Back to Journals » Cancer Management and Research » Volume 11
MicroRNA-218 regulates the chemo-sensitivity of cervical cancer cells through targeting survivin
Authors Yu M, Xu B, Yang H, Xue S, Zhang R, Zhang H, Ying X , Dai Z
Received 27 December 2018
Accepted for publication 10 April 2019
Published 12 July 2019 Volume 2019:11 Pages 6511—6519
DOI https://doi.org/10.2147/CMAR.S199659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Minmin Yu,1,2,* Baozhen Xu3,*, Hui Yang4,*, Songlin Xue,2 Rong Zhang,2 Hongmei Zhang,5 Xiaoyan Ying,1 Zhiqin Dai6
1Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing 210011, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing 210003, People’s Republic of China; 3Department of Obstetrics and Gynecology, Nanjing Lishui People’s Hospital, Nanjing 211200, People’s Republic of China; 4Department of Obstetrics and Gynecology, Huaian Maternal and Child Health Care Hospital, Huaian 223002, People’s Republic of China; 5Department of Clinical Research Center, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing 210003, People’s Republic of China; 6Department of Gynecologic Oncology, Jiangsu Cancer Hospital, Nanjing 210009, People’s Republic of China
*These authors contributed equally to this work
Background: Cervical cancer is one of the most lethal malignancies among women in the world. Every year about 311,365 women die because of cervical cancer. Chemo-resistance is the main reason of the lethal malignancies, and the mechanism of chemo-resistance in cervical cancer still remains largely elusive.
Purpose: Previous studies reported that microRNAs played important biological roles in the chemo-resistance in many types of cancers, in the present study we tried to investigate the biological roles of microRNA-218 in chemo-resistance in cervical cancer cells.
Results: Real-time PCR results indicated microRNA-218 was downregulated in cisplatin-resistant HeLa/DDP and SiHa/DDP cells compared with the mock HeLa and SiHa cells. CCK-8 assay results showed upregulation of microRNA-218 enhanced the cisplatin sensitivity of cervical cancer cells; while downregulation of microRNA-218 decreased the cisplatin sensitivity of cervical cancer cells. Dual-luciferase assay indicated survivin was a direct target of microRNA-218. Western blotting and PCR results indicated the expression of survivin in HeLa/DDP and SiHa/DDP cells was significantly increased compared with HeLa and SiHa cells. Further study indicated induction of microRNA-218 decreased the expression of survivin while inhibition of microRNA-218 increased the expression of survivin in cervical cancer cells. Cell apoptosis results indicated induction of microRNA-218 induced the cell apoptosis in cervical cancer cells.
Conclusion: Our data revealed microRNA-218 enhanced the cisplatin sensitivity in cervical cancer cells through regulation of cell growth and cell apoptosis, which could potentially benefit to the cervical cancer treatment in the future.
Keywords: miR-218, cervical cancer, cisplatin resistant, apoptosis, survivin
Introduction
Cervical cancer (CC) is the fourth most common malignancy cancer amongst women worldwide, and CC is the fourth leading cause of cancer-related death in women.1 It was showed about 569,847 new CC patients were diagnosed every year, and about 311,365 women were died because of CC.1 An important reason of the high mortality is that there were no obvious symptoms until cancer has been progressed to an advanced stage.2 In the current stage, the clinical treatments of CC were mainly including: surgery therapy, chemotherapy, radiotherapy, and the combination therapy.3 For advanced CC patient, chemotherapy was recommended as a standard therapy, however the clinical drug did not show strong therapeutic efficacy.4–6 Like other cancers, the chemo-resistant greatly impacted the therapeutic efficacy of CC patients. Therefore, investigating of the mechanism of the chemo-resistant in CC has the great significance to explore the effective therapy for CC.
