Back to Journals » OncoTargets and Therapy » Volume 11
MicroRNA-140-3p enhances the sensitivity of hepatocellular carcinoma cells to sorafenib by targeting pregnenolone X receptor
Authors Li J, Zhao J, Wang H, Li X, Liu A, Qin Q, Li B
Received 8 July 2018
Accepted for publication 1 August 2018
Published 17 September 2018 Volume 2018:11 Pages 5885—5894
DOI https://doi.org/10.2147/OTT.S179509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Jiaqi Li,1,2 Jing Zhao,2 Huan Wang,2 Xiaohan Li,2 Aixia Liu,2 Qin Qin,1,3 Boan Li1,2
1Basic Medicine College, Navy Military Medical University of Chinese PLA, Shanghai 200433, People’s Republic of China; 2Center for Clinical Laboratory, The 302nd Hospital of Chinese PLA, Beijing 100039, People’s Republic of China; 3Department of Laboratory Medicine, Changhai Hospital, Navy Military Medical University of Chinese PLA, Shanghai 200433, People’s Republic of China
Background: Pregnane X receptor (PXR), which is a member of the nuclear receptor protein family (nuclear receptor subfamily 1 group I member 2 [NR 1I2]), mediates the drug-resistance in the hepatocellular carcinoma (HCC) via enhancing the expression of drug-resistance-related genes which accelerate the clearance of antitumor drugs, eg, sorafenib. However, there are few reports on miRNA targeting PXR participating in the epigenetic regulation of PXR in HCC cells.
Materials and methods: TargetScan 7.2, an online method, was used to predict the miRNAs potentially targeting PXR. The expression of PXR and PXR downstream genes was detected by quantitative real-time PCR (qPCR) and Western blot. The clearance of sorafenib in HCC cells was monitored by liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS). The effects of miRNA on sorafenib’s efficacy were examined by in vitro methods, eg, MTT, and in vivo methods, eg, subcutaneous or intrahepatic tumor model.
Results: By virtual screening, we identified that miR-140-3p possibly targets PXR and then confirmed that the overexpression of miR-140-3p via lentiviral particles inhibited the expression of PXR in HCC cells. The downregulation of PXR’s expression by miR-140-3p led to the reduction of PXR downstream genes’ expression, which finally resulted in the decelerating clearance of sorafenib in HCC cells and enhanced the sensitivity of HCC cells to sorafenib. The effect of miR-140-3p could not modulate the expression of mutated PXR and the effect of miR-140-3p could also be inhibited by miR-140-3p’s inhibitor. Moreover, miR-140-3p enhanced the antitumor effect of sorafenib in both the subcutaneous and intrahepatic HCC tumor models.
Conclusion: Our study suggests that targeting PXR by miR-140-3p is a promising strategy for enhancing sorafenib’s efficacy during HCC treatment.
Keywords: hepatocellular carcinoma, microRNA, pregnenolone X receptor, sorafenib
Introduction
Pregnane X receptor (PXR) is a member of the nuclear receptors (NRs) protein family (classified as NR1I2).1,2 In mammal cells, PXR could function as a transcription factor that could be activated by endogenous or exogenous ligands, in turn leading to the transcription of downstream genes, eg, CYP/CYP450 (CYP450) or ATP-binding cassette (ABC), which often participate in drug-resistance or drug-metaboloisms.3,4 Previously, PXR was considered as one of the key regulators mediating the expression of phase I, II or III drug metabolism enzymes (DMEs) to accelerate the clearance of exogenous drugs to protect the human body and liver organ from drug-induced injuries.5–7 Recently, PXR has also been considered to play an important role in the resistance of antitumor drugs.8,9 Our recent study indicated that sorafenib, an oral molecular-targeted antitumor agent, enhances its own clearance in hepatocellular carcinoma (HCC) cells via enhancing the activation of PXR during treatment.11 Although current researches are focusing on PXR as a potential target to overcome drug-resistance of HCC treatment, the approaches targeting PXR have been poorly developed.
