Back to Journals » OncoTargets and Therapy » Volume 10
MicroRNA-148a suppresses proliferation and invasion potential of non-small cell lung carcinomas via regulation of STAT3
Received 29 September 2016
Accepted for publication 2 November 2016
Published 2 March 2017 Volume 2017:10 Pages 1353—1361
DOI https://doi.org/10.2147/OTT.S123518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Mei He, Yan Xue
Department of Respiratory Medicine, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, People’s Republic of China
Abstract: Lung cancer has the highest morbidity and mortality in the world, and non-small cell lung carcinomas (NSCLC) account for 80% of cases of lung cancer. The mechanism of NSCLC is still largely unknown, and finding novel targets is of great importance for the treatment of NSCLC. The current study was designed to evaluate the role of miR-148a in NSCLC cell proliferation and invasion and to investigate the possible molecular mechanisms. We found that miR-148a expression was decreased in NSCLC tissues and cell lines. Upregulation of miR-148a significantly decreased A549 cell proliferation, and downregulation of miR-148a significantly increased A549 cell proliferation. Upregulation of miR-148a markedly increased apoptotic cell death and inhibited cell invasion potential. Upregulation of miR-148a significantly decreased signal transducer and activator of transcription 3 (STAT3) expression and 3'-untranslated region luciferase activity. Downregulation of miR-148a significantly increased STAT3 expression. Overexpression of STAT3 significantly inhibited the effect of miR-148a on cell viability and invasion potential. In conclusion, we found that miR-148a inhibited NSCLC cell proliferation and invasion potential through the inhibition of STAT3. Our findings highlight miR-148a/STAT3 axis as a novel therapeutic target for the inhibition of NSCLC growth.
Keywords: miR-148a, STAT3, non-small cell lung carcinomas, cell proliferation, invasion
Introduction
It is well known that lung cancer has the highest morbidity and mortality in the world.1,2 It has been reported that lung cancer resulted in 160,000 deaths in the United States in 2014, which accounted for 20% of all cancer-caused deaths.3 Among those, ~80% of lung cancers are classified as non-small cell lung carcinomas (NSCLC) with a fairly low survival rate (5-year survival rate is less than 15%).3–5 Adenocarcinoma and squamous cell carcinoma are the main types of NSCLC.6 Despite great breakthrough in the development of therapeutic techniques, patients with advanced NSCLC have a poor prognosis, and the 5-year survival rate of patients with NSCLC remains poor.7,8 The mechanism of NSCLC is still largely unknown, and finding novel targets is of great importance for the treatment of NSCLC.9
MicroRNAs (miRNAs or miRs) are a class of small non-coding RNAs (20–24 nucleotides) that have been implicated in various biological processes and diseases.10,11 miRNAs function to modulate protein expression through mRNA destabilization and degradation via directly binding the 3′-untranslated region of the target mRNA.12,13 One-third of all mammalian genes have been estimated to be directly or indirectly regulated by miRNAs. It is reported that miRNAs play a role in the regulation of cell growth, proliferation, apoptosis, differentiation, migration, metabolism, etc.14 In particular, dysregulation of miRNAs is believed to function as an important regulator in the pathogenesis of various types of tumors.15,16 Investigation of miRNAs in the development of tumor is useful for diagnosis and therapy of cancer.17 Numerous miRNAs have been found to be dysregulated under the pathological condition of NSCLC.18,19 Recently, studies have found that miR-148a functions to suppress NSCLC.20–22 However, the mechanism underlying the inhibitory effect of miR-148a on NSCLC is still not completely known.
The present study was designed to investigate the mechanism underlying the antitumor effect of miR-148a in NSCLC. We found that miR-148a expression was decreased in NSCLC tissues and cell lines and functioned to suppress NSCLC cell proliferation and invasion through inhibition of signal transducer and activator of transcription 3 (STAT3).
Materials and methods
Chemicals and materials
β-actin was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz). Bax, Bcl-2, STAT3, E-cadherin, and Snail antibodies were obtained from Cell Signaling Technology (CST; Danvers, MA, USA). All of the other chemicals used were of the highest grade available commercially.
