Back to Journals » Cancer Management and Research » Volume 11
MicroRNA-145 inhibits growth of laryngeal squamous cell carcinoma by targeting the PI3K/Akt signaling pathway
Authors Ye D , Zhou C , Deng H , Lin L , Zhou S
Received 23 December 2018
Accepted for publication 1 April 2019
Published 30 April 2019 Volume 2019:11 Pages 3801—3812
DOI https://doi.org/10.2147/CMAR.S199291
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Dong Ye,1,2 Chongchang Zhou,2 Hongxia Deng,2 Lexi Lin,2 Shuihong Zhou1
1Department of Otorhinolaryngology-Head and Neck Surgery, The First Affiliated Hospital of Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Otorhinolaryngology-Head and Neck Surgery, Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China
Purpose: In this study, we used a nude mouse model of human laryngeal squamous cell carcinoma (LSCC) to investigate inhibition of tumor growth by microRNA-145 (miR-145) and the mechanisms underlying this inhibition.
Methods: Tumors were established in nude mice by transplantation of the LSCC AMC-HN-8 cell line. Forty-eight nude mice were randomly divided into groups of eight mice each and treated with high (1.0 optical density [OD]) or low (0.5 OD) doses of miR-145, or relevant control treatments. Tumor growth was observed in each group and used to calculate the inhibition rate. Routine pathological and electron microscopic examinations were used to determine tumor apoptosis and proliferation. Changes in levels of miR-145 and PI3K and Akt protein levels were also analyzed.
Results: MiR-145 inhibited LSCC growth in a dose-dependent manner, as tumor growth was significantly inhibited in mice injected intratumorally with high-dose miR-145 compared with both the untreated and low-dose miR-145 groups (p<0.05). Pathological examination showed increased tumor necrotic and apoptotic changes in treated mice, which was confirmed by electron microscopy. PI3K and Akt protein expression were significantly lower in tumors treated with high-dose miR-145 group compared with those in the untreated and low-dose miR-145 groups (p<0.05).
Conclusions: MiR-145 was associated with inhibited tumor growth in a nude mouse model of LSCC. The underlying mechanism may be inhibition of the PI3K/Akt signaling pathway, which regulates tumor growth, invasion, and metastasis and also plays an important role in tumor angiogenesis and proliferation of tumor stem cells. MiR-145 may act as a tumor suppressor gene and is a promising candidate for cancer treatment.
Keywords: laryngeal squamous cell carcinoma, animal model, miRNA-145, oncology, treatment, mechanism
Introduction
Laryngeal carcinoma is the second most common malignancy of the head and neck, typically presenting as a form of squamous cell carcinoma.1 The incidence of this disease is increasing, but the mechanisms by which it develops are not fully understood. Therefore, new methods for diagnosis and treatment of laryngeal squamous cell carcinoma (LSCC) are essential.
Recently, investigation of the pathogenesis, diagnosis, and treatment of LSCC has increased in interest in recent years. Numerous protein-coding genes have been identified that serve as main effectors and regulatory factors underlying LSCC development and progression. However, the emergence of epigenetic data has allowed for the identification of a large number of post-transcriptional processes that are implicated in disease pathogenesis. Epigenetic control networks based on non-coding RNA recognition have become an important area of research, with a significant focus on the role of microRNAs (miRNAs) in carcinogenesis. MiRNAs are widely expressed in eukaryotic organisms, and regulate a wide range of biological processes including the expression of tumor-associated genes that contribute to development and progression of tumor cells.2
MiR-145, a recently discovered miRNA, plays an important role in tumor inhibition. As a cancer suppressor gene, it inhibits proliferation, invasion, and metastasis of tumor cells,3,4 increases sensitivity to chemotherapeutic drugs,5 and regulates the development and progression of tumors. Decreased miR-145 expression has been reported in a wide range of tumors including prostate, bladder, colon, ovarian, and esophageal cancers, suggesting a potential role in disease pathology.6–10 In-depth studies suggest that miR-145 could be an ideal marker for diagnostic and prognostic evaluations of tumors, and that it may also represent a novel target for cancer treatment.