MicroRNAs (miRNAs) are a series of small, conserved, and non-coding short RNAs. They are formed by 18–25 nucleotides in length.7 Previous studies indicated that miRNAs were dysregulated in human cancers, and they bind to the UTR of messenger RNAs (mRNAs) to regulate the gene expression, which could regulate the human cancer cell growth and differentiation.8–12 Studies also indicated miRNAs played important biological roles in the chemo-resistance of human cancer.13,14 A growing body of evidence suggested miR-218 was significantly downregulated in human cancer tissues compared with the adjacent non-cancerous tissues,15–20 therefore miR-218 played as a tumor-suppressive miRNA in human cancer. Studies also indicated miR-218 inhibited cancer cell growth and invasion through regulation of oncogenic genes,21–25 and miR-218 increased the chemosensitivity of many types of human cancer cells, including esophageal cancer, colorectal cancer, breast cancer, and gastric cancer.26–29 Recently, studies indicated miR-218 was downregulated in CC and miR-218 regulated CC cell growth,30,31 however whether miR-218 regulates cisplatin (DDP) resistance in CC still remains largely elusive.
In the current study, we reported miR-218 was significantly downregulated in DDP-resistant human CC cells compared with their mock CC cells, and upregulation of miR-218 enhanced the DDP sensitivity of human CC cells through cell growth and cell apoptosis regulation by targeting survivin gene. These findings could potentially benefit to the CC treatment in the future.
Materials and methods
Cells culture
Human CC cell line HeLa, HeLa/DDP, SiHa and SiHa/DDP were purchased from Fenghbio Co., Ltd (Hunan, People's Republic of China). The cells were cultured in DMEM (Life Technologies, CA, USA) supplemented with 10% FBS, 1% penicillin and 1% streptomycin (Gibco, NY, USA) in a humidified incubator of 5% CO2 at 37°C. DDP was added to evaluate the chemo-resistant capability of CC cells. DDP was purchased from Sigma (Shanghai, People's Republic of China).
Transfection
MiR-NC, miR-218 mimic, anti-miR-NC, and anti-miR-218 were purchased from Ambion (Austin, TX, USA). Small interfering RNA-218 (siRNA-218) targeting survivin and the siRNA control were purchased from GenePharma (Shanghai, People's Republic of China). The miR-218 mimic, inhibitor, or control were diluted in Opti-MEM medium (Life Technologies, CA, USA) at room temperature (RT) for 15 mins, then transfected human CC cells with miR-218 mimic or inhibitor and cultured for 48 hrs. The expression of miR-218 was examined by qRT-PCR assay. siRNA transfection was performed with Lipofectamine RNAiMAX reagent (Invitrogen, CA, USA) following the manufacturer’s instructions. The expression of survivin was examined by qRT-PCR and western assay.
RNA extraction and real-time PCR
Total RNA was isolated from mock or transfected CC cells by using TRIzol reagent (Invitrogen). MiR Complementary DNA (cDNA) was converted from total RNA by using PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, Tokyo, Japan) and survivin cDNA was synthesized from total RNA using the PrimeScript RT Master Mix Kit (TaKaRa). MiR-218 quantification was performed with specific primers and probes using TaqMan MicroRNA Assays (Applied Biosystems, CA, USA), RNA U6 was used as the internal control. The survivin mRNA expression was measured by using qPCR assay (SYBR Green; Bio-Rad, USA) according to the manufacturer’s instructions; GAPDH was used as the internal control. Relative mRNA expression was calculated by using the 2−ΔΔCT method. The sequences of the specific primers for mRNA amplification were as follows: survivin forward primer, 5ʹ-GGACCACCGCATCTCTACAT-3ʹ and reverse primer, 5ʹ-GACAGAAAGGAAAGCGCAAC-3ʹ; GAPDH forward primer, 5ʹ- TCGACAGTCAGCCGCATC-TTCTTT-3ʹ and reverse primer, 5ʹ-ACCAAATCCGTTG-ACTCCGACCTT-3ʹ. All experiments were performed in triplicate.
miRNA target predictions
To further investigate the potential target of miR-218, potential genes were predicted by searching from targetscan (http://www.targetscan.org) and mirbase targets (http://microrna.sanger.ac.uk/cgi-bin/targets/v5/search.pl). Survivin was predicted as a target of miR-218.