MicroRNAs (miRNAs or miRs) are a series of RNAs which repress the targeted messenger RNAs (mRNAs) via recognizing and interacting their 3′-untranslated regions (3′-UTR), and miRs have critical roles in regulating proliferation, metastasis or apoptosis of human cancer cells.12,13 Many miRs have been applied for regulating cancers’ progress via targeting cancer-related signaling pathways.14,15 In the present work, we identified miRNAs potentially targeting PXR. By screening a series of miRNAs via online tools, PXR would be a target of miR-140-3p. Transfection of miR-140-3p decreased the expression of PXR or its downstream genes and enhanced the sensitivity of HCC cells to sorafenib.
Materials and methods
Plasmids and reagents
The expression vector of PXR with a mutation of miR-140-3p target sequences in 3′-UTR was constructed and purchased from Vigene Corporation, Jinan City, Shandong Province, People’s Republic of China. For cell-based experiments, the mimic miR-140-3p (Cat. no 4464070) and miR-140-3p inhibitor (Cat. no 4464088) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). MHCC97-H (a highly metastatic cell line of HCC) and HepG2 (a cell line of HCC) cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), the culture collection center of the Chinese government. Cells were preserved in our laboratory; maintaining conditions are described in our previous work.16,17 Control miRNA (random molecular agent), was a gift from Dr Mingyang Li in the General Hospital of Chinese PLA. For animal experiments, a vector with miR-140-3p was constructed and purchased from Vigene Corporation. Sequences of miR-140-3p were prepared into lentivirus particles. The antitumor agent sorafenib (Cat. no S7397) was purchased from Selleck Corporation, Houston, TX, USA. For cell culture experiments, sorafenib were carefully dissolved in dimethyl sulfoxide (DMSO) and the concentrations of sorafenib in DMSO were 10, 3, 1, 0.3, 0.1, 0.03 or 0.01 mmol/L. For subcutaneous tumor experiments, sorafenib (4 mg) was dissolved by a mixture of DMSO (15 μL), PEG400 (60 μL) and Tween80 (40 μL). Then, the solution was carefully added with physiological saline to a total volume 2 mL. This sorafenib-containing solution was named as Sor-Sol. Rifampicin (Cat. no HY-B0272), which is a typical agonist of PXR, was purchased from MedChem Express (MCE) (Monmouth Junction, NJ, USA). Rifampicin was dissolved in DMSO and the indicated concentrations of rifampicin in DMSO were 10, 3, 1, 0.3, 0.1, 0.03 or 0.01 mmol/L.
Luciferase reporter gene assay
MHCC97-H or HepG2 cells, which were transfected with control miRNA or miR-140-3p, were seeded in 24-well plates (Corning Incorporated, Corning, NY, USA), and were co-transfected with luciferase reporters (DR3-Luc, ER6-Luc, XREM-Luc or PXRE-Luc).18 After being cultured for 24 hours, cells were harvested and analyzed for luciferase activities, as described by Ma et al.19 For luciferase experiments, the concentrations of rifampicin on HCC cells were 10, 3, 1, 0.3, 0.1, 0.03 or 0.01 μmol/L diluted from rifampicin solution (1:1,000).
RNA extraction and qPCR experiments
MHCC97-H or HepG2 cells were treated with the indicated concentrations of rifampicin (10, 3, 1, 0.3, 0.1, 0.03 or 0.01 μmol/L) for 24 hours. The cells were then harvested and the total RNA samples were extracted and qPCR experiments were performed following protocols provided by the manufacturer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). The expression of drug-resistance-related genes within MHCC97-H cells was examined by qPCR with primers listed in Table 1.
  | Table 1 Primers used in qPCR experiments |
Antibodies and Western blot
Antibodies against PXR (Cat. no ab85451), CYP3A4 (Cat. no ab155029), P-glycoprotein (Cat. no ab235954) and β-actin (Cat. no ab205) were obtained from Abcam (Cambridge, MA, USA). Cells, which were transfected with control miRNA, miR-140-3p mimic, PXRMut or miR-140-3p inhibitor, were harvested to be extracted for Western blot experiments, which were performed following the standard protocol of Western blot experiments.