Ethics statement and clinical specimens
The study was approved by the Ethical Review Committee of Shanxi Provincial People’s Hospital and complied with the Declaration of Helsinki. Twenty-one cases of NSCLC were retrieved from the Pathology Archive of Shanxi Provincial People’s Hospital in 2015. All patients provided their written informed consent. The NSCLC was diagnosed by two independent pathologists according to the World Health Organization classification. Clinical specimens were collected when the surgery was conducted in patients who had not received any chemotherapy or radiotherapy before surgery.
Cell culture
Normal human bronchial epithelial (HBE) and NSCLC cell lines (A549, H1299, H1650, and H460) were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin in an incubator with 5% CO2 at 37°C.
Cell transfection
miR-148a mimics, miR-148a inhibitors (anti-miR-148a), and negative controls were synthesized by GeneBioPharma Company (Shanghai, People’s Republic of China). The STAT3 plasmid and empty vectors were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz). Transient transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols.
RNA isolation and real-time polymerase chain reaction (PCR)
Total RNA was isolated using Trizol reagent (Invitrogen). Total RNA of 500 ng was reversely transcribed using a commercial kit according to the manufacturer’s instructions (Takara, Tokyo, Japan). One microliter of cDNA was used for the performance of real-time PCR with SYBR Green PCR kit (Thermo Scientific, IL, USA). The PCR reaction conditions were as follows: initial denaturation at 95°C for 10 min followed by 30 cycles at 95°C for 1 min, annealing at 53°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. The PCR was conducted using a CFX96 real-time PCR system (BioRad, Hercules, CA, USA). β-actin and U6 small nuclear RNAs were used as internal controls. Results were expressed as folds of control.
Cell proliferation
Cell Counting Kit-8 assay kit was used to determine cell proliferation according to the manufacturer’s protocols. Absorbance at 450 nm was measured using a microplate reader (BioRad).
Luciferase reporter assay
Cells were seeded in 24-well plates and then co-transfected with miR-148a mimics or negative control and the mutant or wild type 3′UTR of STAT3 using Lipofectamine 2000. Forty-eight hours after the transfection, cells were collected, and then relative luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s protocols.
Western blot
Cells were lysed using RIPA lysis buffer (Thermo Scientific), and proteins were extracted. Protein concentration was determined by bicinchoninic acid (BCA) assay (Thermo Scientific). Twenty micrograms of protein was loaded and separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. After separation, proteins were transferred onto PVDF membrane (Millipore, MA, USA), and then blocked using 8% nonfat milk. Membranes were incubated overnight at 4°C with primary antibodies (β-actin: Santa Cruz, 1:1000; STAT3: CST, 1:1000; Bax: CST, 1:1000; Bcl-2: CST, 1:1000; E-cadherin: CST, 1:1000; Snail: CST, 1:1000). After four-time washing, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Thermo Scientific) at 37°C for 30 min. Protein bands were visualized with the enhanced chemiluminescence and captured using BioRad Imaging Systems (BioRad).
Apoptosis
After the treatment, cells were collected and apoptosis was determined using TdT-mediated dUTP nick end labeling (TUNEL) assay kit (Roche, Basel, Switzerland) according to the manufacturer’s protocols. Percentage of apoptotic cells were analyzed using flow cytometry. Results were shown as folds of control.
Caspase-3 and caspase-9 activities
After the treatment, cells were collected and pellets were suspended in chilled cell lysis buffer. Colorimetric assay kits were used to analyze the activities of caspase-3 and caspase-9 (BioVision, Milpitas, CA, USA). In brief, cytosolic protein from the cells was extracted and protein concentration was determined by BCA assay (Thermo Scientific). In each assay, 50 μL cell lysis buffer containing 50–200 μg protein was used. At the end, absorbance at 405 nm was measured using a microplate reader (BioRad).
Statistical analysis
The data were shown as the mean ± SEM, and data analysis was performed using SPSS 16.0 software (Chicago, IL). Significance between more than two groups was analyzed using one-way analysis of variance. Significance of differences between two groups was analyzed by Student’s t-test. P<0.05 was considered to be statistically significant.