Phosphatidylinositol-3-kinase (PI3K) is a kinase that catalyzes the synthesis of phosphatidylinositol lipids. Members of this protein family have been shown to control a variety of cellular functions including immune activation, inflammation, cell membrane transport, autophagy, and glucose transport. The PI3K signaling pathway is an important cellular signal transduction pathway. Akt, also known as protein kinase B, functions downstream of PI3K, serving as an intermediate regulatory link in the PI3K/Akt signal transduction pathway to control the activity of numerous cellular targets. The PI3K/Akt signaling pathway has been implicated in virtually all phases of carcinogenesis, including tumor occurrence, development, treatment, and outcomes. Activation of this pathway has been shown to inhibit apoptosis while promoting cell proliferation, angiogenesis, tumor invasion, and metastasis, highlighting its importance in the development and progression of malignant tumors.11–14
Recent studies of miR-145 in LSCC were limited to laryngeal carcinoma tissues and cell lines and indicated that miR-145 inhibited the growth of most tumors.15–18 However, no relevant studies of miR-145 in a nude mouse model of laryngeal cancer have been performed, and the specific mechanisms of action of miR-145 have not been characterized. Studies have shown that multiple factors can regulate the PI3K/Akt signaling pathway to promote development and progression of laryngeal cancer,19–21, but no studies have evaluated the correlation between the PI3K/Akt signaling pathway and miR-145. We used a nude mouse transplantation model to investigate the role of miR-145 in LSCC. Forty-eight nude mice were randomly divided into six groups: unmanipulated control, glucose solution control, transfection reagent control, non-specific gene sequence control, miR-145 high-dose (1.0 optical density [OD]) treatment, and miR-145 low-dose (0.5 OD) treatment. Tumor growth was observed in each group, and the rate of inhibition was calculated. Routine pathology and electron microscopic examinations were used to evaluate tumor apoptosis and proliferation. Changes in miR-145 levels and in PI3K and Akt protein levels were analyzed by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and ELISA, respectively. These analyses allowed us to investigate the effects of miR-145 on tumor inhibition and to evaluate possible mechanisms of miR-145 activity in the development of human LSCC.
Methods
Materials
BALB/cA nu/nu male nude mice weighing 14–16 g (quality certification number 2007000553984; animal license number SYXK; Shanghai SLAC Laboratory Animal Center, Shanghai, China; 2009-0123), human LSCC AMC-HN-8 cells (Catalog number:BNCC338377, BeNa Culture Collection, Shanghai, China), transfection reagents (Engreen Biosystem, Beijing, China), miR-145 and non-specific gene sequences (Genepharma, Shanghai, China), ELISA kit (SunBio Technology, Beijing, China), RNA extraction kit (Qiagen, Duesseldorf, Germany), GoScript Reverse Transcription System (Promega, Madison, WI USA), GoTaq qPCR master mix (Promega), primary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), secondary antibody (Zhongshan Golden Bridge, Guangzhou, China), micro-spectrophotometer (ND-2000, USA), qPCR machine (Applied Biosystems, Waltham, MA, USA), and Power Wave XS full wavelength enzyme meter (Gene, San Francisco, USA) were used in this study. All animal-related protocols were approved by the Animal Ethical Care and Use Committee of the First Affiliated Hospital of Zhejiang University (Protocol Approval No. AEWC-2015-10), the rules and regulations for the administration of laboratory animals of Zhejiang University, Hangzhou, Zhejiang, China.
Cell culture
Human AMC-HN-8 cells were cultured in RPMI-1640 culture medium with 10% fetal bovine serum containing 100 U/mL penicillin/streptomycin at 37ºC in a 5% CO2 incubator. Cells were passaged using 0.25% pancreatic enzyme digestion. Cells in the logarithmic phase that exhibited superior growth were selected for experiments.
Construction of laryngeal cancer model in nude mice
Forty-eight BALB/c nude mice were obtained from Shanghai SLAC Laboratory Animal Center. A solution containing miR-145 and transfection reagent was prepared according to the kit manufacturer’s instructions, and the transfection efficiency was 87.7%. Each nude mouse was subcutaneously inoculated in the axillary region with 0.2 mL/injection (containing 8.7×106 cells) of AMC-HN-8 cell suspension. The miR-145 primers were as follows: 5ʹ-GTCCAGTTTTCCCAGGAAT-3ʹ (forward) and 5ʹ-TGGTGTCGTGGAGTCG-3ʹ (reverse). Transfection efficiency was determined by qRT-PCR. Tumors were allowed to grow to more than 100 mm3, and the mice were randomly divided into six groups (n=8 each): 1) unmanipulated control, 2) glucose solution control, 3) transfection reagent control, 4) non-specific gene sequence control, 5) miR-145 high-dose (1.0 OD) treatment , and 6) miR-145 low-dose (0.5 OD) treatment. The mice were maintained on sterilized food and water while quarantined in a pathogen-free environment with a 12 hrs light and 12 hrs dark photoperiod in an animal care facility.
Calculation of transplanted tumor volume and inhibition rate
Tumors were allowed to grow to over 100 mm3. The first group received no treatment, and tumors in the remaining five groups were injected with 50 μL of the corresponding solutions twice per week for 3 weeks. Mice were weighed twice weekly, and tumor growth was monitored. The maximum tumor diameter (a) and transverse diameter (b) of the tumors were measured, and tumor volume was calculated as follows: tumor volume (mm3) = (ab2)/2.
The day after the final treatment, the mice were sacrificed by CO2 inhalation, and solid tumors were dissected. The tumor weight difference and tumor inhibition rate (%) of each group were calculated. Tumor inhibition rate = (mean weight of tumors in the control groups − mean weight of tumors in the experimental group)/mean weight of tumors in the control group ×100%.
