Back to Journals » OncoTargets and Therapy » Volume 12
MicroRNA-27a downregulates the expression of Hsp90 and enhances the radiosensitivity in esophageal squamous cell carcinoma
Authors Wang X, An D, Liu X, Wang X, Li B
Received 7 December 2018
Accepted for publication 1 May 2019
Published 23 July 2019 Volume 2019:12 Pages 5967—5977
DOI https://doi.org/10.2147/OTT.S197456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Xintong Wang,1,2 Dianzheng An,1 Xiaomeng Liu,3 Xinlei Wang,4 Baosheng Li1
1Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China; 3University of Jinan, School of Medicine and Life Sciences, Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 4Department of Gastroenterology, Qingdao Hiser Medical Center, Qingdao, Shandong, People’s Republic of China
Purpose: Accumulating evidence suggests that microRNAs (miRNAs) possess diverse cellular regulatory roles in radiation responses. In this study, we aimed to identify the role of miR-27a in esophageal squamous cell carcinoma (ESCC) radiosensitivity by exploring the relationship between miR-27a and heat shock protein 90 (Hsp90).
Materials and methods: We performed quantitative real-time polymerase chain reaction (qRT-PCR) to detect miR-27a expression in the plasma of ESCC patients and healthy volunteers. The expression of Hsp90 and its key client proteins associated with radioresistance were analyzed by Western blotting. Then, the effects of miR-27a on proliferation, apoptosis, cell cycle and radiosensitivity in ESCC cell lines were determined by CCK-8, flow cytometry, and clonogenic survival assay. We also generated subcutaneous tumors to explore whether miR-27a enhanced radiosensitivity in vivo.
Results: In our current study, we found that miR-27a expression was downregulated in the plasma of ESCC patients compared with that of healthy volunteers. Overexpression of miR-27a in ESCC cell lines caused a reduction of Hsp90 mRNA and protein. We also demonstrated that upregulation of miR-27a induced degradation of Hsp90 key client proteins associated with radioresistance. In related functional experiments, miR-27a significantly inhibited growth, increased radiation-induced apoptosis, induced cell cycle arrest in G0/G1 phase and enhanced ESCC radiosensitivity both in vitro and in vivo.
Conclusion: From these findings, we concluded that miR-27a may contribute to radiosensitivity by modulating Hsp90 expression. Moreover, miR-27a-based therapy utilized to target Hsp90 could be contemplated as a compelling alternative for sensitize ESCC to radiotherapy with fewer side effects.
Keywords: miR-27a, Hsp90, esophageal squamous cell carcinoma, radiosensitivity
Introduction
Esophageal carcinoma is the eighth most common cancer and the sixth leading cause of cancer deaths in the world.1 In China and other East Asia countries, esophageal squamous cell carcinoma (ESCC) accounts for 90% of the total esophageal cancers. Radiotherapy is one of the recommend treatments for ESCC. However, the therapeutic outcomes are not satisfactory because of intrinsic tumor radioresistance.2 Therefore, therapeutic strategies to improve the response to radiation in ESCC need to be developed. Investigation of the underlying mechanisms of tumor radioresistance may help us explore more effective therapeutic targets.
One of these therapeutic targets is microRNAs (miRNAs). Increasing evidence suggests that miRNAs are involved in many human diseases, particularly cancer.3 miRNAs function as tumor suppressors or oncogenes are potential targets for cancer treatment.4 Numerous miRNAs have been demonstrated to play an essential role in the prediction and modification of anticancer treatments, including radiotherapy.5,6 Recently, several studies have investigated the important functions of miR-27a as a suppressor in cancer,7 and the expression level of miR-27a has been confirmed significantly reduced in ESCC cell lines and tissues.8,9
Heat shock protein 90 (Hsp90) is explored by cancer cells to support activated oncoproteins that are essential for oncogenic transformation.10 In addition, Hsp90 is abundantly expressed in esophageal cancer and esophageal cancer cell lines.11 Numerous Hsp90 client proteins have been shown to be associated with radiosensitivity.12 Thus, inhibition of Hsp90 provides an approach for the simultaneous targeting of multiple proteins that can possibly serve as determinants of radiosensitivity.13 Notably, several miRNAs have been reported to effect cancer aggression and treatment response by reducing Hsp90 expression,14–16 including miR-27a.17 These findings suggest that inhibition of Hsp90 through miR-27a may not only provide a unique therapeutic pathway, but also promote the efficacy of radiotherapy in ESCC.
