Back to Journals » OncoTargets and Therapy » Volume 12
Microcalcification and BMP-2 in breast cancer: correlation with clinicopathological features and outcomes
Authors Zhang L , Hao C, Wu Y, Zhu Y, Ren Y, Tong Z
Received 17 September 2018
Accepted for publication 4 February 2019
Published 15 March 2019 Volume 2019:12 Pages 2023—2033
DOI https://doi.org/10.2147/OTT.S187835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Li Zhang,1,* Chunfang Hao,1,* Yansheng Wu,2 Yuying Zhu,1 Yulin Ren,1 Zhongsheng Tong1
1Department of Breast Oncology, Key Laboratory of Breast Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, People’s Republic of China; 2Department of Maxillofacial and Otorhinolaryngology Head and Neck Surgery, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Background: Microcalcification is a very important diagnostic information in breast cancer. The purpose of this study was to determine the relationship of clinicopathological features and prognosis of breast cancer with microcalcification and to detect biomarkers related to the possible mechanisms of microcalcifications.
Patients and methods: All 529 subjects with microcalcifications were selected from patients who had been examined using breast mammography. The control group did not have detectable microcalcifications, and was matched in a ratio of 1:3. The clinicopathological factors, progression-free survival (PFS), and overall survival were evaluated by SPSS.
Results: There was a significant difference in tumor size between the two groups, with larger tumors in the calcification group than the control group, and the proportion of patients in the calcification group with tumors of >5 cm was 20.4% vs 17.2% in the control group (P=0.041). The proportion of patients with lymph node metastasis in the calcification group was higher than that of the control group (35% vs 27.9%, P=0.027). The recurrence rate in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) patients with microcalcification was higher than that in the control group (P=0.035 and 0.044). BMP-2 expression was higher in breast cancer tissues, especially in breast cancer tissues with microcalcifications. The recurrence rate in the BMP-2(+) group was higher than that in the BMP-2(-) group both in DCIS and IDC (P=0.044 and 0.049). Microcalcifications and the positive expression of BMP-2 were independent factors affecting the PFS of the breast cancer patients.
Conclusion: Through the analysis of this study, it was found that the prognosis of the patients with microcalcification was relatively poor. BMP-2 was highly expressed in the breast cancer with microcalcification and was associated with poor prognosis.
Keywords: breast cancer, microcalcification, BMP-2, mammography, immunohistochemistry prognosis
Introduction
Breast cancer is a kind of epithelial malignant tumor that has the ability to cause local invasion and distant metastasis.1 In recent years, the incidence of breast cancer has increased significantly and it has become one of the most common malignant tumors, with a trend toward an increased frequency in younger patients.1 Microcalcification is a very important diagnostic information in breast cancer, which can be detected by mammography and pathology. Approximately 50% of nonpalpable breast cancers are detected by mammography exclusively through microcalcification patterns, revealing up to 90% of ductal carcinoma in situ (DCIS).2,3 It has been reported that there is a close relationship between microcalcification and the diagnosis of breast cancer.4 However, there are few reports on the relationship with clinicopathological features, recurrence, invasion, or metastasis. The purpose of this study was to determine the relationship of clinicopathological features and prognosis of breast cancer with microcalcification.
Microcalcifications are mainly composed of calcium oxalate or hydroxyapatite.5,6 The mechanisms that induce the formation of microcalcifications in breast cancer are still not clear. In recent years, studies have shown that this pathological microcalcification may be similar to heterotopic bone formation process.7,8 To detect biomarkers related to the possible mechanisms, we analyzed the expression and roles of BMP-2, Runx2, and osteopontin (OPN), which are the key indicators in the osteogenic pathways, in tissues with microcalcifications.9–11
Patients and methods
Patients
All 529 subjects were selected from patients who had been examined using breast mammography in the Department of Breast Imaging, Tianjin Medical University Cancer Institute and Hospital from January 2008 to June 2009. Written informed consent was obtained from each patient. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tianjin Cancer Hospital.
