Back to Journals » OncoTargets and Therapy » Volume 8
MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1
Authors Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng C, Zhang L, Feng Y, Zhou H, Zhou B, Zeng T
Received 5 May 2015
Accepted for publication 13 July 2015
Published 24 August 2015 Volume 2015:8 Pages 2227—2235
DOI https://doi.org/10.2147/OTT.S87976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Daniele Santini
This paper has been retracted.
Huahua Tu,1,* Gang Wei,2,* Qinghe Cai,1 Xianxiang Chen,1 Zequn Sun,2 Caitao Cheng,1 Linfei Zhang,1 Yong Feng,1 Huadong Zhou,1 Bo Zhou,1 Tiancai Zeng1
1Department of Hepatobiliary Surgery, 2Department of Gastroenterology, Renmin Hospital, Hubei University of Medicine, Shiyan, People’s Republic of China
*These authors contributed equally to this work
Abstract: MircroRNA-212 (miR-212) is proposed as a novel tumor-related miRNA and has been found to be significantly deregulated in human cancers. In this study, the miR-212 expression was found to be obviously downregulated in hepatocellular carcinoma (HCC) tissues as compared with adjacent nontumor tissues. Clinical association analysis indicated that low expression of miR-212 was prominently correlated with poor prognostic features of HCC, including high AFP level, large tumor size, high Edmondson-Steiner grading, and advanced tumor-node-metastasis tumor stage. Furthermore, the miR-212 expression was an independent prognostic marker for predicting both 5-year overall survival and disease-free survival of HCC patients. Our in vitro studies showed that upregulation of miR-212 inhibited cell proliferation and induced apoptosis in HepG2 cells. On the contrary, downregulation of miR-212 promoted cell proliferation and suppressed apoptosis in Huh7 cells. Interestingly, we found that upregulation of miR-212 decreased FOXA1 expression in HepG2 cells. Significantly, FOXA1 was identified as a direct target of miR-212 in HCC. FOXA1 was downregulated in HCC tissues as compared with noncancerous tissues. An inverse correlation between FOXA1 and miR-212 expression was observed in HCC tissues. Notably, FOXA1 knockdown inhibited cell proliferation and induced apoptosis in HepG2 cells. In conclusion, miR-212 is a potent prognostic marker and may suppress HCC tumor growth by inhibiting FOXA1 expression.
Keywords: microRNA-212, hepatocellular carcinoma, proliferation, apoptosis, FOXA1
Introduction
MicroRNA-212 (miR-212) has been considered as a novel tumor-related miRNA and is found to be significantly deregulated in human cancers. The miR-212 expression was reduced in human primary gastric cancer,1–3 lung cancer,4,5 colorectal cancer,6 and acute myeloid leukemia (AML).7 Furthermore, decreased miR-212 expression was correlated with adverse clinicopathological features of cancer, aggressive tumor phenotype,6 and poor prognosis of patients with malignancies.7 Importantly, miR-212 has been considered as a tumor suppressive miRNA by regulating cellular processes such as proliferation and apoptosis in human cancers. Upregulation of miR-212 induced decreased growth of gastric cancer cells by targeting methyl-CpG-binding protein MeCP2.1,2 Ectopic expression of miR-212 increased tumor necrosis factor-related apoptosis-inducing ligand-induced cell death in non-small-cell lung (NSCLC) cells by targeting the antiapoptotic protein PED/PEA-15 (PED).5 miR-212 was associated with the cetuximab resistance of head and neck squamous cell carcinoma.8 And the cell proliferation, migration, and invasion of ovarian cancer were found to be modulated by miR-212 through targeting heparin-binding epidermal growth factor (HBEGF).9 However, the oncogenic role of miR-212 was confirmed in other cancer types. miR-212 was upregulated in pancreatic ductal adenocarcinoma (PDAC)10,11 and oral tumors.12 It was found to exert oncogenic function in PDAC by targeting protein patched homolog 1 (PTCH1) and pRb.10,11 Therefore, the functional significance of miR-212 in cancer initiation and development seems to be cancer-type specific. However, the clinical significance of miR-212 and its related molecular pathways involved in the progression of hepatocellular carcinoma (HCC) remains poorly investigated.
