luciferase reporter plasmid carrying the WT or MUT MTDH binding site. As shown in Figure 4B, the restoration of miR-877 expression prominently reduced the luciferase activity of the plasmid harboring the WT miR-877 site in the 3′-UTR of MTDH in both SW480 and HCT116 cells (P<0.05). However, luciferase activity was unaffected upon transfection with the plasmid carrying the MUT binding sequence in the 3′-UTR.
To further explore the association between miR-877 and MTDH in CRC, we detected MTDH expression in CRC tissues and investigated whether miR-877 expression was negatively correlated with MTDH expression. RT-qPCR and western blotting analyses showed that MTDH mRNA (Figure 4C, P<0.05) and protein (Figure 4D and E, P<0.05) expression levels were significantly upregulated in CRC tissues relative to those in adjacent non-tumor tissues. In addition, an inverse correlation was found between the expression levels of miR-877 and MTDH mRNA in CRC tissues according to Spearman’s correlation analysis (Figure 4F, r=− 0.5381, P<0.0001). Furthermore, transfection with miR-877 mimics resulted in a significant decrease in MTDH expression at both the mRNA (Figure 4G, P<0.05) and protein (Figure 4H, P<0.05) levels in SW480 and HCT116 cells. Thus, MTDH is a direct target of miR-877 in CRC cells.
Inhibition of MTDH simulates tumor suppressor role of miR-877 in CRC cells
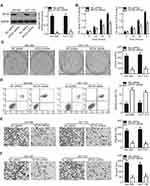
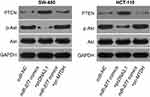
To evaluate whether MTDH is a direct functional downstream target of miR-877 in CRC cells, we explored the functional roles of MTDH. SW480 and HCT116 cells were transfected with MTDH or NC siRNA. Western blotting analysis indicated that MTDH siRNA transfection effectively knocked down the expression of endogenous MTDH in both SW480 and HCT116 cells (Figure 5A, P<0.05). A series of functional assays revealed that MTDH knockdown resulted in the inhibition of cell proliferation (Figure 5B, P<0.05) and colony formation (Figure 5C, P<0.05), induction of cell apoptosis (Figure 5D, P<0.05), and attenuation of cell migration (Figure 5E, P<0.05) and invasion (Figure 5F, P<0.05). These results demonstrate that MTDH knockdown reproduces the results observed for miR-877 overexpression in CRC cells, suggesting that MTDH may act as a direct functional target of miR-877 in CRC cells.
Restoration of MTDH expression counteracts the tumor suppressor role of miR-877 in CRC cells
As MTDH was identified as a direct target of miR-877, rescue experiments were conducted to investigate whether the effects of miR-877 on CRC cells were dependent on MTDH expression. An MTDH overexpression plasmid (pc-MTDH) or empty pcDNA3.1 plasmid was transfected into SW480 and HCT116 cells that were treated with miR-877 mimics. Western blotting analysis showed that the decrease in MTDH protein expression caused by miR-877 upregulation was reversed in SW480 and HCT116 cells co-transfected with pc-MTDH (Figure 6A, P<0.05). The tumor suppressive effects observed upon miR-877 overexpression, including effects on cell proliferation (Figure 6B, P<0.05), colony formation (Figure 6C, P<0.05), apoptosis (Figure 6D, P<0.05), migration (Figure 6E, P<0.05), and invasion (Figure 6F, P<0.05), were partly counteracted upon restoration of MTDH expression. These results demonstrate that miR-877 may act as a tumor suppressor in CRC, at least in part, by reducing MTDH expression.
miR-877 inhibits activation of the PTEN/Akt signaling pathway in CRC cells
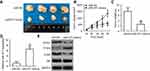
Studies have shown the involvement of MTDH in the regulation of the PTEN/Akt signaling pathway.35,36 We therefore explored the ability of miR-877 to influence the activation of the PTEN/Akt pathway in CRC cells via the inhibition of MTDH expression. Levels of PTEN, p-Akt, and Akt were measured in SW480 and HCT116 cells after co-transfection with miR-877 mimics and pc-MTDH or empty pcDNA3.1 plasmid. The ectopic expression of miR-877 resulted in an increase in PTEN and a decrease in p-Akt protein levels in SW480 and HCT116 cells; no change was observed in the expression level of total Akt protein. Restoration of MTDH expression abolished the changes in PTEN and p-Akt protein expression induced in response to miR-877 upregulation (Figure 7). Thus, miR-877 directly targets MTDH to inhibit the activation of the PTEN/Akt pathway in CRC cells.