Hematoxylin and eosin (H&E) staining examination of tumor necrotic and apoptotic changes
Tumor tissues were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned. The sections were then deparaffinized, stained with H&E, and sealed. Morphological and structural changes of the tumor tissues were observed under a microscope.
Electron microscopic examination of cellular apoptosis
Tumor tissue was fixed using glutaraldehyde, flushed with buffer solution, fixed using osmic acid, gradient dehydrated using acetone, embedded using embedding agent, and prepared for imaging. Changes in organelle structure were observed using a JEM-1101 transmission electron microscope.
qRT-PCR detection of miR-145 gene expression
RNA was extracted from tumor tissue using an RNA extraction kit, and RNA concentration and purity were determined using an ND-2000 nucleic acid meter. cDNA was synthesized from RNA by reverse transcription using the GoScript Reverse Transcription System (Promega) kit. PCR reaction solution was prepared using SYBR Green premix, template, upstream and downstream primers, and ddH2O, and then placed on a StepOnePlus Fluorescence Quantitative PCR system (ABI) for PCR amplification. Reaction conditions were as follows: 95ºC for 2 mins, 95ºC for 1 min, 60ºC for 1 min, and 72ºC for 1 min for a total of 40 cycles, followed by 72ºC for 7 mins. The relative expression of genes was calculated using the 2−ΔΔCt relative quantitative analysis method. GAPDH mRNA level was used as an internal reference.
Analysis of PI3K and Akt protein expression by ELISA
Tumor tissue (100 mg) was washed and placed in a tissue grinder with 1 mL of phosphate-buffered saline to prepare homogenates. Samples were stored at −20°C overnight. Two freeze-thaw cycles were performed to disrupt cell membranes and the homogenates were then centrifuged at 5,000 g for 5 mins at 4ºC. A bicinchoninic acid protein concentration assay kit was used to determine protein concentrations. PI3K and Akt concentrations were determined using an ELISA kit. The concentration (pg/mL) of PI3K and Akt protein in the samples was calculated according to the standard curve prepared per the kit manufacturer’s instructions.
Statistical methods
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for analysis. Data are shown as the means ± standard deviations. Independent samples of two groups were compared using paired Student’s t-test, and the mean values of multiple groups were compared using single-factor variance analysis. P<0.05 was considered statistically significant.
Results
Histological observations and calculation of the inhibition rate of solid tumors
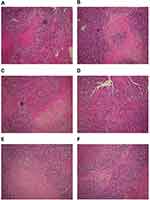
The solid tumors in the treatment groups were smaller than those in the control groups, and this reduction in size was more conspicuous in the high-dose miR-145 treatment group. However, there were no clear differences among the control groups. The tumors in the treatment groups were small with spherical, smooth, pink, and soft features, while those in the control groups were comparatively large with nodular surfaces and less central necrosis (Figure 1). The mean tumor volume, weight, and inhibition rate for each group are shown in Table 1.
 | Table 1 The mean tumor volume, weight and inhibition rate for each group |
 | Figure 1 Growth of solid tumors. Tumors in the treatment groups were smaller than those in the control groups, with the reduction in tumor size more obvious in the high-dose miR-145 treatment group. |
Pathological changes in tumors
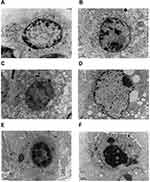
Tumors were generated in nude mice by subcutaneous injection of LSCC strain AMC-HN-8 cells. The tumor cells in the treatment groups showed cell apoptosis, cell shrinkage, cytoplasmic condensation, and formation of apoptotic bodies, as shown using an optical microscope. These features were more pronounced in the high-dose miR-145 (1OD) treatment group. Apoptotic cells were occasionally observed in control groups (Figure 2). Electron microscopic examination of changes in the treatment groups revealed “edge-set” and shrinkage phenomena in nuclear chromatin, accompanied by necrosis and nuclear vacuoles. These nuclear changes were more pronounced in the high-dose miR-145 (1OD)treatment groups. Some cells in the control groups had nuclear cytoplasmic condensation or a slight “edge-set” phenomenon, but there were no significant differences between the control groups (Figure 3).
MiR-145 expression in tumors
MiR-145 treatment significantly increased miR-145 expression in human LSCC AMC-HN-8 tumors transplanted into nude mice in both treatment groups relative to the controls (p<0.05). Furthermore, there was a statistically significant difference between the high- and low-dose miR-145 treatment groups (p<0.05). In contrast, there were no significant differences among the control groups (Figure 4).