The inclusion criteria were as follows: 1) microcalcification was confirmed through preoperative mammography; 2) breast cancer was identified through pathology: the pathological type was DCIS or invasive ductal carcinoma (IDC); 3) the patient did not receive preoperative chemotherapy, radiotherapy, endocrine therapy, or any other anticancer treatments; and 4) the patient had complete clinicopathological and follow-up data, including age, menopausal status, tumor size, lymph node involvement, pathological stage, histological grade, and estrogen receptor (ER), progesterone receptor (PR), HER2, Ki67, and p53 statuses. The exclusion criteria were as follows: 1) the pathological type was neither DCIS nor IDC; 2) the patient had locally advanced or advanced breast cancer and did not undergo operation; 3) the follow-up period was <1 year; and 4) the cause of death was not cancer. The control group, which comprised of patients who did not have detectable microcalcifications on mammography, was matched in a ratio of 1:3. Matching was based on both age at diagnosis and time of surgery (±2 years). All subjects were female.
Evaluation of mammography
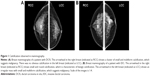
Calcification was judged to be benign or malignant by two experienced imaging doctors in a blinded manner according to the morphology and distribution of calcification (Figure 1).
The American College of Radiology Breast Imaging Reporting and Data System lexicon, fifth edition, was used to classify all mammographic findings with respect to breast tissue density, masses, microcalcifications, architectural distortion, and focal asymmetry. Microcalcification morphology was classified as punctate/amorphous, coarse heterogeneous/fine pleomorphic, or fine linear (branching). Microcalcification distribution was classified as clustered/grouped, linear/segmental, or regional/diffuse.
Evaluation of immunohistochemistry
Formalin-fixed, paraffin-embedded serial tissue sections of full block-face tissue from surgical specimen of each case were selected for BMP-2, OPN, and Runx2 immunohistochemical stains using standard procedures. Briefly, 4-μm tissue sections were subsequently dewaxed and rehydrated using xylene and graded alcohol washes. Antigen retrieval was performed at 121°C for 2 minutes, using citrate buffer, pH 6.0. After serial blocking with hydrogen peroxide and normal goat serum, the sections were incubated with monoclonal anti-BMP-2 (AF355, R&D Systems, Inc., Minneapolis, MN, USA; 1:500), monoclonal anti-OPN (sc-21742, Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:100), and monoclonal anti-Runx2 (sc-390715, Santa Cruz Biotechnology Inc; 1:100) for 30 minutes at room temperature. Then, the sections were sequentially incubated with biotinylated immunoglobulin and peroxidase-conjugated streptavidin.
The ER, PR, and HER2 statuses were determined using the criteria of the American Society of Clinical Oncology/College of American Pathologists.12,13 For ER and PR, nuclear staining in ≥1% of the tumor cells was considered positive. HER2 immunoreactivity was evaluated on a standardized scale from 0 to 3 based on the intensity of membranous staining and the proportion of tumor cells stained, where a strong complete membranous staining in ≥10% of tumor cells (3+) was considered positive. Ki67 and p53 statuses were determined through nuclear staining. Molecular classification of the tumor was performed using the established criteria.14,15 For BMP-2 and OPN, cytoplasmic/membranous staining in ≥10% of the tumor cells was considered positive (Figure 2). For Runx2, nuclear staining in ≥10% of the tumor cells was considered positive.
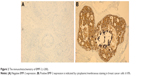  | Figure 2 The immunohistochemistry of BMP-2 (×200). |
Statistical analyses
SPSS 22.0 software was used for statistical analyses. To compare the clinicopathological characteristics of patients, the chi-squared test was used for dichotomous variables. Continuous variables were compared using the independent two-sample t-test. A nonparametric test was used to analyze the ranked data and continuous data that were not normally distributed. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of surgery. Survival curves were plotted using the Kaplan–Meier method, and group differences in the survival curves were investigated by the log-rank test. A Cox proportional hazards model was used to identify variables that were independently associated with OS. All statistical tests were two-sided and a P-value <0.05 was considered statistically significant.