Cell culture and proliferation analysis
HCC cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) with 0.5%–1% fetal bovine serum (FBS) (Thermo Fisher Scientific) in a sterile incubator maintained at 37°C under 5% CO2 condition. For drug antitumor, cells were seeded in 24-well plate (Corning Incorporated) and incubated by the indicated concentrations (10, 3, 1, 0.3, 0.1, 0.03 or 0.01 μmol/L) of sorafenib diluted by sorafenib DMSO solution (final DMSO concentration was 1‰). Then, cells were incubated by sorafenib for 48 hours and harvested for MTT experiments. The inhibition rates of sorafenib on HCC cells were calculated by the methods described by Feng et al.20
Subcutaneous HCC tumor model
Animal experiments were permitted by the Institutional Animal Care and Use Committee of the 302nd Hospital, People’s Liberation Army of China. All animal studies were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines. For subcutaneous tumor experiments,21,22 nude mice (severe combined immune deficiency mice) aged 4–6 weeks were purchased from Si-Bei-Fu Biotechnology Corporation, Beijing, People’s Republic of China. MHCC97-H or HepG2 cells which were cultured in DMEM adding 10% FBS, were injected into nude mice (5×106 cells per inoculation point). After 2–4 weeks’ growth, the tumoral volume reached almost 1,000–1,200 mm3.
Clearance of sorafenib in HCC cells
To examine the clearance of sorafenib in HCC cells, MHCC97-H or HepG2 cells were transfected with miR-140-3p or PXR expression plasmids, and were treated with 1 μmol/L (the IC50 concentration of sorafenib on MHCC97-H cells) for 12 hours. For cultured MHCC97-H cells, cells were harvested after sorafenib treatment at the indicated time points and then harvested for liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS) examination.23 For the subcutaneous tumor model, MHCC97-H or HepG2 cells which were infected with miR-140-3p or PXR, were seeded into nude mice to form subcutaneous tumors. Sor-Sol was injected into subcutaneous tumors (20 μL per tumor) in the subcutaneous tumors of the nude mice formed by HCC cells. Cells or tumor tissues were harvested and the sorafenib was extracted by acetonitrile (ACN) at the indicated time points. The amount of sorafenib in tumor tissues was measured by LC-MS/MS.11,23
In vivo tumor growth
MHCC97-H cells, which were transfected with plasmids, were injected into the nude mice to form subcutaneous tumors or into the liver organs via hepatic portal vein injection.24 Five days after injection, the nude mice were orally administrated 2 mg/kg sorafenib treatment every 2 days. After 10 × treatments for 21 days, the mice were collected. For the subcutaneous tumor model, tumors were harvested and the tumor weights or volumes were examined following the methods described in the references.21,22 For the intrahepatic growth model, mice were harvested and the liver organs with nodules formed by MHCC97-H were collected for taking photographs. Photographs were quantitatively analyzed to determine the total amount of nodules by ImageJ software following the methods described by Xie et al.24 The inhibition rate of each group was calculated as [control group relative nodule area (percentages of nodules to total area of liver organs, %) − administration group relative nodule area]/control group relative nodule area × 100%.
Statistical analysis
Statistical analysis was performed by Bonferroni’s correction with or without two-way ANOVA, SPSS Software (IBM Corporation, Armonk, NY, USA). The IC50 values half-life (t1/2 value) values were calculated by an Origin software (Version No 6.1, OriginLab Corporation, Northampton, MA, USA). A P-value of <0.05 was considered statistically significant.
Results
Transfection of miR-140-3p decreased PXR expression by targeting PXR mRNA’s 3′-UTR
In order to predict the miR potentially targeting PXR, online tools TargetScan and MiRanda database were used, and it was found that PXR was a potential target of miR-140-3p: the italicized font in Figure 1A indicates the miR-140-3p targeted sequence within PXR mRNA’s 3′-UTR (Figure 1). Next, MHCC97-H cells and HepG2 cells were transfected with control miRNA, miR-140-3p mimic, miR-140-3p mimic+ PXRMut or miR-140-3p mimic+ inhibitor for 48 hours and harvested for Western blot. We found that compared with control miRNA, miR-140-3p significantly decreased the expression of PXR (Figure 1B and C) but not PXRMut which had a mutation in the miR-140-3p-binding site (shown in Figure 1A–C); whereas transfection of miR-140-3p’s inhibitor could block the effect of miR-140-3p on PXR’s expression in MHCC97-H cells (Figure 1B) or HepG2 cells (Figure 1C). Therefore, miR-140-3p could repress the expression of PXR in HCC cells by directly targeting PXR mRNA’s 3′-UTR.