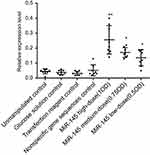 | Figure 4 Effect of miR-145 on miR-145 expression in a nude mouse model of transplanted human LSCC AMC-HN-8 cells, relative to the unmanipulated control group; *p<0.05, **p<0.01. |
PI3K and Akt protein expression in tumors
PI3K and Akt protein expression were significantly reduced in both treatment groups relative to the controls (p<0.05). In addition, PI3K and Akt expression were significantly lower in the high-dose miR-145 treatment groups vs the low-dose miR-145 treatment group (p<0.05). No significant differences were observed among the control groups. PI3K and Akt protein expression levels were positively correlated within the samples (Figure 5).
 | Figure 5 Effect of miR-145 on the expression of PI3K and Akt protein in human LSCC AMC-HN-8 cells transplanted into nude mice, relative to the unmanipulated control group; *p<0.05, **p<0.01. |
Discussion
MiRNAs are important post-transcriptional regulator molecules that exhibit both oncogenic and cancer suppressor functions including regulation of proliferation, differentiation, and apoptosis. Disruption in the normal expression of these molecules can lead to tumorigenesis. MiR-145 has been extensively studied in the context of tumor inhibition, which is an increasingly important step in the processes of tumor diagnosis, prognostic assessment, and targeted therapies.22 MiR-145 may be an effective cancer suppressor due to its ability to regulate growth, apoptosis, invasion, and metastasis of tumor cells. Significant decreases in miR-145 expression have been described in a variety of tumors including ovarian, cervical, breast, and colorectal cancers.23–26 In addition, overexpression of miR-145 has been shown to inhibit proliferation and metastasis of tumor cells, thus exhibiting significant tumor suppressor actions. MiR-145 has also been shown to improve the sensitivity of chemotherapeutic drugs, making miR-145 a promising candidate for use in early detection and prognosis of the disease.
In this study, we used a novel LSCC nude mouse transplantation model to evaluate the effect of miR-145 on tumor development and prognosis. Injection of miR-145 into tumors caused significantly slower tumor growth compared with the control groups. None of the mice died during testing, indicating that miR-145 therapy was not toxic. Furthermore, cells in the experimental group demonstrated typical morphological features of apoptosis, suggesting that miR-145 effectively induced apoptosis and inhibited proliferation of LSCC. The inhibition rate in the high-dose miR-145 treatment group was 56.9% relative to the control group. Furthermore, the inhibition rate was significantly different between the high- and low-dose miR-145 groups. However, inhibition in the low-dose treatment group was significantly higher than that in the control group (p<0.05), indicating a strong dose-dependent effect on tumor inhibition. In contrast, no significant differences in the inhibition rate were observed among the control groups. Pathological changes in the tumors in the treatment groups were characterized by areas of apoptotic cells with cell shrinkage, cytoplasmic condensation, and apoptotic bodies. These effects were markedly greater in the high-dose miR-145 treatment group, although apoptotic cells were also observed to a lesser extent in the other groups. Electron microscopy was used to confirm the gross pathological results.
Our results showed that miR-145 levels were negatively correlated with the expression of PI3K and Akt. Expression of PI3K and Akt proteins was suppressed by miR-145 in a dose-dependent manner, with the high-dose miR-145 treatment group resulting in lower PI3K and Akt expression compared with both the low-dose treatment group and the controls. Higher miR-145 content positively correlated with lower expression of PI3K and Akt, increased inhibition of the PI3K/Akt signaling pathway, and reduced proliferation of the LSCC cell line AMC-HN-8, in addition to a stronger apoptotic effect on cell lines. These results indicated that in an animal model of LSCC in nude mice, miR-145 may promote tumor cell apoptosis and inhibit tumor cell proliferation through its effects on the PI3K/Akt signaling pathway.
These experiments were performed using a model in which a human LSCC cell line was subcutaneously xenografted into nude mice. Using this model, inoculation was convenient and highly reproducible, with an excellent survival rate after transplant inoculation. The transplanted tumor was able to be maintained through continuous passages, and the tumor cells retained the histological and ultrastructural features of LSCC. The experimental period was relatively short, and the tumor growth trends were able to be observed in real time. Similar models have been used as a preliminary screening model to evaluate drug efficacy. Orthotopic xenograft animal models can simulate the tumor microenvironment in a manner similar to that of the primary tumor location. Furthermore, these xenografts can develop metastases capable of spreading from the inoculation site. This outcome is important as it helps to prevent false positives arising from site-specificity of transplantation.27–30 However, implementation of this type of model requires great technical rigor. After consulting the relevant literature, we identified orthotopic transplantation models used for liver, lung, cervical, bladder, and ovarian cancers, but no such model had been developed for laryngeal carcinoma. For future experiments, we will employ a laryngeal carcinoma orthotopic transplantation model to clarify the effects of the microenvironment on the growth of LSCC.
Our findings that miR-145 plays a role in tumor growth are consistent with those of many previous studies. Numerous studies have described potential therapeutic uses for miR-145 including inhibition of proliferation and metastasis of tumor cells. MiR-145 has also been shown to reduce and counteract chemotherapeutic resistance of some drugs, making it an ideal candidate for cancer therapy.
Liu et al,15 reported that LSCC is a very common neoplasm of the head and neck. The expression of miR-145 was lower in LSCC tissues than in their paired normal samples. They showed that miR-145 was downregulated in LSCC cell lines (Hep2 and TU212) compared with a normal bronchial epithelial cell line (16HBE). Knockdown of miR-145 promoted proliferation, invasion, and EMT progression of LSCC cells. Moreover, miR-145 silencing suppressed E-cadherin expression and enhanced N-cadherin and vimentin expression in Hep2 cells.
