Back to Journals » OncoTargets and Therapy » Volume 13
MicroRNA-766 Promotes The Proliferation, Migration And Invasion, And Inhibits The Apoptosis Of Cutaneous Squamous Cell Carcinoma Cells By Targeting PDCD5
Authors Liu P, Shi L, Ding Y, Luan J, Shan X, Li Q, Zhang S
Received 11 July 2019
Accepted for publication 21 October 2019
Published 13 May 2020 Volume 2020:13 Pages 4099—4110
DOI https://doi.org/10.2147/OTT.S222821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Pengyu Liu,1,* Liang Shi,2,* Yan Ding,1 Jiaxi Luan,1 Xiaojun Shan,3 Qinghua Li,1 Shuhua Zhang1
1Department of Hepatobiliary Surgery, Oilfields General Hospital in Daqing, Daqing City, Heilongjiang Province 163000, People’s Republic of China; 2Department of Plastic Hand Surgery, Oilfields General Hospital in Daqing, Daqing City, Heilongjiang Province 163000, People’s Republic of China; 3Department of Thyroid and Breast Surgery, Oilfields General Hospital in Daqing, Daqing City, Heilongjiang Province 163000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Shi
Department of Plastic Hand Surgery, Oilfields General Hospital in Daqing, No. 9, Zhongkang Street, Sa’er’tu District, Daqing City, Heilongjiang Province 163000, People’s Republic of China
Tel +86-13349506617
Email [email protected]
Purpose: This study aimed to investigate the regulatory role and mechanism of microRNA-766 (miR-766) on cutaneous squamous cell carcinoma (CSCC) cells.
Methods: The expression of miR-766 and programmed cell death 5 (PDCD5) was detected in CSCC tissues and CSCC cell lines (A431, SCL-1 and DJM-1 cells) by qRT-RCR. The proliferation, colony-forming ability, apoptosis, migration and invasion of A431 and SCL-1 cells was measured by MTT, colony formation, flow cytometry, wound healing and transwell assay, respectively. The interaction between miR-766 and PDCD5 was detected by dual-luciferase reporter gene assay. The expression of matrix metalloproteinase 2 (MMP-2), MMP-9 and PDCD5 was measured by Western blot. In addition, A431 cells were subcutaneously injected into mice, and the tumor volume and weight were measured.
Results: MiR-766 was upregulated, and PDCD5 was downregulated in CSCC tissues and cells. MiR-766 significantly promoted the proliferation, migration and invasion, and inhibited the apoptosis of A431 and SCL-1 cells. MiR-766 also significantly increased the expression of MMP-2 and MMP-9 in A431 and SCL-1 cells. PDCD5 was a target gene of miR-766. PDCD5 significantly reversed the tumor-promoting effect of miR-766 on A431 and SCL-1 cells. In addition, miR-766 inhibitor inhibited the tumor growth in mice.
Conclusion: MiR-766 inhibitor inhibited the proliferation, migration and invasion, and promoted the apoptosis of CSCC cells via downregulating PDCD5.
Keywords: CSCC, miR-766, proliferation, migration, invasion, apoptosis, PDCD5
Introduction
Cutaneous squamous cell carcinoma (CSCC) is an epidermal keratinocyte-derived skin tumor that is considered as one of the most common types of skin cancer.1 Recently, the incidence of CSCC is still increasing.2 Although substantial improvements have been made on the treatment of CSCC, the prognosis of CSCC remains poor due to metastasis and recurrence.3,4 Hence, new therapeutic methods and targets against CSCC are urgently needed.
MicroRNAs (miRNAs) are small endogenous non-coding RNAs (22–24 nt) that modulate gene expression via binding to the 3ʹ-untranslated region (3ʹ-UTR) of mRNA molecules.5,6 More and more evidence have demonstrated that miRNAs participate in diverse cellular processes, such as cell proliferation, apoptosis and differentiation.7,8 Some miRNAs have been reported to play an important role in the progress of CSCC. For example, miR-221 plays an oncogenic function in CSCC through promoting CSCC cell proliferation and cell cycle.9 Hu et al10 have indicated that miR-186 promotes cell proliferation and suppresses cell apoptosis in CSCC. In addition, growing evidence have indicated that miR-766 acts as a tumor promoter in different types of cancers.11,12 A microarray analysis by Sand et al13 has indicated that miR-766 is highly expressed in CSCC and may play an important role in the molecular pathogenesis of CSCC. However, the specific regulatory effect of miR-766 on CSCC remains unclear.