Results
miR-148a reduced in NSCLC tissues and cell lines
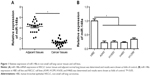
To confirm the expression pattern of miR-148a in NSCLC, we first assessed the expression of miR-148a in NSCLC tissues and cell lines. As shown in Figure 1A, miR-148a expression was reduced in samples of NSCLC tissues, compared with that in the corresponding adjacent normal lung tissues. In addition, in all the NSCLC cell lines, including A549, H1299, H1650, and H460 cell lines, miR-148a expression was markedly lower than that in the HBE cell line (Figure 1B).
miR-148a reduced cell proliferation in A549 cells
In order to evaluate the role of miR-148a in the growth of NSCLC, the effect of upregulation and downregulation of miR-148a on cell proliferation in A549 cells was examined. A549 cells were transfected with miR-148a mimic or inhibitor and then real-time PCR was conducted to detect the miR-148a expression. In Figure 2A, the results showed that transfection of miR-148a mimic induced a significant increase in miR-148a expression. Upregulation of miR-148a expression significantly suppressed A549 cell proliferation (Figure 2B). In Figure 2C, the results showed that transfection of miR-148a inhibitor induced a significant decrease in miR-148a expression. Downregulation of miR-148a expression significantly increased A549 cell proliferation (Figure 2D). The results demonstrated that miR-148a reduced cell proliferation in A549 cells.
miR-148a promoted apoptosis in A549 cells
Role of miR-148a in apoptotic cell death was evaluated in A549 cells. In Figure 3A–D, we showed that transfection of miR-148a mimic significantly increased the mRNA and protein expression of Bax and decreased the mRNA and protein expression of Bcl-2. Moreover, miR-148a mimic notably increased the activities of caspases 3 and 9 (Figure 3E, F). The percentage of TUNEL-positive cells in A549 cells was significantly increased by upregulation of miR-148a (Figure 3G, H). The results indicated that miR-148a promoted apoptosis, which may contribute to the decrease of cell proliferation in A549 cells.
miR-148a decreased the invasion potential in A549 cells
The effect of miR-148a on invasion potential in A549 cells was examined. As shown in Figure 4A–D, the expression of N-cadherin, vimentin, and Snail was notably reduced by miR-148a mimic. The expression of E-cadherin was markedly increased by miR-148a mimic. The results indicated that miR-148a exhibited an inhibitory effect against invasion potential in A549 cells.
Downregulation of STAT3 required for miR-148a-induced inhibition of cell proliferation and invasion in A549 cells
Previous literature has reported that STAT3 is a potential target of miR-148a in gastric tumor.23 In the current study, we also examined whether regulation of STAT3 was involved in the effect of miR-148a on cell proliferation and invasion in A549 cells. We showed that miR-148a mimic significantly decreased the mRNA and protein expression of STAT3 (Figure 5A, B). miR-148a mimic markedly decreased the luciferase activity of 3′-untranslated region of STAT3 (Figure 5C). miR-148a inhibitor notably increased the mRNA and protein expression of STAT3 (Figure 5D, E). The results indicated that STAT3 may play a role in the regulation of proliferation and invasion.
To further confirm the extract role of STAT3, we transfected cells with plasmid carrying STAT3. The results showed that overexpression of STAT3 significantly inhibited miR-148a mimic-induced decrease of cell proliferation (Figure 6A), increase of apoptosis (Figure 6B) and E-cadherin expression (Figure 6C, E), and decrease of Snail expression (Figure 6D, E). The results indicated that downregulation of STAT3 was involved in miR-148a mimic-induced effect on cell proliferation and invasion potential in A549 cells.