Cell viability and apoptosis assays
Cell viability was measured by Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) assay according to the manufacturer’s protocol. Briefly, mock or transfected human CC cells were seeded into the 96-well plate and allowed to attach overnight. Then fresh prepared DDP, mimic, inhibitor, or siRNAs were added into the wells as planned and cultured for 72 hrs in the incubator. At last, CCK-8 solution was added to the 96-well plate, and incubated for another 2 hrs at 37°C. The 450 nm absorbance was measured by using a microplate reader (Molecular Devices, USA). The cell viability was equal to the ratio of treatment group to the control group. The IC50 was calculated as the DDP concentration of 50% reduction on cell viability. The CC cell apoptosis was measured by using the Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, Beijing, People's Republic of China) according to the manufacturer’s instructions. CellTiter-Blue (Promega) was used to measure the cell number. The relative Caspase-3/7 activity was calculated by the ratio of Apo-ONE and CellTiter-Blue signals. All experiments were performed in triplicate.
Luciferase reporter assay
A fragment from the 3’UTR of survivin gene containing the predicted binding site of miR-218 was amplified by PCR from genomic DNA. The amplified fragment was cloned into the UTR downstream of the luciferase gene in the pMIR-reporter luciferase vector (Ambion, USA). A corresponding mutant construct was used as the control. Human CC cells were co-transfected with the testing firefly luciferase reporter plasmid together with a Renilla luciferase plasmid. Then, we harvested the cells and measured the dual-luciferase activities by using Dual-Glo Luciferase Assay System (Promega). Renilla signal was used as an internal control for normalization. All experiments were performed in triplicate.
Western blot
Total protein was extracted from mock or transfected human CC cells by using RIPA lysis buffer (Beyotime, Shanghai, People's Republic of China) according to the manufacturer’s protocol. BCA assay (Beyotime) was performed to determine the protein concentration. First proteins were separated on a SDS-PAGE gel (10%), and then transfer the separated protein onto a PVDF membrane (GE, USA). The PVDF membrane was blocked with 5% BSA for 2 hrs at RT and further incubated with antibodies against survivin or GAPDH for 2 hrs at RT. The PVDF membrane was washed with PBST for three times and further probed with second antibody for 2 hrs at RT. The bands were detected by using the Novex ECL HRP chemiluminescent substrate reagent kit (Invitrogen). Alpha Innotech imaging software was used to take the band image (San Leandro, CA, USA). All experiments were performed in triplicate.
Statistical analysis
A one-way analysis of variance was performed to analyze the statistical difference between groups by using SPSS v13.0 software. The data were expressed as mean±SD. P<0.05 indicated a statistically significant difference. All experiments were performed in triplicate.
Results
miR-218 was associated with DDP chemosensitivity in CC
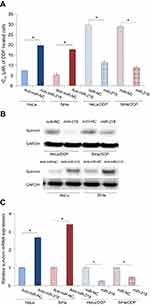
To investigate the potential biological roles of miR-218 on the DDP chemoresistance of CC cells, the expression level of miR-218 was measured in both DDP resistant and mock CC cells. First, we check the DDP resistance capability of the HeLa/DDP and SiHa/DDP cells, the HeLa, HeLa/DDP, SiHa, and SiHa/DDP cells were exposed to different concentrations of DDP ranging from 0 to 100 μM and cultured for 72 hrs, the cell viability was measured by using CCK-8 assay. As shown in Figure 1A, the IC50 of HeLa/DDP and SiHa/DDP cells were much higher than the IC50 of mock HeLa and SiHa cells. The qPCR results indicated the expression of miR-218 was significantly downregulated in DDP resistant HeLa/DDP and SiHa/DDP cells compared to their mock cells (Figure 1B, P<0.05). To further confirm the effect of miR-218 on DDP chemosensitivity, we measured the cell viability of CC cells transfected with miR-218 or anti-miR-218 in the presence of DDP. The results indicated that suppression of miR-218 by anti-miR-218 transfection markedly decreased the DDP chemosensitivity of HeLa and SiHa cells (Figure 1C, P<0.05); while induction of miR-218 by miR-218 mimic transfection markedly improved the DDP chemosensitivity of HeLa/DDP and SiHa/DDP cells (Figure 1D, P<0.05). Our results indicated that deregulation of miR-218 is critically involved in DDP-based chemosensitivity in CC.