Programmed cell death 5 (PDCD5), one of the programmed cell death proteins, is abnormally expressed in many cancers and plays an important role in cell proliferation, apoptosis and metastasis.14–16 Fan et al17 have indicated that PDCD5 can inhibit cell growth and invasion, and promote cell apoptosis in hepatic cancer. However, whether the regulatory effect of miR-766 on CSCC is related to PDCD5 is unknown.
In this study, we explored the function of miR-766 on CSCC cells, as well as related molecular mechanism. Our results suggested that miR-766 could promote the proliferation, migration and invasion, and inhibit the apoptosis of CSCC cells by targeting PDCD5. Our findings may provide new theoretical foundation for the treatment of CSCC.
Materials And Methods
Clinical Samples
CSCC tissues (n = 16) were collected from patients undergoing surgery (29–59 years) in our hospital between October 2016 and September 2018. None of the patients had received chemotherapy and radiotherapy before surgical resection. Normal cutaneous tissues (n = 24) were collected from healthy volunteers. All tissues were immediately stored in liquid nitrogen after the surgery and maintained at −80°C until use. Written informed consent was obtained from each patient. This study was approved by the Ethics Committee of our hospital, and was performed in accordance with the Declaration of Helsinki.
Cell Cultures
Three CSCC cell lines (A431, SCL-1 and DJM-1) and normal human epidermal keratinocyte cell line (HaCaT) were purchased from American Type Culture Collection (ATCC, USA). Cells were cultured in RPMI-1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Sigma, USA). All cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Cell Transfection
The miR-766 mimics, miR-766 inhibitor and the corresponding negative controls (mimics NC and inhibitor NC) were purchased from Genepharma (Shanghai, China). The PDCD5 siRNA1, PDCD5 siRNA2 and siRNA Control were purchased from Invitrogen (USA). The target sequences were shown as follows: PDCD5 siRNA1: 5′-GAGAAUUACCUUAUACAGTT-3′; PDCD5 siRNA2: 5′-AGAAUUACCUUAUACAGA-3′; siRNA Control: 5′-UUCUCCGAACGUGUCACGUTT-3′. The above agents were separately transfected or co-transfected into A431 and SCL-1 cells by Lipofectamine® 3000 Reagent (Invitrogen, USA). The grouping was shown as follows: Control group (no-treated group), NC mimics group (transfected with mimics NC), miR-766 mimics group (transfected with miR-766 mimics), NC inhibitor group (transfected with miR-766 inhibitor NC), miR-766 inhibitor group (transfected with miR-766 inhibitor), siRNA group (transfected with PDCD5 siRNA), siRNA NC group (transfected with siRNA Control), si-NC + miR-766 INC group (transfected with siRNA Control and miR-766 inhibitor NC), siRNA + miR-766 INC group (transfected with PDCD5 siRNA and miR-766 inhibitor NC), siRNA + miR-766 inhibitor group (transfected with PDCD5 siRNA and miR-766 inhibitor), and si-NC + miR-766 inhibitor group (transfected with siRNA Control and miR-766 inhibitor). After 48 h of transfection, cells were used in further assays.
Cell Proliferation Assay
A431 and SCL-1 cells were seeded into 96-well plates at a density of 5 × 103 cells/well. MTT (5 mg/mL, 20 μL, Sigma) was added into each well at 0, 24, 48, 72 and 96 h post-culturing. After 4 h of incubation, 100 μL DMSO was added into each well. The absorbance at 495 nm was measured by using an ELISA reader.
Colony Formation Assay
Colony formation assay was performed to evaluate the colony-forming abilities of A431 and SCL-1 cells. In brief, A431 and SCL-1 cells were seeded into 6-well plates at a density of 1000 cells/well and cultured for 10 days. After fixed in methanol for 15 min, the colonies were stained with crystal violet for 15 min. Positive stained colonies were observed and photographed under an optical microscope.