Discussion
In the present study, we investigated the role of miR-148a in NSCLC cell proliferation and invasion potential and clarified the possible molecular mechanisms. Previous literature has shown that miR-148a functions to regulate the growth of several types of tumors.24 For example, Xu et al found that miR-148a functioned to suppress metastasis and served as a prognostic indicator in triple-negative breast cancer.20 Downregulation of miR-148a was significantly associated with shorter overall survival of gastric cancer patients.25 In contrast, decreased expression of miR-148a was found to predict poor prognosis in ovarian cancer and to be associated with tumor growth and metastasis.26 miR-148a was reported to serve as predictive biomarker for early diagnosis of laryngeal carcinoma.27 Huang reported that circulating miR-148a expression level in plasma samples of NSCLC patients was decreased.28 Yang et al believed that miR-148a could function as a novel biomarkers in NSCLC screening.29 Moreover, Joshi et al found that miR-148a reduced tumorigenesis and increased TNF-related apoptosis-inducing ligand-induced apoptosis in NSCLC.30 These literatures suggest a potent role of miR-148a in the development of NSCLC. However, the exact role and molecular mechanism of miR-148a-induced regulation of NSCLC is far from completely understood.
In the current study, we found that miR-148a expression was decreased in NSCLC tissues and cell lines. Moreover, we showed that upregulation of miR-148a expression decreased cell proliferation in A549 cells and downregulation of miR-148a expression showed an opposite effect. Furthermore, upregulation of miR-148a expression activated apoptotic pathway and promoted apoptosis. The results demonstrated that miR-148a inhibited A549 cell proliferation and downregulation of miR-148a may be required for the development of NSCLC. Furthermore, the effect of miR-148a on invasion potential was evaluated. The results suggested that miR-148a inhibited invasion potential in A549 cells since upregulation of miR-148a decreased N-cadherin, vimentin, and Snail expression and increased E-cadherin.
In the next step, we examined the potential targets that mediated the antitumor effect of miR-148a in NSCLC. The STAT signaling pathway is one of the most important intracellular signaling networks.31 As one of the family members of STATs, STAT3 transduces signals from activated receptors or intracellular kinases to the nucleus, thus activating and regulating gene transcription.31,32 In response to interleukin-6 signal, STAT3 could be activated and is involved in various types of pathological conditions. STAT3 plays important roles in various types of cancer.33–35 Yu et al found that activated STAT3 was correlated with prognosis of NSCLC.36 Disruption of STAT3 by niclosamide reversed radioresistance of human lung cancer.37 Suppression of JAK/STAT3 was found to exert antitumor activity in NSCLC.38 Moreover, miR-148a was identified to function as a tumor suppressor via inactivating STAT3 in human gastric cancer.23
In the present study, we also examined the role of STAT3 in the antitumor effect of miR-148a in NSCLC. We showed that STAT3 was a target of miR-148a in A549 cells. Upregulation of STAT3 significantly suppressed the effect of miR-148a on cell proliferation and invasion potential. The results indicated that downregulation of STAT3 was required for miR-148a-induced inhibition of cell proliferation and invasion potential in A549 cells.
In conclusion, we found that miR-148a inhibited NSCLC cell proliferation and invasion potential through inhibition of STAT3. Our findings identify miR-148a/STAT3 axis as a novel therapeutic target for the inhibition of NSCLC growth.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. | ||
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. | ||
Claassens L, van Meerbeeck J, Coens C, et al. Health-related quality of life in non-small-cell lung cancer: an update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011;29(15):2104–2120. | ||
Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28(10):1721–1726. | ||
Shtivelman E, Hensing T, et al. Molecular pathways and therapeutic targets in lung cancer. Oncotarget. 2014;5:1392–1433. | ||
Scagliotti GV, Novello S. Adjuvant therapy in completely resected non-small-cell lung cancer. Curr Oncol Rep. 2003;5:318–325. | ||
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. | ||