MiR-140-3p inhibits PXR pathway’s activation
To further identify the effect of miR-140-3p on PXR, luciferase experiments were performed. As shown in Table 2, PXR’s agonist rifampicin could induce the transcription factor activity of PXR, whereas miR-140-3p blocked the activity of PXR induced by rifampicin. Moreover, transfection of PXRMut or miR-140-3p’s inhibitor decreased the effect of miR-140-3p on PXR’s transcription factor activity. Accordingly, miR-140-3p inhibited the effect of rifampicin on the mRNA or protein expression of PXR downstream genes, CYP3A4 and MDR-1. Transfection of PXRMut or miR-140-3p’s inhibitor inhibited the effect of miR-140-3p (Table 3 and Figure 2). Therefore, miR-140-3p inhibits PXR pathway activation.
MiR-140-3p decelerates the clearance of sorafenib in HCC cells
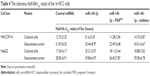
Since PXR, CYP3A4 and MDR-1 are related to the clearance of sorafenib in HCC cells, we then aimed to study the correlation of miR-140-3p and the clearance of sorafenib in HCC cells. We performed sorafenib treatment on HCC cells in vitro or injected Sor-Sol in vivo in HCC tumors and detected the clearance of sorafenib under different conditions. As shown in Table 4, miR-140-3p decelerated the clearance of sorafenib in cultured HCC cells or subcutaneous HCC tumors, the half-life (t1/2 value) of sorafenib in cultured HCC cells correspondingly increased. PXRMut or miR-140-3p’s inhibitor inhibited the effect of miR-140-3p on the clearance of sorafenib. Therefore, our in vitro and in vivo data suggest that miR-140-3p decelerated the clearance of sorafenib in HCC cells.
MiR-140-3p enhances the sensitivity of HCC cells to sorafenib
To determine whether miR-140-3p enhances the in vitro antitumor effect of sorafenib on HCC cells, MTT assays were performed. As shown in Table 5, miR-140-3p enhanced the sensitivity of MHCC97-H and HepG2 cells to sorafenib, and the IC50 value of sorafenib on MHCC97-H or Hepg2 cells were decreased.
Next, the effect of miR-140-3p and sorafenib co-administration on subcutaneous HCC tumors was determined. As shown in Figure 3, treatment of sorafenib could significantly inhibit the subcutaneous growth of HCC cells in nude mice. Infection of miR-140-3p could enhance the antitumor effect of sorafenib on MHCC97-H cells’ subcutaneous growth and PXRMut expressing cells/tumors could not be affected by miR-140-3p (Figure 3D and E and Table 6).
To mimic the in vivo growth of MHCC97-H cells in mouse liver, MHCC97-H cells were injected into nude mice via the hepatic portal vein to form tumor nodules on their livers (Figure 4). We firstly established an oral sorafenib administration method for this intrahepatic HCC tumor model (Figure 4C and Table 6). Then, we performed miR-140-3p and sorafenib co-administration. As shown in Figure 4, MHCC97-H cells could form multi-nodules in the livers via hepatic portal vein injection (Figure 4). The results showed that treatment of sorafenib could significantly inhibit the intrahepatic growth of MHCC97-H cells in the livers of nude mice (Figure 4 and Table 6). Infection of miR-140-3p in MHCC97-H cells could enhance the antitumor effect of sorafenib on MHCC97-H cells’ intrahepatic growth (Figure 4 and Table 6), whereas transfection of PXRMut in MHCC97-H cells could almost block the effect of to miR-140-3p on sorafenib’s antitumor activation. Therefore, miR-140-3p could enhance the sensitivity of HCC cells to sorafenib by targeting PXR.