Cell Apoptosis Assay
Cell apoptosis was detected by using Annexin V-FITC apoptosis detection kit (BD Biosciences, USA). Briefly, A431 and SCL-1 cells were washed with PBS, and stained with Annexin V-FITC and propidium iodide (PI) in dark for 15 min. The cell apoptosis was detected on a flow cytometer (BD Biosciences, USA).
Wound Healing Assay
A431 and SCL-1 cells were seeded into 12-well plates at a density of 1 × 105 cells/well and cultured for 24 h. A plastic pipette was used to scrape a wound. Followed by washing with PBS for twice, cells were cultured in complete medium for 24 h. The scratch wound was observed and photographed under an optical microscopy (100×).
Transwell Assay
Transwell assay was performed using transwell chamber (Corning, USA) pre-coated with Matrigel (BD Biosciences, USA). A431 and SCL-1 cells (1 × 105 cells/well) were harvested and added into the upper chamber. Then, 500 μL medium with 10% FBS was added into the lower chamber. After 24 h of incubation at 37°C, cells on the bottom surface of the lower chamber were fixed with 4% formaldehyde and stained with 0.5% crystal violet (Sigma, USA). The number of stained cells was counted under an optical microscope (400×).
Real-Time Fluorogenic PCR Assays
Total RNA was extracted from tissues and cells by using TRIZOL (Invitrogen, USA). Then, total RNA was reverse-transcribed into cDNA by using Reverse Transcription Kit (ThermoScientific, USA). qRT-PCR (Bio-Rad, USA) was performed by using SYBR Green Mixture (Roche, Switzerland). Primers used for qRT-PCR were shown as follows: miR-766 F: 5′-GCGGCCGCTATACACAGAGGATTGCTTAG-3′, R: 5′-ACGCGTCAGGCAACAGATTTC-3′; U6 F: 5′-CTCGCTTCGGCAGCACATATACT-3′, R: 5′-ACGCTTCACGAATTTGCGTGTC-3′; PDCD5 F: 5′-CAACAGGAAGCAAAGCAC-3′, R: 5′-GATCTTAACTTCTGCCTAGAC-3′; GAPDH F: 5′-GACGGCCGCATCTTCTTGT-3′, R: 5′-CACACCGACCTTCACCATTTT-3′. The primers were synthetized by Sangon Co., Ltd. (Shanghai, China). The relative expression of miR-766 and PDCD5 was normalized to U6 and GAPDH, respectively, and calculated according to the 2−ΔΔCt method.
Western Blot Analysis
Total proteins were extracted from cells using RIPA lysis buffer (Sigma, USA). Proteins (50 μg/lane) were separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membrane (Thermo Fisher, USA). After blocked with PBS containing 5% non-fat milk for 2 h at room temperature, the membrane was incubated with specific primary antibody (PDCD5, 1:1000, SAB2700420; MMP-2, 1:1000, SAB4501891; MMP-9,1:1000, SAB4501896, Sigma Aldrich, USA; β-actin, 1:1000, #3700, Cell Signal, USA) overnight at 4°C. Followed by three times of washing, the membranes were incubated with peroxidase-labeled secondary antibody (anti-rabbit IgG, 1:5000, Sigma, USA) for 2 h at 37°C. The protein bands were visualized by using ECL system (Thermo Fisher).
Dual-Luciferase Reporter Gene Assay
TargetScan was used to predict the targeted relationship between PDCD5 and miR-766. The exact target binding sites were identified. The 3ʹ-untranslated region (3ʹ-UTR) of PDCD5 containing miR-766 binding site was amplified, and then cloned into pmirGLO luciferase vector (Promega, USA), in order to construct wild pmirGLO-WT-PDCD5-3ʹ-UTR (PDCD5-wt) and mutant pmirGLO-MUT-PDCD5-3ʹ-UTR (PDCD5-mut). For luciferase assay, the reporter vectors with miR-766 mimics and miR-766 mimics NC were co-transfected into A431 and SCL-1 cells by Lipofectamine 3000 Reagent, respectively. A431 and SCL-1 cells were grouped as follows: MT + miR-766 mimics group (transfected with PDCD5-mut and miR-766 mimics), MT + NC group (transfected with PDCD5-mut and miR-766 mimics NC), WT + miR-766 mimics group (transfected with PDCD5-wt and miR-766 mimics) and WT + NC group (transfected with PDCD5-wt and miR-766 mimics NC). After 48 h of transfection, the luciferase activity was detected by using a dual-luciferase kit (Promega).