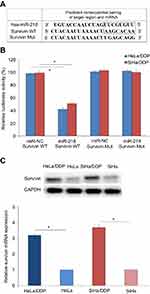
Survivin is a direct target of miR-218
In order to determine the molecular basis of the effect of miR-218 in CC, we used a prediction program to identify putative target genes of miR-218. By using the prediction program, we identified survivin is a potential target of miR-218 (Figure 2A). Further results showed that the expression of survivin was significantly increased in DDP resistant CC cells both in protein and mRNA level (Figure 2C). To confirm whether survivin is a direct target of miR-218 in CC cells, we co-transfected miR-218 and luciferase reporter plasmids with wild type (WT) or mutant type (Mut) of 3ʹ-UTRs of survivin into CC cells. Then, we measured the luciferase values. As shown in Figure 2B, miR-218 remarkably decreased the luciferase activity in WT groups in HeLa/DDP and SiHa/DDP cells and miR-218 did not affect the luciferase activity in Mut groups. These results suggested survivin is a direct target of miR-218 in CC cells.
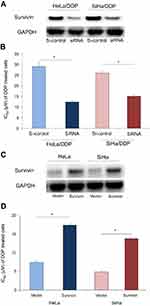
Survivin contributes to DDP chemoresistance in CC
To investigate whether survivin was associated with the DDP chemoresistance in CC, loss of function and gain of function assays were performed. Our results indicated that survivin siRNA transfection significantly decreased the expression of survivin in Hela/DDP and SiHa/DDP cells (Figure 3A), and survivin expression inhibition increased the DDP sensitivity of CC cells (Figure 3B, P<0.05). In Hela and SiHa cells, survivin mimic transfection increased the expression of survivin (Figure 3C), and survivin expression induction decreased the DDP sensitivity of CC cells (Figure 3D, P<0.05). These results suggested survivin functionally regulated the DDP chemosensitivity in CC cells.
miR-218 modulated DDP chemosensitivity of CC cells through targeting survivin
To investigate the effect of miR-218 on DDP chemosensitivity in CC cells, CCK-8 assay was performed. The results indicated that upregulation of miR-218 significantly decreased the IC50 of DDP in HeLa/DDP and SiHa/DDP cells; while downregulation of miR-218 significantly enhanced the IC50 of DDP in HeLa and SiHa cells (Figure 4A, P<0.05). Therefore, miR-218 modulated the DDP chemosensitivity of CC cells in vitro. Then, we performed western and qPCR assays to test the effect of miR-218 on the expression of survivin in CC cells. The results indicated that upregulation of miR-218 decreased the expression of survivin both in mRNA (Figure 4C) and protein (Figure 4B) level in CC cells; while downregulation of miR-218 increased the expression of survivin both in mRNA (Figure 4C) and protein (Figure 4B) level in CC cells. These results suggested that miR-218 modulated DDP chemosensitivity of CC cells through targeting survivin.
miR-218 modulated cell apoptosis of CC cells
Previous study indicated that survivin expression was associated with cell apoptosis, in the present study we suppose miR-218 regulated the CC cells growth through the cell apoptosis regulation. To confirm our hypothesis we measured the effect of miR-218 on CC cell apoptosis. As shown in Figure 5, upregulation of miR-218 in DDP-resistant CC cells significantly induced the cell apoptosis, while downregulation of miR-218 in mock CC cells significantly decreased the cell apoptosis. These findings suggested miR-218 modulated DDP-based chemosensitivity of CC cells might attribute to the cell apoptosis regulation.