miR-877 inhibits growth of colorectal tumors in vivo
To extend these observations in vivo, we established a xenograft model to evaluate whether miR-877 expression affects the growth of CRC cells in vivo. SW480 cells transfected with miR-877 mimics or miR-NC were injected into the dorsal flanks of nude mice. As indicated in Figure 8A and B, the volumes of xenograft tumors were significantly lower in mice treated with miR-877 mimics than in those treated with miR-NC (P<0.05). In addition, treatment with miR-877 mimics caused a notable decrease in tumor weight relative to treatment with miR-NC (Figure 8C, P<0.05). The expression of miR-877 was higher in the miR-877 mimics groups than in the miR-NC group, as detected by RT-qPCR (Figure 8D, P<0.05). Western blotting analysis revealed significant downregulation of the expression of MTDH and p-Akt and upregulation of PTEN in the miR-877 mimics-treated group (Figure 8E). These data suggest that miR-877 restricts the growth of CRC cells in vivo through the suppression of MTDH expression and regulation of the PTEN/Akt pathway.
Discussion
Studies have highlighted the aberrant expression patterns of several miRNAs in CRC.37–39 Dysregulated miRNA expression is associated with CRC pathogenesis, as these miRNAs act as tumor suppressors or oncogenes depending on the biological functions of their target genes.39 Therefore, studies on these miRNAs in CRC may improve our understanding of the mechanisms underlying CRC occurrence and development, which may be helpful for the design of promising therapeutic approaches for patients with CRC. In the present study, we detected the expression of miR-877 in CRC tissues and cell lines and clarified the clinical significance of miR-877 in patients with CRC. We determined the roles of miR-877 in CRC and explored the underlying mechanisms.
miR-877 is downregulated in hepatocellular carcinoma tissues and cell lines,22,23 and a decrease in miR-877 expression is strongly associated with histologic grade and TNM stage.22 Kaplan-Meier analysis previously indicated that low miR-877 expression was significantly correlated with a shorter overall survival and disease-free survival. In addition, multivariate analysis identified miR-877 as an independent biomarker for poor prognosis in patients with hepatocellular carcinoma.22 Low expression of miR-877 has also been detected in blood sera, tissues, and cell lines from subjects with renal cell carcinoma.24 However, the expression status of miR-877 in CRC was previously unclear. Herein, we collected 53 pairs of CRC tissues and adjacent non-tumor tissues and evaluated the expression level of miR-877. The expression of miR-877 was also determined in several human CRC cell lines. Our results showed that the expression of miR-877 was downregulated in CRC tissues and cell lines. The clinical significance of miR-877 expression in patients with CRC was also determined. Low expression of miR-877 was related to lymph node metastasis and TNM stage in CRC patients. These findings suggest that miR-877 may be a potential biomarker for the diagnosis of human malignancies.
miR-877 acts as a tumor suppressor during carcinogenesis and cancer progression. For instance, miR-877 overexpression suppresses hepatocellular carcinoma cell proliferation, colony formation, and metastasis and improves the chemosensitivity of CRC cells to paclitaxel.22,23 In renal cell carcinoma, the restoration of miR-877 expression decreases cell proliferation and migration.24 However, the specific roles of miR-877 in the progression and development of CRC were unknown. In the current study, a series of in vitro and in vivo experiments revealed that miR-877 upregulation attenuated CRC cell proliferation and colony formation activity, induced apoptosis, reduced cell migration and invasion, and restricted tumor growth in vivo. These findings suggest that miR-877 may serve as a promising therapeutic target for the treatment of patients with these cancer types.
Multiple genes, including cyclin-dependent kinase 14 (CDK14),22 Forkhead box protein M1 (FOXM1),23 and eukaryotic elongation factor-2 kinase,24 are known as direct targets of miR-877. MTDH, also known as astrocyte elevated gene-1, was predicted to be a putative target gene of miR-877 through bioinformatics analysis. Luciferase reporter assays revealed that miR-877 directly interacted with the 3′-UTR of MTDH in CRC cells. In CRC tissues, MTDH was overexpressed, and MTDH expression inversely correlated with that of miR-877. In addition, RT-qPCR and western blotting analyses demonstrated a decrease in MTDH expression at both the mRNA and protein levels in CRC cells upon the restoration of miR-877 expression. MTDH knockdown recapitulated the results observed following miR-877 overexpression in CRC cells, while restoration of MTDH expression antagonized the tumor suppressive effects observed upon miR-877 overexpression in CRC cells. These results provide sufficient evidence to demonstrate that MTDH is a direct and functional downstream target of miR-877 in CRC cells.