Tumor Formation In Mice
Male BALB/c mice (4 weeks old, N = 30) were obtained from the Laboratory Animal Centre of Chinese Academy of Sciences (Shanghai, China). Mice were randomly divided into three groups, including Mock group, miR-766 INC group and miR-766 inhibitor group (N = 10 each group). A431 cells transfected with miR-766 INC and miR-766 inhibitor were subcutaneously injected into the right axilla of mice, respectively. Mice in Mock group were subcutaneously injected with normal A431 cells without transfection. The tumor volume was measured at 4, 8, 12, 16 and 20th day post-injection using the formula as follows: volume = length × width2 × 1/2. At the 20th day post-injection, mice were killed by cervical dislocation, and the tumor weight was measured. Animal experiment was approved by the local Ethics Committee of our hospital.
Statistical Analysis
Data were presented as the mean ± SD. All statistical analyses were performed using SPSS 22.0 software. The differences between various groups were analyzed by one-way ANOVA followed by Tukey’s post hoc test. The differences between two groups were analyzed by Student's t-test. Correlation examination was performed by Spearman correlation analysis. P < 0.05 was considered to be statistically significant.
Results
MiR-766 Is Upregulated In CSCC Tissue And CSCC Cells
The results of qRT-PCR showed that the expression of miR-766 in CSCC tissues was significantly higher than that in normal tissues (P < 0.05) (Figure 1A). In addition, miR-766 expression in A431, SCL-1 and DJM-1 cells was also significantly higher than that in HaCaT cells (P < 0.05) (Figure 1B). The results above suggested that the expression of miR-766 was upregulated in CSCC.
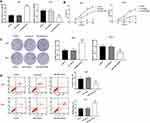
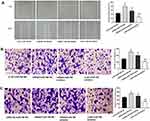
MiR-766 Promotes The Proliferation And Inhibits The Apoptosis Of A431 And SCL-1 Cells
The results of qRT-PCR (Figure 2A) showed that the expression of miR-766 in A431 cells was significantly increased in miR-766 mimics group compared with Control group and NC mimics group (P < 0.01). Meanwhile, miR-766 expression in SCL-1 cells was significantly decreased in miR-766 inhibitor group compared with Control group and NC inhibitor group (P < 0.01). MTT assay (Figure 2B) confirmed that the proliferation ability of A431 cells in miR-766 mimics group was significantly increased at 48 (P < 0.05), 72 (P < 0.01) and 96 h (P < 0.01) post-culturing compared with Control group and NC mimics group. The proliferation ability of SCL-1 cells in miR-766 inhibitor group was significantly decreased at 48 (P < 0.05), 72 (P < 0.01) and 96 h (P < 0.01) post-culturing compared with Control group and NC inhibitor group. Colony formation assay (Figure 2C) showed that the colony number of A431 cells in miR-766 mimics group was significantly higher than that in Control group and NC mimics group (P < 0.01). When compared with Control group and NC inhibitor group, the colony number of SCL-1 cells was significantly decreased in miR-766 inhibitor group (P < 0.01). In addition, miR-766 overexpression significantly inhibited the apoptosis of A431 cells (P < 0.01), whereas the silencing of miR-766 significantly promoted the apoptosis of SCL-1 cells (P < 0.01) (Figure 2D). All these results indicated that miR-766 could promote the proliferation and inhibit the apoptosis of CSCC cells.