In the present study, we detected miR-27a expression in the plasma of ESCC patients. Subsequently, we upregulated miR-27a and then assessed the expression of Hsp90 as well as its key client proteins associated with radioresistance both in vitro and in vivo. In addition, we also conducted functional experiments to investigate whether miR-27a can improve the radiosensitivity of ESCC cells. The results of our study suggest that miR-27a may present a better strategy for the treatment of ESCC patients as a radiosensitizer, through downregulating Hsp90 expression and subsequently disrupting multiple radioresistant signaling pathways simultaneously.
Materials and methods
Patients and samples
The study included 63 patients with newly diagnosed ESCC at Shandong Cancer Hospital between May 2016 and March 2018. Peripheral blood (10 ml) was collected from each patient before treatment. Plasma samples were also collected as a control from 63 healthy volunteers. The samples were snap-frozen in liquid nitrogen and then stored at −80 °C before processing. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Shandong Cancer Hospital. Written informed consent was obtained from each participant.
Culture of ESCC cell lines and cell transfection
Eca109 and Eca9706 were purchased commercially from American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in DMEM medium (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin G and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 atmosphere.
Eca109 and Eca9706 cell lines were cultured to 40–50% confluency and transiently transfected with negative control miRNA (miR-NC) or miR-27a mimics using EndoFectinTM-Max (GeneCopoeia, MD, USA) reagent according to the manufacturer’s instructions. miR-27a mimics and miR-NC were purchased from GenePharma (Shanghai, China).
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from Eca109 and Eca9706 cells using miRNeasy Mini kit (Qiagen). Total plasma RNA was isolated using miRNeasy Serum/Plasma Kit (Qiagen) following the manufacture’s protocols.
For single-stranded complementary DNA synthesis, 500 ng of total RNA was reverse-transcribed using PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, Dalian, China). RT-PCR was performed with UltraSYBR Mixture (CWBIO, Beijing, China) and LC 480 PCR System (Roche, Shanghai, China). Specific primers for Hsp90 were as follows: forward primer (F) 5ʹ-CGCTCCTGTCTTCTGGCTTC-3ʹ and reverse primer (R) 5ʹ-TGGTATCATCAGCAGTAGGGTC-3ʹ. Specific primers for GAPDH were as follows: forward primer (F) 5ʹ-CATGAGAAGTATGACAACAGCCT-3ʹ and reverse primer (R) 5ʹ-AGTCCTTCCACGATACCAAAGT-3ʹ. For quantification of miRNA, 500 ng of total RNA was reverse-transcribed using the miScript II RT kit (Qiagen), and miR-27a PCR amplification was performed with specific primers and the miScript SYBR Green PCR Kit (Qiagen). RNU6B (U6) was used as the endogenous control in cell samples. U6 and miR-39 were used as endogenous and external control in plasma samples. GAPDH was assessed to standardize the expression level of Hsp90 mRNA. All the real time-PCRs were performed in triplicate. Data were presented based on calculating 2−ΔΔCt.
CCK-8 assay
The effect of miR-27a on cell proliferation was evaluated by CCK-8 assay. 2000 cells were grown in complete medium in 96-well plates overnight and then transfected with miR-NC or miR-27a mimics. At 0–4 days after transfection, 10 μl CCK-8 labeling reagent (BestBio, Shanghai, China) was added to each well with 100 μl fresh medium. Four hours after incubation at 37 °C, the spectrometric absorbance of cells at 450 nm wavelength was measured using a microplate reader.
Clonogenic assay
Transfected cells were trypsinized to generate a single cell suspension and seeded in 6-well plates at 500 cells per well. 24 h later, the cells were irradiated with a single dose of 0, 2, 4, 6, 8 Gy using a dedicated small animal X-ray irradiator (X-RAD 225, Precision X-ray, North Branford, CT, USA). The medium was replaced with a fresh one 24 h after irradiation. After incubation for 14 days, colonies were fixed in anhydrous ethanol, stained with crystal violet and the number of colonies containing at least 50 cells was counted. The colony survival fraction was calculated for each treatment.