Here, we demonstrate that reduced miR-212 expression is observed in HCC tissues. The low expression of miR-212 is correlated with poor prognostic features and reduced survival of HCC patients. Furthermore, miR-212 inhibits HCC cell proliferation and induces apoptosis in vitro. Forkhead box protein A1 (FOXA1) is identified as a functional target of miR-212. Mechanistically, our results demonstrate that miR-212 functions as a tumor suppressive miRNA by inhibiting FOXA1 in HCC.
Materials and methods
Clinical samples and cell lines
Eighty-six HCC samples were collected from patients including 69 males and 17 females, who underwent the resection of their primary HCC in the Department of Hepatobiliary Surgery at the People’s Hospital affiliated to Hubei University of Medicine during January 2006 to December 2008, with a median follow-up time of 29.5 months. The clinicopathological data are shown in Table 1. Informed consent was obtained from all patients. No patients received preoperative chemotherapy or embolization. The study was approved by the Hubei University of Medicine Ethics Committee according to the Declaration of Helsinki (as revised in Tokyo 2004).
The human HCC cell lines, HepG2 and Huh7 (the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, People’s Republic of China), were cultured in complete Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific) with 100 units/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich Co., St Louis, MO, USA) in a humidified incubator containing 5% CO2 at 37°C.
Real time quantitative reverse transcription-PCR
Quantitative polymerase chain reaction (PCR) primer against mature miRNA hsa-miR-212-3p (HmiRQP0319) and Homo sapiens snRNA U6 quantitative PCR primer (HmiRQP9001) were purchased from GeneCopoeia (Guangzhou, People’s Republic of China). The PCR amplification to quantify the miR-212 and U6 was performed using TaqMan miRNA Reverse Transcription Kit (Thermo Fisher Scientific) and TaqMan Human MicroRNA Assay Kit (Thermo Fisher Scientific). The relative expression of miR-212 was shown as fold difference relative to U6.
The following primers were used: FOXA1 sense primer 5′-AAT CAT TGC CAT CGT GTG-3′ and antisense primer 5′-CGC GGC TTA AAA TCT GGT AT-3′ and GAPDH sense primer 5′-CAA GCT CAT TTC CTG GTA TGA C-3′ and antisense primer 5′-CAG TGA GGG TCT CTC TCT TCC T-3′. The PCR amplification to quantify the FOXA1 and GAPDH mRNA was performed using an ABI PRISM 7300 Sequence Detection System (Thermo Fisher Scientific) and a SYBR® Premix Ex Taq™ ii (Perfect Real Time) Kit (Takara Bio, Shiga, Japan), as previously reported.13
Cell transfection
miRNA vectors, including miR-212 expression vector (HmiR0269-MR04), the control vector for miR-212 (CmiR0001-MR04), miR-212 inhibitor (HmiR-AN0319-AM04), and the negative control for the miR-212 inhibitor (CmiR-AN0001-AM04), were purchased from GeneCopoeia. The targeted sequences for FOXA1 siRNA duplex (5′-GCA CUG CAA UAC UCG CCU U-3′) or a nonspecific duplex oligonucleotide as a negative control were synthesized using Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, People’s Republic of China). Cells were transfected with the vectors mentioned above using Lipofectamine 2000, according to the manufacturer’s instructions (Thermo Fisher Scientific).
Cell proliferation and apoptosis detection
For the proliferation assay, HCC cells were seeded into 96-well plates at 5,000 cells per well for 24 hours and assessed using a Cell Proliferation ELISA, BrdU (5-bromodeoxyuridine) (chemiluminescent) (Hoffman-La Roche Ltd., Basel, Switzerland). Flow cytometric analysis was carried out with fluorescence activated cell sorting Calibur (Becton Dickinson, San Jose, CA, USA) and Cell Quest software (Becton Dickinson). An Annexin-V-FLUOS Staining Kit (Hoffman-La Roche Ltd.,) was used to analyze the level of apoptosis, following the manufacturer’s instruction. The percentage of apoptotic cells were calculated using the software in the flow cytometry.