MiR-766 Promotes The Migration And Invasion Of A431 And SCL-1 Cells
Wound healing assay (Figure 3A) showed that the migration ability of A431 cells was significantly increased in miR-766 mimics group compared with Control group and NC mimics group (P < 0.01). On the contrary, the migration ability of SCL-1 cells was significantly decreased in miR-766 inhibitor group compared with Control group and NC inhibitor group (P < 0.05). Transwell assay (Figure 3B and C) also confirmed that the migration and invasion ability of A431 cells were significantly increased in miR-766 mimics group compared with Control group and NC mimics group (P < 0.01), while the migration and invasion ability of SCL-1 cells were significantly decreased in miR-766 inhibitor group compared with Control group and NC inhibitor group (P < 0.01). In addition, we also found that miR-766 overexpression significantly increased the expression of MMP-2 and MMP-9 in A431 cells (P < 0.01), while the silencing of miR-766 significantly decreased the expression of MMP-2 and MMP-9 in SCL-1 cells (P < 0.01) (Figure 3D). These results above suggested that miR-766 could promote the migration and invasion of CSCC cells.
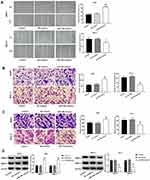
PDCD5 Is A Target Of miR-776
The result of qRT-PCR showed that the expression of PDCD5 in CSCC tissues was significantly lower than that in normal tissues (P < 0.05) (Figure 4A). The expression of miR-766 was negatively correlated with the expression of PDCD5 in CSCC tissues (P < 0.05) (Figure 4B). The mRNA and protein expression of PDCD5 in A431, SCL-1 and DJM-1 cells was significantly lower than that in HaCaT cells (P < 0.05) (Figure 4C and D). In addition, the expression of PDCD5 was significantly decreased in miR-766 mimics group compared with Control group and NC mimics group in A431 and SCL-1 cells (P < 0.05) (Figure 4E). On the contrary, the expression of PDCD5 was significantly increased in miR-766 inhibitor group compared with Control group and NC inhibitor group (P < 0.05) (Figure 4E). TargetScan predicted that the binding site of PDCD5 to miR-766 was at the 3ʹ-UTR region (Figure 4F). The luciferase activity of WT + miR-766 mimics group was significantly decreased compared with WT + NC group (P < 0.05) (Figure 4G). All these results above indicated that miR-776 could negatively regulate its target PDCD5.
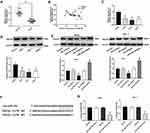
PDCD5 Reverses The Effect Of miR-766 On The Proliferation Of A431 And SCL-1 Cells
Western blot showed that PDCD5 expression in A431 and SCL-1 cells was significantly decreased in siRNA1 group and siRNA2 group compared with Control group and siRNA NC group (P < 0.05) (Figure 5A). Because PDCD5 expression was lower in siRNA2 group than that in siRNA1 group, siRNA2 was selected for the subsequent experiments. MTT and colony formation assay showed that the proliferation and colony number of A431 and SCL-1 cells were significantly increased in siRNA2 + miR-766 INC group and decreased in si-NC + miR-766 inhibitor group compared with si-NC + miR-766 INC group (P < 0.05) (Figure 5B and C). When compared with siRNA2 + miR-766 INC group, the proliferation and colony number of A431 and SCL-1 cells were significantly decreased in siRNA2 + miR-766 inhibitor group and si-NC + miR-766 inhibitor group (P < 0.05) (Figure 5B and C). These results above indicated that PDCD5 could reverse the inhibition effect of miR-766 inhibitor on the proliferation of CSCC cells.
PDCD5 Reverses The Effects Of miR-766 On The Migration And Invasion Of A431 Cells
Wound healing assay (Figure 6A) and transwell assay (Figure 6B and C) showed that the migration and invasion ability of A431 cells were significantly increased in siRNA2 + miR-766 INC group and decreased in si-NC + miR-766 inhibitor group compared with si-NC + miR-766 INC group (P < 0.05). When compared with siRNA2 + miR-766 INC group, the migration and invasion ability of A431 cells were significantly decreased in siRNA2 + miR-766 inhibitor group and si-NC + miR-766 inhibitor group (P < 0.05). These results above suggested that PDCD5 could reverse the inhibition effects of miR-766 inhibitor on the migration and invasion of CSCC cells.