Flow cytometry analysis of apoptosis and cell cycle
Eca109 and Eca9706 cells were seeded into 6-well plates and transfected with miR-NC or miR-27a mimics. After incubation for 24 h, the cells were irradiated with single dose of 6 Gy rays. Both floating and attached cells were collected by centrifugation at 24 h after irradiation. Apoptosis analysis was assessed using Annexin V-FITC Apoptosis detection kit (Becton Dickson, Franklin Lakes, NJ, USA). The cells were washed twice with cold PBS and resuspended in 1×Binding Buffer sufficiently, stained with Annexin V-FITC and PI according to the manufacturer’s protocol. Cell samples were analyzed within 1 h by flow cytometry (FACS, Becton Dickson, Franklin Lakes, NJ, USA).
Cell cycle distribution was measured using the same treatment as above. The cells were digested by trypsin, washed with PBS, fixed with 75% cold ethanol at 4 °C for 48 h and dyed with PI for more than 30 min. The results were analyzed by flow cytometry.
Protein extraction and Western blotting
Protein were extracted 48 h after Eca109 and Eca9706 cells were transfected with miR-NC or miR-27a mimics. For combination, cells were irradiated with single dose of 6 Gy rays after 24 h of transfection and the protein were extracted 24 h after irradiation. Then cells were washed with PBS, and added to RIPA Lysis Buffer and PMSF (Beyotime, Shanghai, China). Equal amounts of protein were separated by SDS-PAGE and electrotransferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% non-fat dry milk that was dissolved with 1×TBST and then incubated with the indicated primary antibodies at 4 °C overnight. After washing with 1×TBST three times, the membranes were incubated with secondary antibody for 1 h at room temperature. Specific antigen-antibody interactions were detected with enhanced chemiluminescence.
Primary antibodies used were anti-Hsp90α rabbit monoclonal antibody, anti-phospho-EGFRTyr1068 rabbit monoclonal antibody, anti-phospho-c-RafSer338 rabbit monoclonal antibody, anti-phospho-AktSer473 rabbit monoclonal antibody, anti-cleaved PARP rabbit monoclonal antibody and anti-β-actin rabbit monoclonal antibody (Cell signaling technology, Beverly, MA, USA).
Animal experiments
2×106 transfected ESCC cells suspended in 100 μl of phosphate-buffered saline were injected subcutaneously into the right hindlimbs of BALB/c nude mice (female, 4–5 weeks old, Beijing HFK Bioscience Co., Ltd, China). After 10 days, when the tumor diameters reached approximately 5–6 mm, the nude mice were randomly divided into two groups (n=6 each). NC agomir or miR-27a agomir (RiboBio Co., Ltd, Guangzhou, China) was then directly injected into the implanted tumor at a dose of 5 nmol per mouse every 3 days for 15 days. The mice were irradiated with a single dose of 6 Gy 24 h after the first injection. Tumor size was measured every 3 days since the first day of injection of agomir. The tumor volume was calculated according to the formula: tumor volume =0.5×length×width2. At 48 h after the last injection, the mice were sacrificed and the tumors were resected, weighed and measured. After weighing and measuring they were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin according to standard histological procedures. All animal studies were approved by Shandong Hospital Experimental Animal Care Commission and conducted in compliance with UKCCCR guidelines.
Immunohistochemistry
Tumor sections were deparaffinized in xylene and rehydrated in graded alcohols and distilled water. Then the sections were treated with 3% hydrogen peroxide for 15 min to eliminate endogenous peroxidase activity. Following antigen retrieval, goat serum was used to block the nonspecific binding. Then the sections were incubated with primary antibody (Hsp90, p-EGFR and cleaved PARP) overnight at 4 °C. Negative controls were incubated with the negative control antibody under the same condition. After that the sections were washed and stained with secondary antibodies for 1 h, followed by incubation with conjugated HRP streptavidin for 1 h. Finally, the sections were incubated with diaminobenzidine and counterstained with hematoxylin. Images were obtained under a light microscope.