Gao et al,16 indicated that miR-145-5p plays a critical role in inhibiting the progression of LSCC by suppressing FSCN1. Both miR-145-5p and FSCN1 are important potential prognostic markers and therapeutic targets for the treatment of LSCC.
Zhao et al,17 analyzed miR-145 and MYO5A expression in 132 patients with LSCC, and evaluated associations between this expression and clinicopathological features. They observed a regulatory relationship between miR-145b and MYO5A by dual luciferase reporter assay. The role of the miR-145/MYO5A pathway in proliferation, metastasis, and apoptosis was examined in vitro. The predictive potential of MYO5A for neck lymph node metastasis and for prognosis was determined during patient follow-up. Their results showed downregulation of miR-145 in LSCC, which was negatively correlated with MYO5A suppression of LSCC progression and metastasis. MiR-145 directly regulated MYO5A expression in vitro and suppressed LSCC proliferation and invasion while promoting apoptosis by inhibiting MYO5A.
Zhu et al,18 showed that miR-145 was significantly downregulated in LSCC tissues and cells. MiR-145 overexpression and curcumin treatment both markedly suppressed cell proliferation, migration, and invasion, and induced cell cycle arrest and apoptosis in LSCC cells. Moreover, curcumin treatment reversed anti-miR-145-mediated increases in cell viability, migration, and invasion, and reversed inhibition of apoptosis in LSCC cells. Curcumin treatment increased miR-145-induced inhibition of the PI3K/Akt/mTOR pathway, and reversed anti-miR-145-mediated activation of the PI3K/Akt/mTOR pathway in LSCC cells.
Many other studies also support the potential use of miR-145 as an effective treatment for various cancers. Li et al,31 reported that miR-145 expression was significantly reduced in rectal cancer tissues. Treatment of rectal cancer cells with miR-145 effectively inhibited proliferation and metastasis, thereby providing a theoretical basis for treatment of rectal cancer by targeting the tumor suppressor miR-145.
MiR-145 substitution therapy for treatment of colorectal cancer was mediated by polyethylene imide, resulting in enhanced apoptosis and reduced proliferation.32 MiR-145 treatment appears to reduce cancer cell resistance to apoptosis, resulting in enhanced sensitivity to chemotherapeutic drugs such as gefitinib in patients with non-small-cell lung cancer.33
Pancreatic cancer is a major cause of cancer deaths worldwide. Despite recent progress in both research and treatment, difficulty in early detection and lack of effective treatments results in poor prognosis. Gemcitabine is a first-line drug for treating pancreatic cancer, although resistance is a serious problem. Expression of p70S6K1 has been shown to play an important role in this resistance.5 MiR-145 directly inhibits the expression of p70S6K1 in pancreatic cancer, making it an effective therapeutic agent for the treatment of pancreatic cancer.
In ovarian cancer, miR-145 was imported into SKOV3/PTX ovarian cancer cells, resulting in decreased intracellular cyclin-dependent kinase 6 and Sp1 levels.34 Levels of ATP binding cassette subfamily B1 and retinoblastoma 1 were also reduced. These changes may induce aggregation of antitumor drugs in cells, in addition to G1 cell cycle blockade, consequently improving the sensitivity of tumor cells to taxols both in vitro and in vivo. Reactivation of miR-145 expression by demethylation mediated by the DNA methylase inhibitor 5-azos-2-deoxycytidine may also increase the susceptibility of tumor cells to taxols.
Tumor development is a complex process involving multiple factors and stages, typically arising from the accumulation of multiple gene mutations. As a result, the etiology and biological mechanisms underlying the proliferation of tumor cells are complex and include inactivation of cancer suppressor genes, overexpression of oncogenes, uncontrolled cell cycling, and tolerance to apoptosis. MiR-145 inhibits tumor growth by inhibiting the expression of oncogenes, blocking the cell cycle, and promoting apoptosis of tumor cells. An important mechanism of the rapid proliferation of tumor cells is the activation of proto-oncogenes. MiR-145 inhibits the expression of multiple oncogenes and proteins. Previous studies have investigated the mechanisms underlying miR-145 activity in vitro, including studies of non-small-cell lung,33 thyroid,35 liver,36,37 and pancreatic cancers.34 However, no studies have evaluated the effects of miR-145 in LSCC or the relationship between the PI3k/Akt signaling and miR-145 activity in LSCC pathology.
MiR-145 exhibits tumor-inhibiting properties, but the mechanisms by which it is involved in proliferation, metastasis, and invasion of tumor cells have not been characterized. Patients with bladder cancer express low levels of miR-145, which inhibits tumor cell proliferation by targeting FSCN1.7 In prostate cancer, miR-145 inhibits proliferation, invasion, and metastasis of tumor cells via FSCN1-.38
Overexpression of miR-145 in MCF-7 breast cancer cells significantly decreased proliferation compared with MCF10A normal mammary epithelial cells.39 RTKN expression is also decreased in MCF-7 cells, indicating that miR-145 inhibits proliferation of breast cancer cells by targeting RTKN.