Discussion
Advanced HCC is one of the foremost cancers in People’s Republic of China nowadays.25–27 Due to the limitation of current diagnostic and therapeutic strategies, most patients could not be treated by the radical ablation.28,29 Due to the fact that advanced HCC is insensitive to chemotherapy30 and radiotherapy,31 the prognosis or clinical outcome of patients with advanced HCC is poor. Although some kinds of local ablation therapies, eg, radiofrequency ablation (RFA)32 or transarterial chemoembolization (TACE),33 could decelerate the progress of HCC to some extent, the rapid and aggressive recurrence of HCC after RFA or TACE treatment affect the application of these kinds of local ablation therapies. Therefore, molecular-targeted agents play a central role in advanced HCC treatment. Sorafenib is the only choice for advanced HCC treatment.34–36 Although the application of sorafenib enhances the survival of HCC patients, sorafenib-resistance has become one of the major obstacles in improving its efficacy.37–39 Thus, it is urgent to develop new strategies to overcome sorafenib-resistance.
Previous work has often focused on the antitumor drug-resistance by regulating multi-drug resistance genes, such as ABCs, eg, MDR-1/ABCB1 (ABC Subfamily B Member 1).40–49 However, the role of PXR, which is the transcription factor of ABCs and mediates the transcription of ABCs in antitumor agents-resistance, is not very clear. PXR expressed in the liver organ or intestine organ could mediate drug clearance in general; however, the tumor-specific expression of PXR could inhibit the therapeutic efficacy of anticancer agents by accelerating these drugs during treatment.9–11 As a central regulator of drug metabolism or clearance, PXR alters the absorption and clearance of xenobiotics (eg, antitumor drugs), leading to the reduced efficacy of the treatments.9–11 In our previous work, we showed that the endogenous PXR is a master regulator of sorafenib-resistance in HCC cells.11 Sorafenib can bind to and activate PXR and facilitate the expression of PXR downstream genes’ expression.11 By examining the clinical specimens, we found that a high level of PXR is correlated with poor prognosis of advanced HCC patients receiving sorafenib treatment. However, the regulatory mechanism of endogenous PXR mRNA level is not clear.
MiR is an important component of gene expression regulation.50–56 MiRs are important regulators of HCC proliferation and metastasis.57–63 In the present work, we firstly identified miR-140-3p as a potential miRNA target for PXR. Then, the regulatory effect of miR-140-3p on PXR was identified. Results showed that overexpression of miR-140-3p via lentiviral vector decreased the expression of PXR and PXR downstream genes in HCC cells; whereas overexpression of PXR with mutated miR-140-3p target sequence or transfection of miR-140-3p’s inhibitor blocked the effect of miR-140-3p on PXR’s expression. Moreover, miR-140-3p also enhanced the sensitivity of HCC cells to sorafenib. The effect of sorafenib on HCC cells were examined by in vitro methods or in vivo experiments. Our data illustrated that miR-140-3p administration was a promising strategy to enhance the antitumor effect of sorafenib on HCC cells. Interestingly, miR-140-3p could decelerate the clearance of sorafenib in cultured HCC cells or HCC subcutaneous tumor by decreased PXR’s expression. This result could extend our knowledge of miRs regulating sorafenib clearance and provide useful methods in metabolism-related drug-resistance researches.
Moreover, this work reported on miR-140-3p targeting PXR and such reports of miRs targeting PXR are rare. Beside PXR, researchers often focus on the miRs targeting ABCs.64,65 Awortwe et al and Rao et al reported that miR-655-3p or miR-148a could modulate the drug-resistance by targeting ABCs, eg, MRP3 or ABCG2.65,66 Following on from the paper by Sharma et al that concluded that miR-18a-5p is a negative regulator and research by Vachirayonstien et al that stated that miR-30c-1-3p could be a suppressor of PXR, our results extend the knowledge of miRs on the PXR signaling pathway.67,68
Conclusion
This work for the first time reported that PXR is a target of miR-140-3p. MiR-140-3p suppresses the PXR pathway and enhances the sensitivity of HCC cells to sorafenib. Downregulation of PXR by miR-140-3p may be a promising strategy for enhancing sorafenib-based HCC treatment.