Statistical analysis
Statistical Package for Social Sciences software (SPSS Version 22.0) was used for all statistical analysis. All data were shown as mean ± SD. Data were analyzed by Student’s t-test when comparing two groups and by one-way ANOVA when comparing more than two groups. Differences with P-value <0.05 were considered statistically significant. Experiments were performed at least three times.
Results
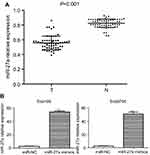
miR-27a expression was downregulated in ESCC patients’ plasma
Previous studies confirmed that the expression level of miR-27a significantly reduced in ESCC tissues and cell lines.8,9 Here we also found that miR-27a level was much lower in 63 ESCC patients’ plasma than healthy volunteers (0.57±0.03 vs 0.79±0.06; P<0.001) (Figure 1A). There was also a good correlation between miR-27a level in plasma and tumor T stage, lymph node metastasis (P<0.05). All the results reveal the potential role of miR-27a as a tumor suppressor in ESCC. In addition, miR-27a expression was upregulated in miR-27a mimics transfected ESCC cells (Figure 1B).
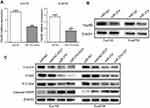
miR-27a downregulated Hsp90 expression and induced degradation of Hsp90 key client proteins associated with radioresistance
The inhibition of Hsp90 is a multitarget approach to radiosensitization.13 EGFR, Akt, Raf-1 are client proteins of Hsp90 that have been reported to be associated with radiosensitivity.18–20 In order to assess the functional relevance of miR-27a, we detected the alteration in the expression of Hsp90 and these key client proteins. As shown in Figure 2A, the mRNA level of Hsp90 was significantly decreased in Eca109 (5.44±0.27 vs 2.78±0.18, P=0.004) and Eca9706 (3.77±0.29 vs 1.71±0.30, P=0.023) cells after miR-27a mimics transfection. The protein level of Hsp90 was also significantly decreased in both cell lines (Figure 2B). What’s more, miR-27a or in combination with radiation induced degradation of p-EGFR, p-Akt and p-c-Raf (Figure 2C). However, miR-NC plus 6 Gy radiation had a limited effect on the level of these key proteins. Cleaved PARP is considered to be a hallmark of apoptosis. As shown in Figure 2C, moderate PARP cleavage was seen when transfected with miR-NC following 6 Gy radiation. Encouragingly, it was obviously elevated in both cell lines transfected with miR-27a mimics or in combination with radiation. These results indicated that miR-27a downregulated Hsp90 expression, and subsequently induced degradation of Hsp90 key client proteins associated with radioresistance.
miR-27a inhibited the proliferation and sensitized ESCC cells to radiation
CCK-8 assay was used to evaluate the effect of miR-27a on proliferation ability of ESCC cells. As shown in Figure 3A, miR-27a significantly inhibited the proliferation of Eca109 and Eca9706 cells. We then evaluated if upregulation of miR-27a could influence the colony forming ability of ESCC cells. The result of clonogenic assay (Figure 3B) showed that miR-27a significantly increased the antitumor effect of radiation at 4 Gy, 6 Gy and 8 Gy in both of the two cell lines (Eca109: P=0.021 at 4 Gy, P=0.023 at 6 Gy, P=0.034 at 8 Gy; Eca9706: P=0.004 at 4 Gy, P=0.027 at 6 Gy, P=0.007 at 8 Gy). As shown of the results above, upregulation of miR-27a inhibited growth and enhanced radiosensitivity in ESCC cells.
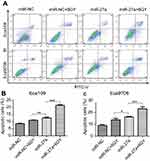
miR-27a promoted radiation-induced apoptosis and induced G0/G1 arrest in ESCC cell lines
Apoptosis is one of the major factors regulating radioresistance. To investigate whether miR-27a acts as a radiosensitizer through regulation of apoptosis, we examined the apoptosis following radiation (Figure 4A). The result showed that the proportion of total apoptotic cells in ESCC cells treated with miR-27a mimics alone (Eca109: P=0.005, Eca9706: P=0.043), or in combination with 6 Gy radiation (P<0.001) increased significantly compared with the cells treated with miR-NC following 6 Gy radiation (Figure 4B and C). Therefore, overexpression of miR-27a significantly promoted radiation-induced apoptosis in ESCC cells. The result indicated that miR-27a may be used as a radiosensitizer in the radiotherapy of ESCC patients to improve the antitumor effect of radiation.