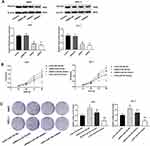
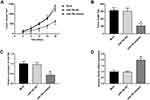
MiR-766 Inhibitor Inhibits The Tumor Growth In Mice
The anti-tumor effect of miR-766 inhibitor on CSCC was further evaluated in mice. As shown in Figure 7A, the tumor volume in miR-766 inhibitor group was significantly lower than that in Mock and miR-766 INC group beginning from the 8th day post-injection (P < 0.05). After the injection for 20 days, the tumor weight in miR-766 inhibitor group was significantly lower than that in Mock and miR-766 INC group (P < 0.05) (Figure 7B). In addition, qRT-PCR showed that the expression of miR-766 in miR-766 inhibitor group was significantly lower than that in Mock and miR-766 INC group (P < 0.05) (Figure 7C). On the contrary, the expression of PDCD5 in miR-766 inhibitor group was significantly higher than that in Mock and miR-766 INC group (P < 0.05) (Figure 7D). The above results indicated that miR-766 inhibitor could inhibit the tumor growth in mice.
Discussion
CSCC is a malignant tumor with poor prognosis.18 The incidence of CSCC is increasing in the past years.2 It is urgent to explore the molecular mechanisms involved in CSCC to better understanding CSCC and identify novel therapeutic targets. In the present study, we demonstrated that miR-766 could promote the proliferation, migration and invasion, and inhibit the apoptosis of CSCC cells by targeting PDCD5.
Up to now, massive studies have confirmed that miRNAs play important roles in various cancers, including CSCC.19 Some studies have suggested that miRNAs are abnormal expressed in CSCC.20,21 MiR-766 is highly expressed in many kinds of cancers, such as hepatocellular carcinoma,22 breast cancer23 and colorectal cancer.12 In this study, we detected the expression of miR-766 in CSCC tissues and CSCC cells (A431, SCL-1 and DJM-1), and found that miR-766 expression was highly expressed in both CSCC tissues and CSCC cells. MiRNAs have been reported to participate in the regulation of cancer cell proliferation, apoptosis, migration and invasion.7,8 For instance, miR-217 overexpression induces the growth, cell cycle and invasion of CSCC cells via targeting PTRF.24 MiR-199a inhibits the proliferation and migration of CSCC cells through regulating CD44-Ezrin pathway.25 Zhang et al26 have indicated that miR-15b suppresses the proliferation and promotes the apoptosis of CSCC cells through regulating survivin expression. Wang et al27 have confirmed that miR-199a-5p promotes the invasion of CSCC cells through inhibiting E-cadherin expression. In the present study, we demonstrated that miR-766 could promote the proliferation, migration and invasion, and inhibit the apoptosis of CSCC cells. Moreover, tumor formation experiment in mice confirmed that miR-766 inhibitor could inhibit the tumorigenesis in vivo. All these findings indicated that miR-766 may be a potential therapeutic target for CSCC. In addition, more and more researches have demonstrated that MMP-2 and MMP-9 play dominant roles in tumor metastasis.28 Our results showed that miR-766 overexpression increased the expression of MMP-2 and MMP-9 in CSCC cells, while silencing of miR-766 decreased the expression of MMP-2 and MMP-9. These results further confirmed that miR-766 could promote the migration and invasion of CSCC cells.
Programmed cell death (PCD) is an active dead process regulated by a series of intracellular programs. At present, twelve members of PDCD protein family are identified, including PDCD1-PDCD12.16 It has reported that PDCD5 is lowly expressed in various tumors, such as lung cancer,29 gastric cancer30 and liver cancer.31 In this study, we also confirmed that PDCD5 expression was downregulated in CSCC tissue and cells. It has reported that PDCD5 promotes the apoptotic process of gastric cancer cells.30 Han et al32 have indicated that PDCD5 plays anti-tumor role in osteosarcoma cells via inducing cell apoptosis and G(2) phase arrest. Xu et al33 have reported that PDCD5 suppresses the proliferation and induces the apoptosis of lung cancer cells. Zhao et al have proved that PDCD5 overexpression suppresses the metastasis of osteosarcoma cells through targeting TGF-β1/Smad pathway.14 Fan et al17 have demonstrated that PDCD5 reduces cell invasion in hepatic cancer. In the present study, we confirmed that miR-776 negative regulated its target PDCD5. In addition, we further explored whether the regulatory effect of miR-766 on CSCC was associated with PDCD5. Our results showed that PDCD5 reversed the tumor-promoting effects of miR-766 on the proliferation, migration and invasion of A431 and SCL-1 cells. These findings further suggested that miR-766 could promote the proliferation, migration and invasion, and inhibit the apoptosis of CSCC cells through targeting PDCD5.