Results
Clinicopathological characteristics of patients with microcalcification
The information of the breast cancer patients who had been examined by breast mammography in the Department of Breast Imaging, Tianjin Medical University Cancer Institute and Hospital from January 2008 to June 2009 were collected. There were 529 cases with microcalcification (calcification group), all of which were female, single sided, and the average age was 47 (25–76) years old. Among them, there were 412 (77.9%) cases of DCIS and 117 (22.1%) cases of IDC. Lymph node metastasis (LNM) was found in 185 (35%) cases, ER status was positive in 407 (72%) cases, PR status was positive in 391 (73.9%) cases, and HER2 status was positive in 145 (27.4%) cases. In total, 317 (59.9%) cases were classified as Luminal A type, 84 (15.9%) cases were classified as Luminal B type, 53 (10.0%) cases were classified as HER2 positive type, and 75 (14.2%) cases were classified as basal-like type. The control group, comprising patients without detectable microcalcification through mammography, was matched in a ratio of 1:3 (1,587 cases). The clinicopathological features are detailed in Table 1.
The results showed that there was a significant difference in tumor size between the two groups, with larger tumors in the calcification group than the control group, and the proportion of patients in the calcification group with tumors of >5 cm was 20.4% vs 17.2% in the control group (P=0.041). The proportion of patients with LNM in the calcification group was higher than that of the control group (35% vs 27.9%, P=0.027). There was no significant difference in age, menopausal status, family history, pathological type, histological grade, ER, PR, HER2, molecular typing, p53, and Ki67 between the two groups.
Survival analysis of patients with microcalcification
Due to the great difference in the prognosis of patients with DCIS and IDC, we analyzed survival separately according to the pathological types. We analyzed the recurrence rate in patients with DCIS and found that the recurrence rate in patients with microcalcification was higher than that in the control group (P=0.035) and the 5- and 7-year PFS of the two groups were 93.6% vs 96.9% and 86.5% vs 93.6%, respectively (Figure 3A). There was no significant difference in OS between the two groups (P=0.257; Figure 3B). For patients with IDC, we found that the recurrence rate in the calcification group was higher than that in the control group (P=0.044) and the 5- and 7-year PFS of the two groups were 82.9% vs 89.4% and 79.2% vs 85.8%, respectively (Figure 3C). There was also no significant difference in OS between the two groups of patients with IDC (P=0.183; Figure 3D).
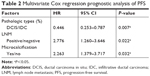
In multivariate Cox regression analysis, after adjusting for pathological type, LNM, and histological grading, microcalcification was still an independent factor affecting the PFS of the breast cancer patients (HR: 2.263, 95% CI: 1.379–3.717, P=0.032; Table 2).
Expression of BMP-2, Runx2, and OPN in patients with microcalcification
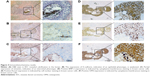
It was reported that the mechanism of microcalcification is ectopic bone formation, and BMP-2, Runx2, and OPN are key indicators in osteogenic pathways. To analyze the expression of E-cadherin, Vimentin, BMP-2, Runx2, and OPN in calcification tissue through immunohistochemistry, we randomly selected 100 cases (DCIS: 50 cases, IDC: 50 cases) from the 529 patients with microcalcification, and 100 cases (DCIS: 50 cases, IDC: 50 cases) without microcalcification served as the control group. Figure 4A shows that H&E stain in IDC revealed calcification in the lumen. E-cadherin expression is indicative of an epithelial phenotype, Vimentin expression is indicative of an interstitial phenotype, positive BMP-2 expression is indicated by cytoplasmic/membranous staining, positive Runx2 expression is indicated by nuclear staining, and positive OPN expression is indicated by cytoplasmic/membranous staining. Staining in ≥10% of tumor cells was considered positive. The statistical results showed that the epithelial marker (E-cadherin) of breast cancer with microcalcification was significantly weakened (Figure 4B) and the interstitial marker (Vimentin) was more highly expressed (Figure 4C), suggesting the occurrence of epithelial–mesenchymal transition (EMT). Irrespective of DCIS or IDC, the proportions of patients who were positive for BMP-2, Runx2, and OPN expression in breast cancer tissues with microcalcifications were significantly higher than those in the control group (P<0.05; Figure 4D–F; Table 3), indicating that these three key indicators (BMP-2, Runx2, and OPN) of osteogenic pathways may be involved in the formation of microcalcifications in breast cancer.
  | Table 3 Expression of BMP-2, Runx2, and OPN in patients with microcalcification |
Relationship between expression of BMP-2 and clinicopathological parameters in breast cancer
From the immunohistochemical results, we found that the BMP-2 is a key indicator involved in the formation of calcifications in osteogenic pathways. Moreover, it is reported that it plays an important role in promoting invasion and metastasis of malignant tumors. Therefore, we speculated that the poor prognosis of breast cancer patients with microcalcification may be related to the high expression of BMP-2. The correlation between BMP-2 and the clinicopathological parameters of breast cancer was analyzed. The results showed that the expression of BMP-2 was closely related to tumor size, LNM, and Ki67 expression (P<0.05), but had no significant correlation with age, family history, histological classification, molecular typing, and p53 (P>0.05; Table 4).