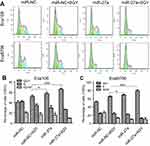
Flow cytometry was performed to measure the status of cell cycle (Figure 5A). After the miR-27a mimics transfected Eca109 cells were treated with 6 Gy radiation, the proportion of cells in G0/G1 phase significantly increased (P<0.001), and the proportion of cells in S phase (P=0.006) and G2/M phase (P=0.02) significantly decreased compared with miR-NC transfected cells following 6 Gy radiation (Figure 5B). The same changes of the proportion of cells in G0/G1 phase (P<0.001) and S phase (P<0.001) were observed in Eca9706, whereas no significant changes were detected in the proportion of cells in G2/M phase (Figure 5C). These results suggested that miR-27a might promote radiation-induced apoptosis by inducing cell cycle arrest in G0/G1 phase in ESCC cells.
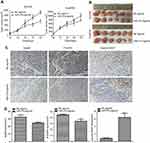
miR-27a enhanced radiosensitivity of ESCC in xenograft models
To explore whether miR-27a enhanced the radiosensitivity of ESCC in vivo, we generated subcutaneous tumors in nude mice using Eca109 and Eca9706 cells, and treated the mice with an intro-tumor injection of NC agomir or miR-27a agomir. After radiation, the tumors of miR-27a agomir treated group grew evidently slower compared with the NC agomir treated group (Figure 6A and B). To investigate the underlying mechanisms of miR-27a mediated radiosensitivity in ESCC xenograft models, we performed IHC to detect the expression of Hsp90, p-EGFR and cleaved PARP. EGFR was suggested to predict the radiosensitivity and prognosis of ESCC.21 As shown in Figure 6C and D, the expression levels of Hsp90 and p-EGFR were decreased, while the expression level of cleaved PARP was increased in the tumors injected with the miR-27a agomir compared with these levels in the tumors injected with NC agomir.
Discussion
miR-27a was recently reported to be downregulated in ESCC cell lines and tissues.8,9 However, whether miR-27a contributes to radiosensitivity in ESCC was an open question. In the present study, we confirmed that miR-27a expression was also downregulated in ESCC patients’ plasma. Subsequently, we showed that miR-27a downregulated Hsp90 expression and then induced degradation of Hsp90 key client proteins associated with radioresistance. Also, miR-27a inhibited growth, increased radiation-induced apoptosis and enhanced radiosensitivity in ESCC both in vitro and in vivo. Our study highlights the possibility that miRNAs-based therapy could be utilized to target Hsp90, thereby improving the response of ESCC patients to radiotherapy.
Radiotherapy is an important modality in ESCC treatment. However, tumor intrinsic radioresistance accounts for the high recurrence and poor survival of ESCC patients. Therefore, it is an urgent problem to find effective radiosensitizers to improve strategies of radiotherapy. Recent studies have shown that miRNAs play an important role in radiation-induced gene expression, cell-signaling events and regulation of damage-response pathways.22 In the context of ESCC, miR-21, miR-96, miR-205 and miR-193a-3 were demonstrated to promote the development of radioresistance, while miR-98, miR-124, miR-381 and miR-338-5p sensitized ESCC cells to radiation.23 Therefore, miRNAs may have significant potential as targets for the development of new therapeutic strategies to overcome radioresistance in ESCC. In our study, a significant increase in the expression of cleaved PARP, a marker of apoptosis, was observed both in vitro and in vivo. Besides, upregulation of miR-27a following radiation significantly inhibited growth, enhanced apoptosis and induced cell cycle arrest in G0/G1 phase in ESCC cells. It is well known that cellular radiosensitivity is determined by numbers of fundamental process, including apoptosis and cell cycle phase distribution.13 Our data of these functional experiments indicated that miR-27a could be a good candidate therapeutic molecular target for improving the radiosensitivity of ESCC.