Author contributions
All authors made substantial contributions to the design and conception; acquisition, analysis or interpretation of data. All authors took part in either drafting or revising the manuscript. At the same time, all authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The author reports no conflicts of interest in this work.
References
Gotoh S, Miyauchi Y, Moore R, Negishi M. Glucose elicits serine/threonine kinase VRK1 to phosphorylate nuclear pregnane X receptor as a novel hepatic gluconeogenic signal. Cell Signal. 2017;40:200–209. | ||
Seow CL, Lau AJ. Differential activation of pregnane X receptor by carnosic acid, carnosol, ursolic acid, and rosmarinic acid. Pharmacol Res. 2017;120:23–33. | ||
Kliewer SA. Nuclear receptor PXR: discovery of a pharmaceutical anti-target. J Clin Invest. 2015;125(4):1388–1389. | ||
Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. | ||
Watkins RE, Wisely GB, Moore LB, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292(5525):2329–2333. | ||
Chen T, Chen Q, Xu Y, et al. SRC-3 is required for CAR-regulated hepatocyte proliferation and drug metabolism. J Hepatol. 2012;56(1):210–217. | ||
Buchman CD, Chai SC, Chen T. A current structural perspective on PXR and CAR in drug metabolism. Expert Opin Drug Metab Toxicol. 2018;14(6):635–647. | ||
Thakur JK, Arthanari H, Yang F, et al. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 2008;452(7187):604–609. | ||
Oladimeji PO, Chen T. PXR: more than just a master xenobiotic receptor. Mol Pharmacol. 2018;93(2):119–127. | ||
Mackowiak B, Hodge J, Stern S, Wang H. The roles of xenobiotic receptors: beyond chemical disposition. Drug Metab Dispos. 2018;46(9):1361–1371. | ||
Feng F, Jiang Q, Cao S, et al. Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma. Biochim Biophys Acta. 2018;1862(4):1017–1030. | ||
Hines PJ. MicroRNAs in functional and dysfunctional pain. Science. 2017;356(6343):1134–1136. | ||
Li L, Liang Y, Kang L, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33(3):368.e7–385.e7. | ||
Ji Q, Xu X, Li L, et al. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. Cell Death Dis. 2017;8(10):e3103. | ||
Liang Y, Xu X, Wang T, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8(7):e2928. | ||
Chen Y, Feng F, Gao X, et al. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells. Curr Cancer Drug Targets. 2015;15(3):176–187. | ||
Hou J, Hong Z, Feng F, et al. A novel chemotherapeutic sensitivity-testing system based on collagen gel droplet embedded 3D-culture methods for hepatocellular carcinoma. BMC Cancer. 2017;17(1):729. | ||
Zhao J, Bai Z, Feng F, et al. Cross-talk between EPAS-1/HIF-2α and PXR signaling pathway regulates multi-drug resistance of stomach cancer cell. Int J Biochem Cell Biol. 2016;72:73–88. | ||
Ma H, Yao Y, Wang C, et al. Transcription factor activity of estrogen receptor α activation upon nonylphenol or bisphenol A treatment enhances the in vitro proliferation, invasion, and migration of neuroblastoma cells. Onco Targets Ther. 2016;9:3451–3463. | ||
Feng F, Lu YY, Zhang F, et al. Long interspersed nuclear element ORF-1 protein promotes proliferation and resistance to chemotherapy in hepatocellular carcinoma. World J Gastroenterol. 2013;19(7):1068–1078. | ||
Jia H, Yang Q, Wang T, et al. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim Biophys Acta. 2016;1860(7):1417–1430. | ||
An L, Li DD, Chu HX, et al. Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and Notch reversal. Pharmacol Res. 2017;124:105–115. | ||
Wu M, Zhao G, Zhuang X, et al. Triclosan treatment decreased the antitumor effect of sorafenib on hepatocellular carcinoma cells. Onco Targets Ther. 2018;11:2945–2954. | ||