Western blot
The following primary antibodies were used in the immunoblotting assays: FOXA1 (#3333, Epitomics Inc., Burlingame, CA, USA) and GAPDH (G8140; United States Biological, Salem, MA, USA). Horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA, USA) were used at a 1:1,000–1:5,000 dilution and detected using a Western Blotting Luminol Reagent (sc-2048; Santa Cruz Biotechnology Inc., Dallas, TX, USA), as described in previous study.14 Western blot results were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Luciferase reporter assay
The 3′-untranslated region (UTR) sequence of FOXA1 predicted to interact with miR-212 or a mutated sequence within the predicted target sites was synthesized and inserted into the XbaI and FseI sites of the pGL3 control vector (Promega Corporation, Fitchburg, WI, USA). These constructs were named as wild-type (wt) FOXA1-3′UTR or mutant (mt) FOXA1-3′UTR, respectively. For the reporter assay, HepG2 cells were seeded into 24-well plates and transfected with the above constructs and miR-212 expression vector, miR-212 inhibitor, control vector, or negative control. After 48 hours, the cells were harvested, and Renilla luciferase activity was measured using the Dual-Luciferase® Reporter Assay System (Promega Corporation), according to the manufacturer’s instructions. Results were obtained from three independent experiments performed in duplicate.
Immunohistochemical staining
Immunohistochemistry was performed on paraformaldehyde-fixed paraffin sections. FOXA1 (#3333, Epitomics Inc.) antibody was used in immunohistochemistry with streptavidin peroxidase conjugated (SP-IHC) method, and it was performed as previously reported.15 The percentage of positive cells was graded as per the following criteria: 0, <10%; 1, 10%–30%; 2, 31%–50%; 3, >50%.
Statistical analysis
Results are expressed as mean ± SEM. Significance was established, with the SPSS statistical package for Windows Version 13 (SPSS, Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software, Inc, San Diego, CA, USA), using a Pearson chi-squared test, the multivariant Cox regression analysis, a Kaplan–Meier plot, a log-rank test, a Spearman’s rank correlation coefficient, or a two-tailed Student’s t-test when appropriate. Difference were considered significant when P<0.05.
Results
Clinical significance of miR-212 in HCC cases
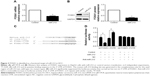
Initially, we tested the miR-212 expression in a retrospective cohort of 86 HCC tissues and matched adjacent nontumor tissues using quantitative reverse transcription (qRT)-PCR. The miR-212 expression in HCC tissues was significantly lower than that in matched adjacent nontumor liver tissues (P<0.05, Figure 1A). The expression level of miR-212 was considered either low (n=43) or high (n=43) according to the cutoff value, which was defined as the median expression level of miR-212 in this cohort. As shown in Table 1, miR-212 was expressed at prominently lower levels in HCC patients with high AFP level (P=0.008), large tumor size (P=0.007), high Edmondson-Steiner grading (P=0.017), and advanced tumor-node-metastasis tumor stage (P=0.009). Further, 86 HCC patients with survival information were analyzed using Kaplan–Meier estimation. Low expression of miR-212 was indeed associated with reduced overall survival and disease-free survival of HCC patients (P<0.05, respectively, Figure 1B and C). Furthermore, multivariate Cox regression analysis indicated that miR-212 expression was an independent factor for predicting both 5-year overall and disease-free survival of HCC patients (P=0.005 and 0.001, respectively, Table 2). These data indicate that miR-212 is a potent biomarker for the prognosis of HCC patients.