In this study, we also explored the possible mechanism of miR-27a regulating the sensitivity of radiotherapy. It is truly challenging to develop a single therapeutic modality that can target a single factor which is linked to multiple pro-proliferative factors. One-pot Hsp90-targeting should enable regulation of multiple pathways and related proteins for comprehensive tumor regression and treatment.24 Hsp90 has been established as a determinant of tumor radiosensitivity. Identifying miRNAs that inhibit Hsp90 expression is crucial for effective Hsp90 targeting. Previous studies have confirmed that miR-27a partially shares the seed sequence to bind to the 3ʹ-untranslated region (3ʹ-UTR) or amino acid coding sequences (CDS) region of Hsp90 gene.25 Herein, we demonstrated that miR-27a reduced Hsp90 expression not only at the levels of translation, but also transcription. To determine whether Hsp90 downregulation by miR-27a can result in weakened radiosensitivity signaling, we evaluated the expression of p-EGFR, p-Akt and p-c-Raf. Intriguingly, both Eca109 and Eca9706 cells exhibited degradation of these key client proteins associated with radioresistance. Several signaling pathways related to radioresistance may be blocked simultaneously. In addition, downregulation of EGFR and Akt were associated with decreased phosphorylation of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and delayed DNA repair.26 Consequently, sensitivity to radiotherapy was enhanced. The results of our in vivo experiment also indicate that miR-27a plays as a tumor suppressor and improves the radiosensitivity in ESCC. All the data indicate that the inhibition of Hsp90 by miR-27a may have broader application against ESCC radioresistance.
Nowadays, targeted biological therapies that selectively interfere with cancer cell growth signals may improve patient survival by enhancing the effects of radiation with little damage to normal tissue. For example, artificial miRNAs are basically designed to utilize the cellular machinery for expression, processing and targeting of mRNAs. It can be used to target and inhibit Hsp90 by delivering plasmid containing miRNAs construct against Hsp90.24 In addition, it has been established that exosomes contain miRNAs and can be manipulated to transfer miRNAs representing therapeutics to recipient cell.27 O’Brien et al have found that miR-134 in exosomes reduced Hsp90 expression, ultimately adding value to anti-cancer agents.16 Taken together, the design of these delivery systems ensures the stability of synthetic miRNAs in the circulation and provides tumor-specific targeting and delivery. What’ more, it is integral to translate miRNAs-based therapy utilized to target Hsp90 to the clinic.
To our knowledge, this is the first comprehensive report concerning the role of miR-27a in radiosensitivity in ESCC by modulating Hsp90 expression. In general, miRNAs modulate the gene expression through base pairing to complementary sites in the 3ʹ-UTR of their target miRNAs. However, recent study showed that miRNAs can regulate target miRNAs by binding to the CDS region.28 The detailed mechanism underlying the control of gene expression via the CDS regions has not yet been identified, although the homology between the miR-27a seed sequence and CDS regions of Hsp90 gene may be important for the regulation of those expressions. Further investigations are certainly necessary to understand the complete mechanism.
Conclusion
In the present study,we reported a novel role for miR-27a as a key regulator of radiosensitivity and showed that miR-27a sensitized ESCC to radiation by downregulating Hsp90 expression. All of these data suggest that miR-27a may be a potential therapeutic target for improving the response of ESCC patients to radiation therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81874224) and the grants from the Key Scientific and Technological Innovation Project of Shandong Province, China (No. 2017CXZC1206).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Koshy M, Esiashvilli N, Landry JC, et al. Multiple management modalities in esophageal cancer: combined modality management approaches. Oncologist. 2004;9(2):147–159.
3. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi:10.1038/nature03702
4. Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi:10.1016/j.addr.2014.05.009
5. Wang P, Zhang J, Zhang L, et al. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145(5):1133–1143. doi:10.1053/j.gastro.2013.07.048
6. Weidhaas JB, Babar I, Nallur SM, et al. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67(23):11111–11116. doi:10.1158/0008-5472.CAN-07-2858
7. Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. doi:10.1073/pnas.0510565103
8. Jiang Y, Duan Y, Zhou H. MicroRNA-27a directly targets KRAS to inhibit cell proliferation in esophageal squamous cell carcinoma. Oncol Lett. 2015;9(1):471–477. doi:10.3892/ol.2014.2701
9. Zhu L, Wang Z, Fan Q, et al. microRNA-27a functions as a tumor suppressor in esophageal squamous cell carcinoma by targeting KRAS. Oncol Rep. 2014;31(1):280–286. doi:10.3892/or.2013.2807
10. Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18(1):64–76. doi:10.1158/1078-0432.CCR-11-1000
11. Wu X, Wanders A, Wardega P, et al. Hsp90 is expressed and represents a therapeutic target in human oesophageal cancer using the inhibitor 17-allylamino-17-demethoxygeldanamycin. Br J Cancer. 2009;100(2):334–343. doi:10.1038/sj.bjc.6604855
12. Dote H, Burgan WE, Camphausen K, et al. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66(8):9211–9220. doi:10.1158/0008-5472.CAN-06-2181
13. Camphausen K, Tofilon PJ, Camphausen KTP. Inhibition of Hsp90: a multitarget approach to radiosensitization. Clin Cancer Res. 2007;13(15):4326–4330. doi:10.1158/1078-0432.CCR-07-0632
14. Choghaei E, Khamisipour G, Falahati M, et al. Knockdown of microRNA-29a changes the expression of heat shock proteins in breast carcinoma MCF-7 cells. Oncol Res. 2016;23(1):69–78. doi:10.3727/096504015X14478843952906
15. Pan J, Jiang F, Zhou J, et al. HSP90: a novel target gene of miRNA-628-3p in A549 cells. Biomed Res Int. 2018;2018:4149707. doi:10.1155/2018/4149707
16. O’Brien K, Lowry MC, Corcoran C, et al. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6(32):32774–32789. doi:10.18632/oncotarget.5192
17. Kariya A, Furusawa Y, Yunoki T, et al. A microRNA-27a mimic sensitizes human oral squamous cell carcinoma HSC-4 cells to hyperthermia through downregulation of Hsp110 and Hsp90. Int J Mol Med. 2014;34(1):334–340. doi:10.3892/ijmm.2014.1758
18. Park JS, Jun HJ, Cho MJ, et al. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin Cancer Res. 2006;12(16):4989–4999. doi:10.1158/1078-0432.CCR-05-2259
19. Tanno S, Yanagawa N, Habiro A, et al. SerineThreonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res. 2004;64(10):3486–3490. doi:10.1158/0008-5472.CAN-03-1788
20. Pfeifer A, Mark G, Leung S, et al. Effects of c-raf-1 and c-myc expression on radiation response in an in vitro model of human small-cell-lung carcinoma. Biochem Biophys Res Commun. 1998;252(2):481–486. doi:10.1006/bbrc.1998.9660
21. Zhao L, He LR, Xi M, et al. Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J Transl Med. 2012;10:249–257. doi:10.1186/1479-5876-10-249
22. Korpela E, Vesprini D, Liu SK. MicroRNA in radiotherapy: miRage or miRador? Br J Cancer. 2015;112(5):777–782. doi:10.1038/bjc.2015.6
23. Malhotra A, Sharma U, Puhan S, et al. Stabilization of miRNAs in esophageal cancer contributes to radioresistance and limits efficacy of therapy. Biochimie. 2018;156:148–157. doi:10.1016/j.biochi.2018.10.006
24. Pore SK, Choudhary A, Rathore B, et al. Hsp90-targeted miRNA-liposomal formulation for systemic antitumor effect. Biomaterials. 2013;34(28):6804–6817. doi:10.1016/j.biomaterials.2013.05.054
25. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi:10.1016/j.cell.2004.12.035
26. Lee KM, Choi EJ, Kim IA. microRNA-7 increases radiosensitivity of human cancer cells with activated EGFR-associated signaling. Radiother Oncol. 2011;101(1):171–176. doi:10.1016/j.radonc.2011.05.050
27. Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
28. Ott CE, Grunhagen J, Jager M, et al. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3ʹ UTR and coding-sequence binding sites. PLoS One. 2011;6(1):e16250. doi:10.1371/journal.pone.0016250
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.