MTDH is overexpressed in CRC tissues and cell lines. MTDH expression is correlated with age, tumor location, Union for International Cancer Control (UICC) stage, TNM stage, Duke’s stage, and histological differentiation.27–29 Patients with CRC with high MTDH expression exhibit poorer postoperative disease-specific survival than those with low MTDH expression. Cox regression analysis has shown that the MTDH expression level is an independent prognostic factor in patients with CRC.29 MTDH has oncogenic roles in colorectal carcinogenesis and cancer development, affecting cell proliferation, cell cycle progression, apoptosis, migration, invasion, and metastasis, as well as the epithelial‑mesenchymal transition.30–34 In the present study, miR-877 directly targeted MTDH and inhibited the aggressive behavior of CRC cells. Thus, MTDH silencing using miR-877 may serve as an attractive therapeutic strategy for CRC treatment. Despite these findings, in the current study, we did not determine the association between miR-877 expression and the prognosis of CRC patients. Additionally, the influence of miR-877 on metastasis in vivo was not examined. These issues will be investigated in future studies.
Taken together, the results of the present study confirmed the downregulation of miR-877 expression in CRC tissues and cell lines. miR-877 inhibited the malignant progression of CRC cells by directly targeting MTDH and regulating the PTEN/Akt signaling pathway. These findings may provide a novel strategy facilitating the development of valuable techniques for CRC prevention and therapy.
Acknowledgments
This study was supported by The Nature Scientific Foundation of China (grant number: 81702956), The Strategy-Oriented Special Project of Center South University in China (grant number: ZLXD2017003), and The XiangYa-Peking University Wei Ming Clinical and Rehabilitation Research Fund (grant number: xywm2015I21).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi:10.3322/caac.20038
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi:10.1002/ijc.25516
3. Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer. 2016;15(3):195–203. doi:10.1016/j.clcc.2016.02.008
4. Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105–114.
5. Zhou Y, Feng X, Liu YL, et al. Down-regulation of miR-126 is associated with colorectal cancer cells proliferation, migration and invasion by targeting IRS-1 via the AKT and ERK1/2 signaling pathways. PLoS One. 2013;8(11):e81203. doi:10.1371/journal.pone.0081203
6. Dopeso H, Rodrigues P, Bilic J, et al. Mechanisms of inactivation of the tumour suppressor gene RHOA in colorectal cancer. Br J Cancer. 2018;118(1):106–116. doi:10.1038/bjc.2017.420
7. Augustus GJ, Ellis NA. Colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am J Pathol. 2018;188(2):291–303. doi:10.1016/j.ajpath.2017.07.023
8. Mendell JT, Olson EN. microRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–1187. doi:10.1016/j.cell.2012.02.005
9. Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18(5–6):282–289. doi:10.1016/j.drudis.2012.10.002
10. Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi:10.1073/pnas.0307323101
11. Link A, Kupcinskas J. microRNAs as non-invasive diagnostic biomarkers for gastric cancer: current insights and future perspectives. World J Gastroenterol. 2018;24(30):3313–3329. doi:10.3748/wjg.v24.i30.3313
12. Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. microRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 2018. doi:10.1016/j.mam.2018.07.003
13. Bryzgunova OE, Konoshenko MY, Laktionov PP. microRNA-guided gene expression in prostate cancer: literature and database overview. J Gene Med. 2018;20(5):e3016. doi:10.1002/jgm.v20.5
14. Banelli B, Forlani A, Allemanni G, Morabito A, Pistillo MP, Romani M. microRNA in glioblastoma: an overview. Int J Genomics. 2017;2017:7639084. doi:10.1155/2017/7639084
15. Qadir MI, Faheem A. miRNA: a diagnostic and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene Expr. 2017;27(3):197–204. doi:10.1615/CritRevEukaryotGeneExpr.2017019494
16. To KK, Tong CW, Wu M, Cho WC. microRNAs in the prognosis and therapy of colorectal cancer: from bench to bedside. World J Gastroenterol. 2018;24(27):2949–2973. doi:10.3748/wjg.v24.i27.2949
17. Wu QB, Sheng X, Zhang N, Yang MW, Wang F. Role of microRNAs in the resistance of colorectal cancer to chemoradiotherapy. Mol Clin Oncol. 2018;8(4):528–532. doi:10.3892/mco.2018.1578
18. Shirafkan N, Mansoori B, Mohammadi A, Shomali N, Ghasbi M, Baradaran B. microRNAs as novel biomarkers for colorectal cancer: new outlooks. Biomed Pharmacother. 2018;97:1319–1330. doi:10.1016/j.biopha.2017.11.046
19. Masuda T, Hayashi N, Kuroda Y, Ito S, Eguchi H, Mimori K. microRNAs as biomarkers in colorectal cancer. Cancers. 2017;9:9. doi:10.3390/cancers9090124
20. Xie T, Huang M, Wang Y, Wang L, Chen C, Chu X. microRNAs as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell Physiol Biochem. 2016;40(1–2):62–76. doi:10.1159/000452525
21. Yang S, Sun Z, Zhou Q, et al. MicroRNAs, long noncoding RNAs, and circular RNAs: potential tumor biomarkers and targets for colorectal cancer. Cancer Manag Res. 2018;10:2249–2257. doi:10.2147/CMAR.S166308
22. Yan TH, Qiu C, Sun J, Li WH. miR-877-5p suppresses cell growth, migration and invasion by targeting cyclin dependent kinase 14 and predicts prognosis in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(10):3038–3046. doi:10.26355/eurrev_201805_15061
23. Huang X, Qin J, Lu S. Up-regulation of miR-877 induced by paclitaxel inhibits hepatocellular carcinoma cell proliferation though targeting FOXM1. Int J Clin Exp Pathol. 2015;8(2):1515–1524.