miR-212 inhibits HCC cell proliferation and induces apoptosis
HepG2 cells were transduced with the miR-212 expression and control vectors, respectively. As measured by qRT-PCR, the miR-212 expression was significantly upregulated by miR-212 expression vector (P<0.05, Figure 2A). BrdU incorporation assays were performed to test the effect of altering miR-212 levels on HCC cell proliferation. We found that upregulation of miR-212 significantly inhibited HepG2 cell proliferation (P<0.05, Figure 2B). Furthermore, as determined by flow cytometry assays, the percentage of apoptotic HepG2 cells significantly elevated after upregulation of miR-212 (P<0.05, Figure 2C). Next, downregulation of miR-212 in Huh7 cells was performed and was confirmed using qRT-PCR (P<0.05, Figure 2D). As expected, downregulation of miR-212 obviously promoted HCC cell proliferation and inhibited apoptosis (P<0.05, respectively, Figure 2E and F). Thus, miR-212 inhibits cell proliferation and induces apoptosis in HCC cells.
FOXA1 is identified as a functional target of miR-212
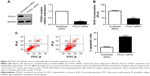
To disclose the molecular mechanisms by which miR-212 inhibits HCC tumor growth, predicted target genes of miR-212 were retrieved and analyzed using publicly available databases (TargetScan 6.2 and MiRanda). FOXA1, an important transcription factor in liver development and cancer progression,16 was predicted as one of the targets of miR-212. As measured by qRT-PCR and Western blot, both the levels of FOXA1 mRNA and protein were significantly reduced by upregulation of miR-212 in HepG2 cells (P<0.05, respectively, Figure 3A and B). Next, we investigated whether the miR-212 directly interacted with the 3′-UTR of FOXA1 mRNA using a Dual-Luciferase® Reporter Assay System. As expected, miR-212 significantly inhibited the luciferase activity of FOXA1 containing a wild-type (wt) 3′-UTR but did not suppress the activity of FOXA1 with a mutant (mt) 3′-UTR (P<0.05, Figure 3C and D). When anti-miR-212 was transfected, an increase in luciferase activity of wt FOXA1 3′-UTR was observed. However, with the mt FOXA1 3′-UTR constructs, there was no relative increase in activity (P<0.05, Figure 3C and D). Thus, our data strongly suggest that FOXA1 is a target of miR-212 in HCC.
miR-212 inhibits cell growth by targeting FOXA1 in HCC cells
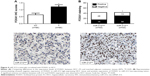
The expression of FOXA1 was further detected using immunohistochemistry in the previous cohort of 86 HCC specimens. FOXA1 was demonstrated to be significantly higher in HCC tissues as compared with that in noncancerous tissues (P<0.05, Figure 4A). The immunoreactivity of FOXA1 was considered either negative (score 0) or positive (scores 1–3). In these cases, the expression of FOXA1 was detected in 69.8% (30/43) of the HCC samples with low expression of miR-212, whereas only 34.9% (15/43) of the HCC specimens with high expression of miR-212 showed a positive FOXA1 signal (P<0.05, Figure 4B). Furthermore, Spearman correlation analysis indicated that miR-212 was inversely correlated with FOXA1 expression in HCC tissues (r=-0.562, P<0.001).
Next, HepG2 cells that were transfected with scrambled siRNA or FOXA1 siRNA were subjected to Western blot for FOXA1. As assessed by immunoblotting, FOXA1 was knocked down by a specific siRNA (P<0.05, Figure 5A). Furthermore, FOXA1 knockdown inhibited cell proliferation and induces apoptosis in HepG2 cells (P<0.05, respectively, Figure 5B and C). In sum, these data indicate that miR-212 suppresses HCC cell proliferation and induces apoptosis by inhibiting FOXA1.