Conclusion
In conclusion, miR-766 was upregulated and PDCD5 was downregulated in CSCC tissues and cells. MiR-766 could promote the proliferation, migration and invasion, and inhibit the apoptosis of CSCC cells through downregulating PDCD5. Our research provides a novel regulatory mechanism of miR-766 in CSCC, and points out a novel therapeutic target for CSCC.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi:10.1056/NEJM200103293441306
2. Zhou L, Wang Y, Ou C, et al. microRNA-365-targeted nuclear factor I/B transcriptionally represses cyclin-dependent kinase 6 and 4 to inhibit the progression of cutaneous squamous cell carcinoma. Int J Biochem Cell Biol. 2015;65:182–191. doi:10.1016/j.biocel.2015.06.009
3. Zhou J, Liu R, Luo C, et al. MiR-20a inhibits cutaneous squamous cell carcinoma metastasis and proliferation by directly targeting LIMK1. Cancer Biol Ther. 2014;15(10):1340–1349. doi:10.4161/cbt.29821
4. Gastaldi C, Bertero T, Xu N, et al. miR-193b/365a cluster controls progression of epidermal squamous cell carcinoma. Carcinogenesis. 2014;35(5):1110–1120. doi:10.1093/carcin/bgt490
5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
6. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi:10.1038/nature02871
7. Liu C, Wang J, Hu Y, Xie H, Liu M, Tang H. Upregulation of kazrin F by miR-186 suppresses apoptosis but promotes epithelial-mesenchymal transition to contribute to malignancy in human cervical cancer cells. Chin J Cancer Res. 2017;29(1):45–56. doi:10.21147/j.issn.1000-9604.2017.01.06
8. Liu X, Li J, Yu Z, Sun R, Kan Q. miR-935 promotes liver cancer cell proliferation and migration by targeting SOX7. Oncol Res. 2017;25(3):427–435. doi:10.3727/096504016X14747300207374
9. Gong ZH, Zhou F, Shi C, et al. miRNA-221 promotes cutaneous squamous cell carcinoma progression by targeting PTEN. Cell Mol Biol Lett. 2019;24:9. doi:10.1186/s11658-018-0131-z
10. Hu X, Liu Y, Ai P, et al. MicroRNA-186 promotes cell proliferation and inhibits cell apoptosis in cutaneous squamous cell carcinoma by targeting RETREG1. Exp Ther Med. 2019;17(3):1930–1938. doi:10.3892/etm.2019.7154
11. Li X, Shi Y, Yin Z, Xue X, Zhou B. An eight-miRNA signature as a potential biomarker for predicting survival in lung adenocarcinoma. J Transl Med. 2014;12:159. doi:10.1186/1479-5876-12-159
12. Li YC, Li CF, Chen LB, et al. MicroRNA-766 targeting regulation of SOX6 expression promoted cell proliferation of human colorectal cancer. Onco Targets Ther. 2015;8:2981–2988. doi:10.2147/OTT.S89459
13. Sand M, Skrygan M, Georgas D, et al. Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2012;68(3):119–126. doi:10.1016/j.jdermsci.2012.09.004
14. Zhao H, Peng C, Lu X, et al. PDCD5 inhibits osteosarcoma cell metastasis via targeting TGF-beta1/Smad signaling pathway and is associated with good prognosis. Am J Transl Res. 2019;11(2):1116–1128.