 |
Figure 5 The caspase-3/7 activity CC cells with indicated treatment were measured in the presence of DDP. The data were shown as the mean±SD. *P<0.05. |
Discussion
DDP is one of the broadest used anticancer drugs in the treatment of many types of cancers in clinical.32 Chemo-resistance is the major impediment to effective cancer therapy in clinical. It was reported a few genes and pathways were involved in the chemo-resistance of DDP by regulation of cell proliferation, cell apoptosis, drug efflux, and cancer angiogenesis.33,34 Besides continuous and multiple DDP treatment also caused lots of side effects,35 therefore enhance the sensitivity of CC cells to DDP provided a new sight for resolving the challenge. However, the mechanism of chemo-resistance in the treatment of CC is still not clearly understood currently.
In 1993, the first miRNA was identified. MiRNAs were noncoding, single-stranded RNAs, and widely present in eukaryotes.36 Previous studies indicated that miRNAs were aberrantly expressed in many types of tumors. They regulated many biological processes, including cell proliferation, cell apoptosis, and stress response.37,38 Studies also have documented miRNAs played an important role in chemo-resistance in cancers.39,40,41 For example, researchers had reported that miR-21, miR-200b, and miR-15b regulated the chemo-resistance in human cancer cells.42–44 MiR-218 is an intronic miRNA, and it was encoded within intronic sequences of tumor suppressor gene SLIT2 and SLIT3.25 It was reported miR-218 was downregulated in various human cancers, including non-small cell lung cancer, oral cancer, and CC.22,24,45–50 Studies also indicated miR-218 was associated in the chemoresistant in cancer cells.51–53 However, the biological role of miR-218 in chemo-resistance in CC cells was not fully understood. In order to investigate the role of miR-218 in chemo-resistance in CC cells, we performed various assays in DDP resistant HeLa/DDP, SiHa/DDP and their mock HeLa, SiHa cells. We found miR-218 was downregulated in DDP resistant HeLa/DDP, SiHa/DDP cells compared with their mock cells, and upregulation of miR-218 significantly enhanced the DDP sensitivity of CC cells. By using miR target tools we found survivin is a target gene of miR-218, and dual-luciferase assay results indicated survivin is a direct target gene of miR-218.
Survivin is a member of the apoptosis inhibitor family, it is encoded by Baculoviral inhibitor of apoptosis repeat containing 5.54 Survivin was being found dysregulated in many types of human cencers, including breast, lung cancer, prostate cancer, gastric cancer, and colon cancer.55 Previous study indicated survivin exhibited anti-apoptotic capability through the inhibition of caspase-9 activity.56 Studies also indicated survivin played important biological roles in chemo-resistance in cancer cells, and survivin would be a biomarker for chemo-resistance in cancer treatment.56,57 In the present study, we reported the expression of survivin was significantly upregulated in DDP resistant CC cells, and knock-down of survivin enhanced the DDP sensitivity of CC cells. We also reported upregulation of miR-218 decreased the expression of survivin, while downregulation of miR-218 increased the expression of survivin. As mentioned below, survivin could inhibit apoptosis in cells. We supposed miR-218 regulated the CC cell apoptosis by the regulation of survivin. Our results indicated that upregulation of miR-218 significantly increased the CC cell apoptosis to DDP.
Taken it together, in the present study we reported miR-218 was an important mediator of the DDP chemo-resistance in CC. MiR-218 enhanced the DDP sensitivity by cell proliferation and cell apoptosis regulation, which might through the survivin regulation in CC. These findings suggested that combination treatment of DDP with miRNAs could potentially benefit to the CC treatment in the future. However, we should note that cervical tumorigenesis and chemo-resistance are complicated and complicated processes, and a lot of other factors were involved in the complicated process. Therefore, further studies are needed to investigate the precise mechanisms of DDP chemo-resistance in CC.