Discussion
Increasing studies have reported that miRNAs regulates carcinogenesis-related gene expression, indicating a new insight in the initiation and progression of HCC.17,18 Decreased miR-212 expression has been confirmed in various types of cancers, including gastric cancer,1–3 lung cancer,4,5 colorectal cancer,6 and AML.7 Accordingly, our study demonstrated that the miR-212 expression in HCC tissues was significantly downregulated as compared with that in adjacent nontumor tissues. Furthermore, our results showed that reduced expression of miR-212 was associated with poor prognostic features of HCC. Importantly, miR-212 was identified as an independent prognostic marker for predicting 5-year overall survival and disease-free survival of HCC patients. These results indicate that miR-212 plays an important role in the progression of HCC, and may serve as a promising prognostic biomarker for HCC patients.
The prognostic significance of miR-212 expression in human malignancies stimulates scientists to dig out its biological functions and underlying mechanisms. miR-212 has been found to inhibit the growth of gastric cancer cells by targeting MeCP2 and promote the cell death of non-small-cell lung cells by regulating PED.1,5 However, in PDAC, miR-212 was found to promote cell growth and invasion by targeting hedgehog signaling pathway receptor patched-1.11 These studies suggest that miR-212 functions as a tumor suppressive or oncogenic miRNA in accordance with tumor types. In this study, we evaluated the biological functions of miR-212 through gain- and loss-of-function experiments. Our results demonstrated that miR-212 had an inhibitory effect on HCC cell growth by suppressing cell proliferation and inducing apoptosis. Due to these obvious effects of miR-212 on HCC cells proliferation and apoptosis, we further investigated its underlying mechanisms in HCC. Deregulation of miRNAs has been considered as a critical factor in the initiation and progression of HCC by serving as a negative regulator of oncogenic protein.19,20 The expression of FOXA1 was found to be upregulated in liver cancer cells21 and promoted the expression of Yes-associated protein and alpha fetoprotein in HCC,16 suggesting its oncogenic role in HCC. Furthermore, the expression of FOXA1 was found to be a downstream mediator of lncRNA MT1DP, which inhibited cell proliferation and transformative phenotype of liver cancer cells.16 In this study, we found that miR-212 significantly reduced the levels of both FOXA1 mRNA and protein in HepG2 cells. Herein, we validated FOXA1 as a direct functional target of miR-212 in HCC. Furthermore, the expression of FOXA1 in HCC tissues was obviously higher as compared with that in matched normal nontumor tissues. Otherwise, a significant inverse correlation between miR-212 and FOXA1 expression was observed in HCC tissues. Importantly, FOXA1 knockdown showed the same regulatory effect as upregulation of miR-212 in HepG2 cells with decreased cell proliferation and increased apoptosis. Altogether, our results suggest that FOXA1 is a downstream target of miR-212, and miR-212 may suppress cell proliferation and induce apoptosis by targeting FOXA1 in HCC. Moreover, the study by Yu et al suggested that Yes-associated protein was a downstream target of FOXA1.16 It is interesting to mention here that another study by Liang et al showed that the H3K4 demethylase retinoblastoma binding protein 2 was a downstream target of miR-212 in HCC cells and miR-212 inhibited HCC cell proliferation and induced cellular senescence by modulating the expression of retinoblastoma binding protein 2.22 This suggests that FOXA1 is not the only target of miR-212 in HCC and miR-212 can exert its tumor suppressive role by modulating various downstream pathways in HCC cells.
In conclusion, we find that miR-212 is downregulated in HCC tissues. The low expression of miR-212 is correlated with poor prognostic features and reduced survival of HCC patients. We demonstrate that miR-212 may reduce HCC cell growth by suppressing FOXA1 expression. In sum, we consider that miR-212 may potentially act as a clinical biomarker, and may also be a therapeutic target, in HCC.
Acknowledgment
This study was supported by Educational Commission of Hubei Province (grant D20082405).
Disclosure
The authors report no conflicts of interest in this work.