15. Wang W, Song XW, Zhao CH. Roles of programmed cell death protein 5 in inflammation and cancer (Review). Int J Oncol. 2016;49(5):1801–1806. doi:10.3892/ijo.2016.3706
16. Li P, Fei H, Wang L, Xu H, Zhang H, Zheng L. PDCD5 regulates cell proliferation, cell cycle progression and apoptosis. Oncol Lett. 2018;15(1):1177–1183. doi:10.3892/ol.2017.7401
17. Fan GL, Yao Y, Yao L, Li Y. PDCD5 transfection increases cisplatin sensitivity and decreases invasion in hepatic cancer cells. Oncol Lett. 2015;9(1):411–417. doi:10.3892/ol.2014.2645
18. Ahamad MS, Siddiqui S, Jafri A, Ahmad S, Afzal M, Arshad M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS One. 2014;9(10):e110003. doi:10.1371/journal.pone.0110003
19. Konicke K, Lopez-Luna A, Munoz-Carrillo JL, et al. The microRNA landscape of cutaneous squamous cell carcinoma. Drug Discov Today. 2018;23(4):864–870. doi:10.1016/j.drudis.2018.01.023
20. Anglicheau D, Muthukumar T, Suthanthiran M. MicroRNAs: small RNAs with big effects. Transplantation. 2010;90(2):105–112. doi:10.1097/TP.0b013e3181e913c2
21. Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18(3):215–222. doi:10.1097/PPO.0b013e318250c001
22. Yang C, Ma X, Guan G, et al. MicroRNA-766 promotes cancer progression by targeting NR3C2 in hepatocellular carcinoma. Faseb J. 2019;33(1):1456–1467. doi:10.1096/fj.201801151R
23. Wang Q, Selth LA, Callen DF. MiR-766 induces p53 accumulation and G2/M arrest by directly targeting MDM4. Oncotarget. 2017;8(18):29914–29924. doi:10.18632/oncotarget.15530
24. Bai M, Zhang M, Long F, Yu N, Zeng A, Wang X. MiR-217 promotes cutaneous squamous cell carcinoma progression by targeting PTRF. Am J Transl Res. 2017;9(2):647–655.
25. Wang SH, Zhou JD, He QY, Yin ZQ, Cao K, Luo CQ. MiR-199a inhibits the ability of proliferation and migration by regulating CD44-Ezrin signaling in cutaneous squamous cell carcinoma cells. Int J Clin Exp Pathol. 2014;7(10):7131–7141.
26. Zhang SH, Li ZY, Liu ZJ, Miao GY, Liu BG. MicroRNA15b regulates apoptosis of cutaneous squamous cell carcinoma SCL-1 cell line: a mechanism study. Eur Rev Med Pharmacol Sci. 2017;21(2):227–233.
27. Wang S, Cao KE, He Q, Yin Z, Zhou J. miR-199a-5p induces cell invasion by suppressing E-cadherin expression in cutaneous squamous cell carcinoma. Oncol Lett. 2016;12(1):97–101. doi:10.3892/ol.2016.4602
28. Che YL, Luo SJ, Li G, et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett. 2015;359(2):241–249. doi:10.1016/j.canlet.2015.01.019
29. Spinola M, Meyer P, Kammerer S, et al. Association of the PDCD5 locus with lung cancer risk and prognosis in smokers. J Clin Oncol. 2006;24(11):1672–1678. doi:10.1200/JCO.2005.04.4339
30. Yang YH, Zhao M, Li WM, Lu YY, Chen YY, Kang B. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis. 2006;11(6):993–1001. doi:10.1007/s10495-006-6714-6
31. Fu DZ, Cheng Y, He H, Liu HY, Liu YF. PDCD5 expression predicts a favorable outcome in patients with hepatocellular carcinoma. Int J Oncol. 2013;43(3):821–830. doi:10.3892/ijo.2013.1993
32. Han XR, Sun Y, Bai XZ. The anti-tumor role and mechanism of integrated and truncated PDCD5 proteins in osteosarcoma cells. Cell Signal. 2012;24(8):1713–1721. doi:10.1016/j.cellsig.2012.04.011
33. Xu S, Sui G, Yuan L, Zou Z. Expression of programmed cell death 5 protein inhibits progression of lung carcinoma in vitro and in vivo via the mitochondrial apoptotic pathway. Mol Med Rep. 2014;10(4):2059–2064. doi:10.3892/mmr.2014.2454
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.