Conclusion
Our data suggested microRNA-218 enhanced the DDP sensitivity in CC cells through regulation of cell growth and cell apoptosis, which could potentially benefit to the CC treatment in the future.
Abbreviation list
DDP, cisplatin; CC, cervical cancer; miRNAs, microRNAs; mRNA, messenger RNA; siRNA, small interfering RNA; cDNA, complementary DNA; CCK-8, Cell Counting Kit-8; WT, wild type; Mut, mutant type; BIRC5, Baculoviral inhibitor of apoptosis repeat containing 5.
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant Nos. 81472431 and 81300323 ) and Jiangsu Provincial Medical Talent (No. ZDRCA2016072 ), Funded by Jiangsu Provincial Key Research and Development Program (No. BE2015606), supported by Nanjing Science and Technology Development Foundation (Grant No. 201611001) and Nanjing Medical Science and Technology Development Foundation (Grant No. YKk16190), supported by Municipal Key Projects of Applied Research and Science and Technology of Huaian (Grant No. HAS2015024).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Shi JF, Chen JF, Canfell K, et al. Estimation of the costs of cervical cancer screening, diagnosis and treatment in rural Shanxi Province, China: a micro-costing study. BMC Health Serv Res. 2012;12:123. doi:10.1186/1472-6963-12-123
3. Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi:10.1016/j.ccr.2004.05.029
4. Lukka H, Johnston M. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer: a meta-analysis. Clin Oncol. 2004;16:160–161. doi:10.1016/j.clon.2004.01.002
5. Martín-Martínez A, Molano F, Lloret M, Falcón-Vizcaino O, García-Hernández JA. Concurrent chemotherapy and radiotherapy for cervical cancer. Eur J Gynaecol Oncol. 2003;24:160–162.
6. Thomas GM. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–1200. doi:10.1056/NEJM199904153401509
7. Chang RK, Li X, Mu N, et al. MicroRNA expression profiles in non‑epithelial ovarian tumors. Int J Oncol. 2018;52:55–66. doi:10.3892/ijo.2017.4200
8. Jiang YW, Chen LA. microRNAs as tumor inhibitors, oncogenes, biomarkers for drug efficacy and outcome predictors in lung cancer (Review). Mol Med Report. 2012;5:890–894. doi:10.3892/mmr.2012.776
9. Kim J, Yao F, Xiao Z, Sun Y, Ma L. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37:5–15. doi:10.1007/s10555-017-9712-y
10. Nair VS, Maeda LS, Ioannidis JPA. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi:10.1093/jnci/djs027
11. Bhome R, Del Vecchio F, Lee GH, et al. Exosomal microRNAs (exomiRs): small molecules with a big role in cancer. Cancer Lett. 2018;420:228–235. doi:10.1016/j.canlet.2018.02.002
12. Glud M, Rossing M, Hother C, et al. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010;20:479–484. doi:10.1097/CMR.0b013e32833e32a1
13. Shi M, Du L, Liu D, et al. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J Pathol. 2012;228:148–157. doi:10.1002/path.3997
14. Yang H, Xiaoli W, Kaihua W, et al. MicroRNA-497 regulates cisplatin chemosensitivity of cervical cancer by targeting transketolase. Am J Cancer Res. 2016;6:2690–2699.