References
Wada R, Akiyama Y, Hashimoto Y, Fukamachi H, Yuasa Y. miR-212 is downregulated and suppresses methyl-CpG-binding protein MeCP2 in human gastric cancer. Int J Cancer. 2010;127(5):1106–1114. | ||
Xu L, Wang F, Xu XF, et al. Down-regulation of miR-212 expression by DNA hypermethylation in human gastric cancer cells. Med Oncol. 2011;28(Suppl 1):S189–S196. | ||
Wu WY, Xue XY, Chen ZJ, et al. Potentially predictive microRNAs of gastric cancer with metastasis to lymph node. World J Gastroenterol. 2011;17(31):3645–3651. | ||
Incoronato M, Urso L, Portela A, et al. Epigenetic regulation of miR-212 expression in lung cancer. PLoS One. 2011;6(11):e27722. | ||
Incoronato M, Garofalo M, Urso L, et al. miR-212 increases tumor necrosis factor-related apoptosis-inducing ligand sensitivity in non-small-cell lung cancer by targeting the antiapoptotic protein PED. Cancer Res. 2010;70(9):3638–3646. | ||
Meng X, Wu J, Pan C, et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology. 2013;145(2):426–436. e421–e426. | ||
Sun SM, Rockova V, Bullinger L, et al. The prognostic relevance of miR-212 expression with survival in cytogenetically and molecularly heterogeneous AML. Leukemia. 2013;27(1):100–106. | ||
Hatakeyama H, Cheng H, Wirth P, et al. Regulation of heparin-binding EGF-like growth factor by miR-212 and acquired cetuximab-resistance in head and neck squamous cell carcinoma. PLoS One. 2010;5(9):e12702. | ||
Wei LQ, Liang HT, Qin DC, Jin HF, Zhao Y, She MC. MiR-212 exerts suppressive effect on SKOV3 ovarian cancer cells through targeting HBEGF. Tumour Biol. 2014;35(12):12427–12434. | ||
Park JK, Henry JC, Jiang J, et al. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406(4):518–523. | ||
Ma C, Nong K, Wu B, et al. miR-212 promotes pancreatic cancer cell growth and invasion by targeting the hedgehog signaling pathway receptor patched-1. J Exp Clin Cancer Res. 2014;33:54. | ||
Scapoli L, Palmieri A, Lo Muzio L, et al. MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol. 2010;23(4):1229–1234. | ||
Huang Y, Guo W, Kan H. TPX2 is a prognostic marker and contributes to growth and metastasis of human hepatocellular carcinoma. Int J Mol Sci. 2014;15(10):18148–18161. | ||
Liu C, Billadeau DD, Abdelhakim H, et al. IQGAP1 suppresses TbetaRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123(3):1138–1156. | ||
Tu K, Zheng X, Zan X, Han S, Yao Y, Liu Q. Evaluation of Fbxw7 expression and its correlation with the expression of c-Myc, cyclin E and p53 in human hepatocellular carcinoma. Hepatol Res. 2012;42(9):904–910. | ||
Yu W, Qiao Y, Tang X, et al. Tumor suppressor long non-coding RNA, MT1DP is negatively regulated by YAP and Runx2 to inhibit FoxA1 in liver cancer cells. Cell Signal. 2014;26(12):2961–2968. | ||
Kan H, Guo W, Huang Y, Liu D. MicroRNA-520 g induces epithelial-mesenchymal transition and promotes metastasis of hepatocellular carcinoma by targeting SMAD7. FEBS Lett. 2015;589(1):102–109. | ||
Zhu Z, Zhang X, Wang G, Zheng H. Role of microRNAs in hepatocellular carcinoma. Hepat Mon. 2014;14(8):e18672. | ||
Liu Z, Tu K, Liu Q. Effects of microRNA-30a on migration, invasion and prognosis of hepatocellular carcinoma. FEBS letters. 2014; 588(17):3089–3097. | ||
Gramantieri L, Fornari F, Callegari E, et al. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12(6A):2189–2204. | ||
Wang J, Park JS, Wei Y, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell. 2013;51(2):211–225. | ||
Liang X, Zeng J, Wang L, et al. Histone demethylase retinoblastoma binding protein 2 is overexpressed in hepatocellular carcinoma and negatively regulated by hsa-miR-212. PLoS One. 2013;8(7):e69784. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.