Xie H, Tian S, Yu H, et al. A new apatinib microcrystal formulation enhances the effect of radiofrequency ablation treatment on hepatocellular carcinoma. Onco Targets Ther. 2018;11:3257–3265. | ||
Kim GA, Lim YS, Han S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67(5):945–952. | ||
Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the National Cancer Database. J Clin Oncol. 2018;36(6):600–608. | ||
Gomaa A, Waked I. Management of advanced hepatocellular carcinoma: review of current and potential therapies. Hepatoma Res. 2017;3(6):112–122. | ||
Zhang H, Li XX, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross-talk in hepatocellular carcinoma. Hepatology. 2018;67(6):2271–2286. | ||
Cast A, Valanejad L, Wright M, et al. C/EBPα-dependent preneoplastic tumor foci are the origin of hepatocellular carcinoma and aggressive pediatric liver cancer. Hepatology. 2018;67(5):1857–1871. | ||
Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8(2):256–265. | ||
Liu R, Zhao D, Zhang X, et al. A20 enhances the radiosensitivity of hepatocellular carcinoma cells to 60Co-γ ionizing radiation. Oncotarget. 2017;8(54):93103–93116. | ||
Xie H, Yu H, Tian S, et al. MEIS-1 level in unresectable hepatocellular carcinoma can predict the post-treatment outcomes of radiofrequency ablation. Oncotarget. 2018;9(20):15252–15265. | ||
Xie H, Yu H, Tian S, et al. What is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? A systematic review and network meta-analysis. Oncotarget. 2017;8(59):100508–100523. | ||
Rim CH, Yoon WS. Leaflet manual of external beam radiation therapy for hepatocellular carcinoma: a review of the indications, evidences, and clinical trials. Onco Targets Ther. 2018;11:2865–2874. | ||
Raufi A, Tirona MT. Prospect of the use of checkpoint inhibitors in hepatocellular cancer treatments. Cancer Manag Res. 2017;9:19–27. | ||
Roskoski R. The role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders. Pharmacol Res. 2018;129:65–83. | ||
Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–622. | ||
Zhang Y, Li D, Jiang Q, et al. Novel ADAM-17 inhibitor ZLDI-8 enhances the in vitro and in vivo chemotherapeutic effects of Sorafenib on hepatocellular carcinoma cells. Cell Death Dis. 2018;9(7):743. | ||
Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89–101. | ||
Lu X, Zhou D, Hou B, et al. Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer Manag Res. 2018;10:1231–1241. | ||
Tang L, Zhang C, He H, et al. Associations between ABCG2 gene polymorphisms and gefitinib toxicity in non-small cell lung cancer: a meta-analysis. Onco Targets Ther. 2018;11:665–675. | ||
Fang Y, Sun J, Zhong X, et al. ES2 enhances the efficacy of chemotherapeutic agents in ABCB1-overexpressing cancer cells in vitro and in vivo. Pharmacol Res. 2018;129:388–399. | ||
Patel A, Li TW, Anreddy N, et al. Suppression of ABCG2 mediated MDR in vitro and in vivo by a novel inhibitor of ABCG2 drug transport. Pharmacol Res. 2017;121:184–193. | ||
Jin W, Liao X, Lv Y, et al. MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death Dis. 2017;8(8):e2980. | ||
Shubbar MH, Penny JI. Effect of amyloid beta on ATP-binding cassette transporter expression and activity in porcine brain microvascular endothelial cells. Biochim Biophys Acta. 2018;1862(10):2314–2322. | ||
Dartier J, Lemaitre E, Chourpa I, et al. ATP-dependent activity and mitochondrial localization of drug efflux pumps in doxorubicin-resistant breast cancer cells. Biochim Biophys Acta. 2017;1861(5 Pt A):1075–1084. | ||
Kim SW, Hasanuzzaman MD, Cho M, et al. Role of 14-3-3 sigma in over-expression of P-gp by rifampin and paclitaxel stimulation through interaction with PXR. Cell Signal. 2017;31:124–134. | ||