Socinski MA. Seeking new options for the treatment of small-cell lung cancer. Lung cancer (Amsterdam, Netherlands). Lung Cancer. 2005;50(Suppl 1):S25–S26. | ||
Ranganathan K, Sivasankar V. MicroRNAs – biology and clinical applications. Journal of oral and maxillofacial pathology. J Oral Maxillofac Pathol. 2014;18(2):229–234. | ||
Li MH, Fu SB, Xiao HS. Genome-wide analysis of microRNA and mRNA expression signatures in cancer. Acta Pharmacol Sin. 2015;36(10):1200–1211. | ||
Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. | ||
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. | ||
Rottiers V, Najafi-Shoushtari SH, Kristo F, et al. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb Symp Quant Biol. 2011;76:225–233. | ||
Zimmerman AL, Wu S. MicroRNAs, cancer and cancer stem cells. Cancer Lett. 2011;300(1):10–19. | ||
Kishikawa T, Otsuka M, Ohno M, Yoshikawa T, Takata A, Koike K. Circulating RNAs as new biomarkers for detecting pancreatic cancer. World J Gastroenterol. 2015;21(28):8527–8540. | ||
Wang P, Zhuang L, Zhang J, et al. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol Oncol. 2013;7(3):334–345. | ||
Wang X, Chen Z. MicroRNA-19a functions as an oncogenic microRNA in non-small cell lung cancer by targeting the suppressor of cytokine signaling 1 and mediating STAT3 activation. Int J Mol Med. 2015;35(3):839–846. | ||
Sun W, Ma Y, Chen P, Wang D. MicroRNA-10a silencing reverses cisplatin resistance in the A549/cisplatin human lung cancer cell line via the transforming growth factor-beta/Smad2/STAT3/STAT5 pathway. Mol Med Rep. 2015;11(5):3854–3859. | ||
Xu X, Zhang Y, Jasper J, et al. MiR-148a functions to suppress metastasis and serves as a prognostic indicator in triple-negative breast cancer. Oncotarget. 2016;7(15):20381–20394. | ||
Li J, Song Y, Wang Y, Luo J, Yu W. MicroRNA-148a suppresses epithelial-to-mesenchymal transition by targeting ROCK1 in non-small cell lung cancer cells. Mol Cell Biochem. 2013;380(1–2):277–282. | ||
Zheng B, Liang L, Wang C, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17(24):7574–7583. | ||
Yu B, Lv X, Su L, et al. MiR-148a functions as a tumor suppressor by targeting CCK-BR via inactivating STAT3 and Akt in human gastric cancer. PloS One. 2016;11(8):e0158961. | ||
Li Y, Deng X, Zeng X, Peng X. The role of Mir-148a in cancer. J Cancer. 2016;7(10):1233–1241. | ||
Qiu X, Zhu H, Liu S, et al. Expression and prognostic value of microRNA-26a and microRNA-148a in gastric cancer. J Gastroenterol Hepatol. Epub 2016 Aug 16. | ||
Gong L, Wang C, Gao Y, Wang J. Decreased expression of microRNA-148a predicts poor prognosis in ovarian cancer and associates with tumor growth and metastasis. Biomed Pharmacother. 2016;83:58–63. | ||
Wu Y, Yu J, Ma Y, Wang F, Liu H. miR-148a and miR-375 may serve as predictive biomarkers for early diagnosis of laryngeal carcinoma. Oncol Lett. 2016;12(2):871–878. | ||
Huang MX. Down-expression of circulating micro ribonucleic acid (miRNA)-148/152 family in plasma samples of non-small cell lung cancer patients. J Cancer Res Ther. 2016;12(2):671–675. | ||
Yang JS, Li BJ, Lu HW, et al. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36(4):3035–3042. | ||
Joshi P, Jeon YJ, Lagana A, et al. MicroRNA-148a reduces tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc Natl Acad Sci U S A. 2015;112:8650–8655. | ||
Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30(2):88–106. | ||
Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. | ||
Yeh CM, Chang LY, Lin SH, et al. Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant JAK/STAT signaling, predicts prognosis in gastric cancer. Sci Rep. 2016;6:31690. | ||
Xiang M, Kim H, Ho VT, et al. Gene expression-based discovery of atovaquone as a STAT3 inhibitor and anti-cancer agent. Blood. Epub 2016 Aug 16. | ||
Duan J, Yue W, Yu JE, et al. In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFkappaB signaling in pancreatic cancer cells. Sci Rep. 2016;6:31672. | ||
Yu Y, Zhao Q, Wang Z, Liu XY. Activated STAT3 correlates with prognosis of non-small cell lung cancer and indicates new anticancer strategies. Cancer Chemother Pharmacol. 2015;75(5):917–922. | ||
You S, Li R, Park D, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 2014;13(3):606–616. | ||
Zhu F, Dai C, Fu Y, et al. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget. 2016;7(8):9462–9476. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.