DNA fragmentation is an important feature of apoptosis, and occurs as a result of DFF40-mediated degradation of chromosomal DNA. Under normal circumstances, DFF40 and DFF45 form an inactive complex within the cell. When apoptosis occurs, a series of enzymes trigger a signaling cascade that includes activation of caspase-3, resulting in the release of DFF40 from DFF45 and subsequent DNA fragmentation. Activation of DFF40 is mediated by direct inhibition of DFF45 expression by miR-145, resulting in DNA fragmentation and apoptosis in colon cancer.40
Given the complex regulatory role attributed to miRNAs, it is not surprising that these molecules regulate events involved in the onset and development of cancers. MiR-145 expression is significantly decreased in HCC cell lines and is negatively correlated with the expression of insulin receptor substrate 1 (IRS1), a key factor in the tumorigenic insulin-like growth factor pathway. Western blotting and luciferase reporter assays have confirmed that IRS1 is a direct target of miR-145.36 Increased miR-145 expression inhibits tumor cell proliferation by lowering IRS1 levels, thereby inhibiting the downstream Akt/FOXO1 signaling pathway. Lower miR-145 expression in HCC cells has been suggested as a potential mechanism of carcinogenesis.36
Numerous studies have shown significantly lower expression of miR-145 in tumor tissues compared with normal controls. However, a small number of tumor types, including esophageal adenocarcinoma (EAC) and glioma, exhibit increased expression of miR-145, resulting in enhanced cell proliferation, invasion, and metastasis. In one study, no significant difference was observed in cell proliferation or 5-fluorouracil resistance between miR-145 treatment and control treatment in EAC cells. Similarly, miR-145 was also shown to enhance the resistance of SK-GT-4 cells to cisplatin. There were obvious differences in cell invasion, adhesion, and apoptotic effects among OE33, FLO-1, and SK-GT-4 cell lines, and invasion was significantly greater in the miR-145 treatment group than in the control group. MiR-145 overexpression in esophageal squamous cell carcinoma prevents proliferation and invasion, whereas overexpression of miR-145 in EAC cells enhances invasion and prevents apoptosis.41 MiR-145 expression was increased, and srGAP1 decreased, in IM3 invasive glioblastoma cells, whereas downstream G-proteins remained active and thus promoted an invasive phenotype.42
Previous studies have reported a wide range of tumor-related factors as targets of miR-145 including PI3K/AKT,33 ROCK1,43 caspases,44 c-MYC,45 RTKN,39 RREB1,46 ER-alpha,47 DFF45,40 MUC1,48 JAM-A,49 fascin,49 ELK1,50 OCT4,51 SOX2,51 KLF4,51 YES,52 STAT1,52 IRS-1,53 FLI1,54 TNFSF10,10 and N-cadherin.55,56 This suggests a broad regulatory role for miR-145, with significant implications for normal cellular function and carcinogenesis.
Conclusions
Great progress has been made in elucidating the role of miR-145 in the development and progression of tumors, which has provided a new avenue for cancer research. MiR-145 plays an important role in proliferation, apoptosis, invasion, and metastasis of tumor cells, as well as tumor angiogenesis and development of tumor stem cells.57 The data presented in this study, in combination with previous reports, demonstrated that miR-145 inhibited the growth of LSCC AMC-HN-8 cells via the PI3K/Akt signaling pathway. These data suggested that miR-145 represents a potentially effective target for the treatment of LSCC.
Abbreviation list
BCA, bicinchoninic acid; EAC, esophageal adenocarcinoma; LSCC, laryngeal squamous cell carcinoma; miRNA, microRNA; OD, optical density; PBS, phosphate-buffered saline; PI3K, phosphatidylinositol-3-kinase; qRT-PCR, quantitative real-time polymerase chain reaction.
Ethics approval and consent to participate
This study was approved by the Animal Ethical Care and Use Committee of the First Affiliated Hospital of Zhejiang University, Hangzhou, Zhejiang, China.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author upon request.
Acknowledgments
We give our sincere gratitude to Dr. Zhisen Shen for advice on writing and improving the manuscript. This work was supported by grants from the Natural Science Foundation of Ningbo (grant nos. 2013A610217; 2017A610236; 2018A610361), the Leading and Top Talents Training Projects of Ningbo (NBLJ201801032), Ningbo Health Branding Subject Fund (PPXK2018-02), and the Medical and Health Training Project of Zhejiang Province (grant nos. 2015RCB025; 2018RC063). The funders had no role in the design of the study, data collection, and analysis, interpretation of data, decision to publish, or preparation of the manuscript.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work-up. Otolaryngol Clin North Am. 2008;41(4):673–695. doi:10.1016/j.otc.2008.01.016
2. Babashah S, Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. 2011;47(8):1127–1137. doi:10.1016/j.ejca.2011.02.008
3. Lu Y, Chopp M, Zheng X, Katakowski M, Buller B, Jiang F. MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep. 2013;29(1):67–72. doi:10.3892/or.2012.2084
4. Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Trans Res. 2010;2(2):170–180.