Khabou B, Durand-Schneider AM, Delaunay JL, et al. Comparison of in silico prediction and experimental assessment of ABCB4 variants identified in patients with biliary diseases. Int J Biochem Cell Biol. 2017;89:101–109. | ||
Xiao J, Egger ME, McMasters KM, Hao H. Differential expression of ABCB5 in BRAF inhibitor-resistant melanoma cell lines. BMC Cancer. 2018;18(1):675. | ||
Ruhl R, Rana S, Kelley K, et al. microRNA-451a regulates colorectal cancer proliferation in response to radiation. BMC Cancer. 2018;18(1):517. | ||
Gao S, Zhao ZY, Wu R, Zhang Y, Zhang ZY. Prognostic value of microRNAs in colorectal cancer: a meta-analysis. Cancer Manag Res. 2018;10:907–929. | ||
Shahid S, Kim G, Johnson NR, et al. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature. 2018;553(7686):82–85. | ||
Hydbring P, Wang Y, Fassl A, et al. Cell-cycle-targeting MicroRNAs as therapeutic tools against refractory cancers. Cancer Cell. 2017;31(4):576.e8–590.e8. | ||
Cassels L, Barde YA. Scaling pain threshold with microRNAs. Science. 2017;356(6343):1124–1125. | ||
Fernandes J, Vieira AS, Kramer-Soares JC, et al. Hippocampal microRNA-mRNA regulatory network is affected by physical exercise. Biochim Biophys Acta. 2018;1862(8):1711–1720. | ||
Sun T. Long noncoding RNAs act as regulators of autophagy in cancer. Pharmacol Res. 2018;129:151–155. | ||
Wang R, Yu Z, Chen F, et al. miR-300 regulates the epithelial-mesenchymal transition and invasion of hepatocellular carcinoma by targeting the FAK/PI3K/AKT signaling pathway. Biomed Pharmacother. 2018;103:1632–1642. | ||
Zhang X, Luo P, Jing W, Zhou H, Liang C, Tu J. circSMAD2 inhibits the epithelial-mesenchymal transition by targeting miR-629 in hepatocellular carcinoma. Onco Targets Ther. 2018;11:2853–2863. | ||
Luo C, Yin D, Zhan H, et al. microRNA-501-3p suppresses metastasis and progression of hepatocellular carcinoma through targeting LIN7A. Cell Death Dis. 2018;9(5):535. | ||
Chen Z, Zuo X, Zhang Y, et al. MiR-3662 suppresses hepatocellular carcinoma growth through inhibition of HIF-1α-mediated Warburg effect. Cell Death Dis. 2018;9(5):549. | ||
Liao X, Zhu G, Huang R, et al. Identification of potential prognostic microRNA biomarkers for predicting survival in patients with hepatocellular carcinoma. Cancer Manag Res. 2018;10:787–803. | ||
Vasuri F, Fittipaldi S, de Pace V, et al. Tissue miRNA 483-3p expression predicts tumor recurrence after surgical resection in histologically advanced hepatocellular carcinomas. Oncotarget. 2018;9(25):17895–17905. | ||
O’Brien KP, Khan S, Gilligan KE, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–2149. | ||
Benson EA, Eadon MT, Desta Z, et al. Rifampin regulation of drug transporters gene expression and the association of microRNAs in human hepatocytes. Front Pharmacol. 2016;7:111. | ||
Awortwe C, Kaehler M, Rosenkranz B, Cascorbi I, Bruckmueller H. MicroRNA-655-3p regulates Echinacea purpurea mediated activation of ABCG2. Xenobiotica. 2018;48(10):1050–1058. | ||
Rao ZZ, Zhang XW, Ding YL, Yang MY. miR-148a-mediated estrogen-induced cholestasis in intrahepatic cholestasis of pregnancy: Role of PXR/MRP3. PLoS One. 2017;12(6):e0178702. | ||
Sharma D, Turkistani AA, Chang W, Hu C, Xu Z, Chang TKH. Negative regulation of human pregnane X receptor by microRNA-18a-5p: evidence for suppression of microRNA-18a-5p expression by rifampin and rilpivirine. Mol Pharmacol. 2017;92(1):48–56. | ||
Vachirayonstien T, Yan B. MicroRNA-30c-1-3p is a silencer of the pregnane X receptor by targeting the 3′-untranslated region and alters the expression of its target gene cytochrome P450 3A4. Biochim Biophys Acta. 2016;1859(9):1238–1244. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.