15. Kikkawa N, Hanazawa T, Fujimura L, et al. miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC). Br J Cancer. 2010;103:877–884. doi:10.1038/sj.bjc.6605811
16. Nohata N, Hanazawa T, Kikkawa N, et al. Tumour suppressive microRNA-874 regulates novel cancer networks in maxillary sinus squamous cell carcinoma. Br J Cancer. 2011;105:833–841. doi:10.1038/bjc.2011.311
17. Kano M, Seki N, Kikkawa N, et al. miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi:10.1002/ijc.25284
18. Moriya Y, Nohata N, Kinoshita T, et al. Tumor suppressive microRNA-133a regulates novel molecular networks in lung squamous cell carcinoma. J Hum Genet. 2012;57:38–45. doi:10.1038/jhg.2011.126
19. Hidaka H, Seki N, Yoshino H, et al. Tumor suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. Oncotarget. 2012;3:44–57. doi:10.18632/oncotarget.417
20. Yoshino H, Chiyomaru T, Enokida H, et al. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104:808–818. doi:10.1038/bjc.2011.23
21. Kinoshita T, Hanazawa T, Nohata N, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget. 2012;3:1386–1400. doi:10.18632/oncotarget.709
22. Alajez NM, Lenarduzzi M, Ito E, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011;71:2381–2391. doi:10.1158/0008-5472.CAN-10-2754
23. Uesugi A, Kozaki K, Tsuruta T, et al. The tumor suppressive microRNA miR-218 targets the mTOR component rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765. doi:10.1158/0008-5472.CAN-11-0368
24. Tie J, Pan Y, Zhao L, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the ROBO1 receptor. PLoS Genet. 2010;6:e1000879. doi:10.1371/journal.pgen.1000879
25. Tatarano S, Chiyomaru T, Enokida H, et al. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol. 2011;39:13–21. doi:10.3892/ijo.2011.1012
26. Jingjing L, Wangyue W, Qiaoqiao X, Jietong Y. MiR-218 increases sensitivity to cisplatin in esophageal cancer cells via targeting survivin expression. Open Med. 2016;11:31–35. doi:10.1515/med-2016-0007
27. Li PL, Zhang X, Wang LL, et al. MicroRNA-218 is a prognostic indicator in colorectal cancer and enhances 5-fluorouracil-induced apoptosis by targeting BIRC5. Carcinogenesis. 2015;36:1484–1493. doi:10.1093/carcin/bgv145
28. Hu Y, Xu K, Yagüe E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res Treat. 2015;151:269–280. doi:10.1007/s10549-015-3372-9
29. Zhang Z, Kong Y, Yang W, et al. MicroRNA‑218 enhances gastric cancer cell cisplatin sensitivity by targeting surviving. Exp Ther Med. 2018;16:4796–4802. doi:10.3892/etm.2018.6802
30. Banno K, Iida M, Yanokura M, et al. MicroRNA in cervical cancer: oncomiRs and tumor suppressor miRs in diagnosis and treatment. Sci World J. 2014;2014:178075. doi:10.1155/2014/178075
31. Nishikawa R, Goto Y, Sakamoto S, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Cancer Sci. 2014;105:802–811. doi:10.1111/cas.12441
32. Brewer CA, Blessing JA, Nagourney RA, Mcmeekin DS, Lele S, Zweizig SL. Cisplatin plus gemcitabine in previously treated squamous cell carcinoma of the cervix: a phase ii study of the gynecologic oncology group. Gynecol Oncol. 2006;100:385–388. doi:10.1016/j.ygyno.2005.09.009
33. Zhang B, Liu M, Tang HK, et al. The expression and significance of MRP1, LRP, TOPOIIβ, and BCL2 in tongue squamous cell carcinoma. J Oral Pathol Med. 2012;41:141–148. doi:10.1111/j.1600-0714.2011.01066.x
34. Olszewski U, Hamilton G. A better platinum-based anticancer drug yet to come? Anticancer Agents Med Chem. 2010;10:293–301.
35. Kostova I. Platinum complexes as anticancer agents. Recent Pat Anticancer Drug Discov. 2006;1:1–22.
36. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854.
37. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi:10.1038/nrc1997
38. Fanini F, Vannini I, Amadori D, Fabbri M. Clinical implications of micrornas in lung cancer. Semin Oncol. 2011;38:776–780. doi:10.1053/j.seminoncol.2011.08.004
39. Donzelli S, Mori F, Biagioni F, et al. MicroRNAs: short non-coding players in cancer chemoresistance. Mol Cell Ther. 2014;2:16. doi:10.1186/2052-8426-2-16
40. Garofalo M, Croce CM. MicroRNAs as therapeutic targets in chemoresistance. Drug Resist Updat. 2013;16:47–59. doi:10.1016/j.drup.2013.05.001
41. Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT, Zhang CP. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol. 2010;46:317–322. doi:10.1016/j.oraloncology.2010.02.002
42. Ren W, Wang X, Gao L, et al. MiR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol Cell Biochem. 2014;390:253–262. doi:10.1007/s11010-014-1976-8
43. Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi:10.1158/1078-0432.CCR-08-3053
44. Sun L, Yao Y, Liu B, et al. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31:432–445. doi:10.1038/onc.2011.263
45. Venkataraman S, Birks DK, Balakrishnan I, et al. MicroRNA 218 acts as a tumor suppressor by targeting multiple cancer phenotype-associated genes in medulloblastoma. J Biol Chem. 2013;288:1918–1928. doi:10.1074/jbc.M112.396762
46. Tu Y, Gao X, Li G, et al. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046–6055. doi:10.1158/0008-5472.CAN-13-0358
47. Mathew LK, Skuli N, Mucaj V, et al. miR-218 opposes a critical RTK-HIF pathway in mesenchymal glioblastoma. Proc Natl Acad Sci USA. 2014;111:291–296. doi:10.1073/pnas.1314341111
48. Wu DW, Cheng YW, Wang J, Chen CY, Lee H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010;70:10392–10401. doi:10.1158/0008-5472.CAN-10-2341
49. Kogo R, How C, Chaudary N, et al. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget. 2015;6:1090–1100. doi:10.18632/oncotarget.2836
50. Wu DW, Chuang CY, Lin WL, Sung WW, Cheng YW, Lee H. Paxillin promotes tumor progression and predicts survival and relapse in oral cavity squamous cell carcinoma by microRNA-218 targeting. Carcinogenesis. 2014;35:1823–1829. doi:10.1093/carcin/bgu102
51. Xie J, Yu F, Li D, Zhu X, Zhang X, Lv Z. MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in non-small cell lung cancer by targeting RUNX2. Tumour Biol. 2016;37:1197–1204. doi:10.1007/s13277-015-3831-2
52. Tian H, Hou L, Xiong YM, et al. miR-218 suppresses tumor growth and enhances the chemosensitivity of esophageal squamous cell carcinoma to cisplatin. Oncol Rep. 2015;33:981–989. doi:10.3892/or.2014.3657
53. Zarogoulidis P, Petanidis S, Kioseoglou E, Domvri K, Anestakis D, Zarogoulidis K. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell Signal. 2015;27:1576–1588. doi:10.1016/j.cellsig.2015.04.009
54. Chen DQ, Pan BZ, Huang JY, et al. HDAC 1/4-mediated silencing of microRNA-200b promotes chemoresistance in human lung adenocarcinoma cells. Oncotarget. 2014;5:3333–3349. doi:10.18632/oncotarget.1948
55. Waligórska-Stachura J, Jankowska A, Waśko R, et al. Survivin–prognostic tumor biomarker in human neoplasms-review. Ginekol Pol. 2012;83:537–540.
56. Marusawa H, Matsuzawa S, Welsh K, et al. HBXIP functions as a cofactor of survivin in apoptosis suppression. Embo J. 2003;22:2729–2740. doi:10.1093/emboj/cdg263
57. Singh N, Krishnakumar S, Kanwar RK, Cheung CH, Kanwar JR. Clinical aspects for survivin: a crucial molecule for targeting drug-resistant cancers. Drug Discov Today. 2015;20:578–587. doi:10.1016/j.drudis.2014.11.013
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.