24. Shi Q, Xu X, Liu Q, Luo F, Shi J, He X. microRNA-877 acts as a tumor suppressor by directly targeting eEF2K in renal cell carcinoma. Oncol Lett. 2016;11(2):1474–1480. doi:10.3892/ol.2015.4072
25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
26. Zheng H, Guo Z, Zheng X, Cheng W, Huang X. microRNA-144-3p inhibits cell proliferation and induces cell apoptosis in prostate cancer by targeting CEP55. Am J Transl Res. 2018;10(8):2457–2468.
27. Song H, Li C, Li R, Geng J. Prognostic significance of AEG-1 expression in colorectal carcinoma. Int J Colorectal Dis. 2010;25(10):1201–1209. doi:10.1007/s00384-010-1009-3
28. Gnosa S, Shen YM, Wang CJ, et al. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. J Transl Med. 2012;10:109. doi:10.1186/1479-5876-10-233
29. Jiang T, Zhu A, Zhu Y, Piao D. Clinical implications of AEG-1 in liver metastasis of colorectal cancer. Med Oncol. 2012;29(4):2858–2863. doi:10.1007/s12032-012-0186-z
30. Sun X, Zhai H, Chen X, Kong R, Zhang X. MicroRNA-1271 suppresses the proliferation and invasion of colorectal cancer cells by regulating metadherin/Wnt signaling. J Biochem Mol Toxicol. 2018;32:2. doi:10.1002/jbt.2018.32.issue-2
31. Wang Z, Wang L, Hu J, et al. RARRES3 suppressed metastasis through suppression of MTDH to regulate epithelial-mesenchymal transition in colorectal cancer. Am J Cancer Res. 2015;5(6):1988–1999.
32. Wang B, Shen ZL, Jiang KW, et al. microRNA-217 functions as a prognosis predictor and inhibits colorectal cancer cell proliferation and invasion via an AEG-1 dependent mechanism. BMC Cancer. 2015;15:437. doi:10.1186/s12885-015-1584-3
33. Song HT, Qin Y, Yao GD, Tian ZN, Fu SB, Geng JS. Astrocyte elevated gene-1 mediates glycolysis and tumorigenesis in colorectal carcinoma cells via AMPK signaling. Mediators Inflamm. 2014;2014:287381. doi:10.1155/2014/287381
34. Huang S, Wu B, Li D, et al. Knockdown of astrocyte elevated gene-1 inhibits tumor growth and modifies microRNAs expression profiles in human colorectal cancer cells. Biochem Biophys Res Commun. 2014;444(3):338–345. doi:10.1016/j.bbrc.2014.01.046
35. Li J, Li C, Li H, et al. microRNA30a5p suppresses tumor cell proliferation of human renal cancer via the MTDH/PTEN/AKT pathway. Int J Mol Med. 2018;41(2):1021–1029. doi:10.3892/ijmm.2017.3269
36. Li L, Zhang H. microRNA-379 inhibits cell proliferation and invasion in glioma via targeting metadherin and regulating PTEN/AKT pathway. Mol Med Rep. 2017. doi:10.3892/mmr
37. Ke SB, Qiu H, Chen JM, Shi W, Chen YS. microRNA-202-5p functions as a tumor suppressor in colorectal carcinoma by directly targeting SMARCC1. Gene. 2018. doi:10.1016/j.gene.2018.08.064
38. Park YR, Seo SY, Kim SL, et al. microRNA-206 suppresses PGE2-induced colorectal cancer cell proliferation, migration, and invasion by targeting TM4SF1. Biosci Rep. 2018;38. doi:10.1042/BSR20180664
39. Yang L, Wl M, Km Y, Tie J, Yz N, Hj X. miR-340-5p is a potential prognostic indicator of colorectal cancer and modulates ANXA3. Eur Rev Med Pharmacol Sci. 2018;22(15):4837–4845. doi:10.26355/eurrev_201808_15619
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.