5. Lin Y, Ge X, Wen Y, et al. MiRNA-145 increases therapeutic sensibility to gemcitabine treatment of pancreatic adenocarcinoma cells. Oncotarget. 2016;7(43):70857–70868. doi:10.18632/oncotarget.12268
6. Arndt GM, Dossey L, Cullen LM, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9:374. doi:10.1186/1471-2407-9-374
7. Chiyomaru T, Enokida H, Tatarano S, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102(5):883–891. doi:10.1038/sj.bjc.6605570
8. Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67(18):8699–8707. doi:10.1158/0008-5472.CAN-07-1936
9. Kano M, Seki N, Kikkawa N, et al. miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127(12):2804–2814. doi:10.1002/ijc.25284
10. Zaman MS, Chen Y, Deng G, et al. The functional significance of microRNA-145 in prostate cancer. Br J Cancer. 2010;103(2):256–264. doi:10.1038/sj.bjc.6605742
11. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi:10.1038/nature04869
12. Hirsch FR, Lippman SM. Advances in the biology of lung cancer chemoprevention. J Clin Oncol. 2005;23(14):3186–3197. doi:10.1200/JCO.2005.14.209
13. Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol. 2009;34(6):1749–1757.
14. Yang M, Wang H, Zhou M, et al. The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget. 2016;7(47):76656–76666. doi:10.18632/oncotarget.12307
15. Liu S, Duan W. Long noncoding RNA LINC00339 promotes laryngeal squamous cell carcinoma cell proliferation and invasion via sponging miR-145. J Cell Biochem. 2018. Epub ahead of print. doi:10.1002/jcb.28110.
16. Gao W, Zhang C, Li W, et al. Promoter methylation-regulated miR-145-5p inhibits laryngeal squamous cell carcinoma progression by targeting FSCN1. Mol Ther. 2019;27(2):365–379. Epub 2018 Sep 27. doi:10.1016/j.ymthe.2018.09.018
17. Zhao X, Zhang W, Ji W. MYO5A inhibition by miR-145 acts as a predictive marker of occult neck lymph node metastasis in human laryngeal squamous cell carcinoma. Onco Targets Ther. 2018;11:3619–3635. eCollection 2018. doi:10.2147/OTT.S164597
18. Zhu X, Zhu R. Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway. Onco Targets Ther. 2018;11:3521–3531. eCollection 2018. doi:10.2147/OTT.S159236
19. Zhu Y, Yan L, Zhu W, Song X, Yang G, Wang S. MMP2/3 promote the growth and migration of laryngeal squamous cell carcinoma via PI3K/Akt-NF-κB-mediated epithelial-mesenchymal transformation. J Cell Physiol. 2019. Epub ahead of print. doi:10.1002/jcp.28242.
20. Ni HS, Hu SQ, Chen X, Liu YF, Ni TT, Cheng L. Tra2β silencing suppresses cell proliferation in laryngeal squamous cell carcinoma via inhibiting PI3K/AKT signaling. Laryngoscope. 2018. Epub ahead of print. doi:10.1002/lary.27716.
21. Jiang T, Zhou ML, Fan J. Inhibition of GLUT-1 expression and the PI3K/Akt pathway to enhance the chemosensitivity of laryngeal carcinoma cells in vitro. Onco Targets Ther. 2018;11:7865–7872. eCollection 2018. doi:10.2147/OTT.S176818
22. Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Ann Biol Clin (Paris). 2010;68(3):263–272. doi:10.1684/abc.2010.0429
23. Dip N, Reis ST, Srougi M, Dall’Oglio MF, Leite KR. Expression profile of microRNA-145 in urothelial bladder cancer. Int Braz J Urol. 2013;39(1):
24. Du L, Pertsemlidis A. microRNAs and lung cancer: tumors and 22-mers. Cancer Metastasis Rev. 2010;29(1):109–122. doi:10.1007/s10555-010-9204-9
25. Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi:10.1146/annurev.med.59.053006.104707
26. Huang L, Lin JX, Yu YH, Zhang MY, Wang HY, Zheng M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PloS One. 2012;7(3):e33762. doi:10.1371/journal.pone.0033762
27. Lee SE, Bairstow SF, Werling JO, et al. Paclitaxel nanosuspensions for targeted chemotherapy - nanosuspension preparation, characterization, and use. Pharm Dev Technol. 2014;19(4):438–453. doi:10.3109/10837450.2013.789911
28. Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17(4):343–359.
29. Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451–452. doi:10.1038/nrc3972
30. Hoffman RM. Patient-Derived Mouse Models of Cancer. Berlin: Springer Intl; 2017.
31. Li C, Xu N, Li YQ, Wang Y, Zhu ZT. Inhibition of SW620 human colon cancer cells by upregulating miRNA-145. World J Gastroenterol. 2016;22(9):2771–2778. doi:10.3748/wjg.v22.i9.2771
32. Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71(15):5214–5224. doi:10.1158/0008-5472.CAN-10-4645
33. Zhong M, Ma X, Sun C, Chen L. MicroRNAs reduce tumor growth and contribute to enhance cytotoxicity induced by gefitinib in non-small cell lung cancer. Chem Biol Interact. 2010;184(3):431–438. doi:10.1016/j.cbi.2010.01.025
34. Zhu X, Li Y, Xie C, et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int J Cancer. 2014;135(6):1286–1296. doi:10.1002/ijc.28774
35. Boufraqech M, Zhang L, Jain M, et al. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21(4):517–531. doi:10.1530/ERC-14-0077
36. Wang Y, Hu C, Cheng J, et al. MicroRNA-145 suppresses hepatocellular carcinoma by targeting IRS1 and its downstream Akt signaling. Biochem Biophys Res Commun. 2014;446(4):1255–1260. doi:10.1016/j.bbrc.2014.03.107
37. Liu Y, Wu C, Wang Y, et al. MicroRNA-145 inhibits cell proliferation by directly targeting ADAM17 in hepatocellular carcinoma. Oncol Rep. 2014;32(5):1923–1930. doi:10.3892/or.2014.3424
38. Fuse M, Nohata N, Kojima S, et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38(4):1093–1101. doi:10.3892/ijo.2011.919
39. Wang S, Bian C, Yang Z, et al. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34(5):1461–1466.
40. Zhang J, Guo H, Qian G, et al. MiR-145, a new regulator of the DNA fragmentation factor-45 (DFF45)-mediated apoptotic network. Mol Cancer. 2010;9:211. doi:10.1186/1476-4598-9-254
41. Derouet MF, Liu G, Darling GE. MiR-145 expression accelerates esophageal adenocarcinoma progression by enhancing cell invasion and anoikis resistance. PloS One. 2014;9(12):e115589. doi:10.1371/journal.pone.0115589
42. Koo S, Martin G, Toussaint LG. MicroRNA-145 promotes the phenotype of human glioblastoma cells selected for invasion. Anticancer Res. 2015;35(6):3209–3215.
43. Lei P, Xie J, Wang L, Yang X, Dai Z, Hu Y. microRNA-145 inhibits osteosarcoma cell proliferation and invasion by targeting ROCK1. Mol Med Rep. 2014;10(1):155–160. doi:10.3892/mmr.2014.2195
44. Ostenfeld MS, Bramsen JB, Lamy P, et al. miR-145 induces caspase-dependent and -independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene. 2010;29(7):1073–1084. doi:10.1038/onc.2009.395
45. Sachdeva M, Zhu S, Wu F, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106(9):3207–3212. doi:10.1073/pnas.0808042106
46. Kent OA, Chivukula RR, Mullendore M, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24(24):2754–2759. doi:10.1101/gad.1950610
47. Spizzo R, Nicoloso MS, Lupini L, et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010;17(2):246–254. doi:10.1038/cdd.2009.117
48. Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70(1):378–387. doi:10.1158/0008-5472.CAN-09-2021
49. Gotte M, Mohr C, Koo CY, et al. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29(50):6569–6580. doi:10.1038/onc.2010.386
50. Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–710. doi:10.1038/nature08195
51. Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137(4):647–658. doi:10.1016/j.cell.2009.02.038
52. Gregersen LH, Jacobsen AB, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS One. 2010;5(1):e8836. doi:10.1371/journal.pone.0008836
53. La Rocca G, Badin M, Shi B, et al. Mechanism of growth inhibition by MicroRNA 145: the role of the IGF-I receptor signaling pathway. J Cell Physiol. 2009;220(2):485–491. doi:10.1002/jcp.21796
54. Zhang J, Guo H, Zhang H, et al. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene Friend leukemia virus integration 1 gene. Cancer. 2011;117(1):86–95. doi:10.1002/cncr.25522
55. Zhang XF, Zhang XQ, Chang ZX, Wu CC, Guo H. microRNA145 modulates migration and invasion of bladder cancer cells by targeting Ncadherin. Mol Med Rep. 2018;17(6):8450–8456. doi:10.3892/mmr.2018.8910
56. Mo D, Yang D, Xiao X, Sun R, Huang L, Xu J. MiRNA-145 suppresses lung adenocarcinoma cell invasion and migration by targeting N-cadherin. Biotechnol Lett. 2017;39(5):701–710. doi:10.1007/s10529-017-2290-9
57. Ye D, Shen Z, Zhou S. Function of microRNA-145 and mechanisms underlying its role in malignant tumor diagnosis and treatment. Cancer Manage Res. 2019;11:969–979. doi:10.2147/CMAR.S191696
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.