Relationship between expression of BMP-2 and prognosis
Several studies have found that the expression of BMP-2 was associated with the prognosis of malignant tumors. However, there is a lack of more detailed studies in breast cancer. We divided the patients into BMP-2(+) and BMP-2(−) groups. By analyzing the recurrence rate of DCIS, it was found that the recurrence rate in the BMP-2(+) group was higher than that in the BMP-2(−) group (P=0.044). The 5- and 7-year PFS rates of the two groups were 83.2% vs 94.7% and 75.3% vs 91.5%, respectively (Figure 5A). There was no significant difference in OS between the two groups (P=0.264; Figure 5B). For the IDC group, we found that the BMP-2(+) group had a higher rate of progression than the BMP-2(−) group (P=0.049). The 5- and 7-year PFS rates were 76.2% vs 94.2% and 72.5% vs 91.2%, respectively (Figure 5C), and the mortality rate in the BMP-2(+) group was relatively higher, but was not statistically significant (P=0.302; Figure 5D).
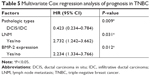
A Cox proportional hazard model was used to identify variables that were independently associated with PFS. The results showed that the positive expression of BMP-2 was an independent factor after adjusting for pathological type and LNM (HR: 2.234, 95% CI: 1.334–3.766, P=0.012; Table 5).
Discussion
Breast cancer is one of the most common malignant tumors in women and seriously endangers health. According to statistics, about 40% of breast cancer shows calcification on mammography and is sometimes the only indication of tumor.16,17 Microcalcifications are also very common in breast cancer. It is reported that 90% of DCIS have signs of microcalcifications on mammography.18,19 However, the mechanism behind the formation of microcalcifications is not yet very clear. In the past, the pathological point of view was that microcalcification in breast cancer tissue was mainly attributed by the necrosis of the cancer tissue. However, in recent years, some studies reported that its formation may be similar to the formation of bone tissue.20 Bone-related proteins in breast cancer may be involved in the formation of microcalcification. It has been reported that BMP-2, OPN, and osteonectin are expressed in the breast tissue. Moreover, the high expression of OPN or osteonectin was closely related to microcalcification in breast cancer.21–23 In bone tissue, BMP-2 could regulate the expression of bone matrix proteins such as OPN, Runx2, and osteocalcin through receptor signaling pathway, thereby affecting the formation of bone mineralization. The main chemical components of microcalcification in breast cancer are carbon hydroxyapatite.20 Most of the microcalcifications are located in the lumen of DCIS and can also be found in IDC. It has been reported that microcalcifications in breast cancer were closely related to recurrence of DCIS and the invasion or metastasis of IDC.24
In order to elucidate the relationship between microcalcification and the prognosis of breast cancer, we collected data from 529 subjects with microcalcification who were selected from the patients examined using breast mammography in the Department of Breast Imaging, Tianjin Medical University Cancer Institute and Hospital from January 2008 to June 2009. The results of our study showed that there was a significant difference in tumor size between the two groups, with larger tumors in the calcification group than in the control group, and the proportion of patients in the calcification group with tumors of >5 cm was 20.4% vs 17.2% in the control group (P=0.041). The proportion of patients with LNM in the calcification group was higher than that of the control group (35% vs 27.9%, P=0.027). There was no significant difference in age, menopausal status, family history, pathological type, histological grade, ER, PR, HER2, molecular typing, p53, and Ki67 between the two groups. It could be seen that patients with microcalcification often had larger tumor and higher rates of LNM, which meant that the malignant degree of tumors was higher in this subgroup of patients. Some other studies have indicated a relationship between HER2 expression and presence of microcalcifications. In our study, we did not find the significant difference between the two groups. That may because all 529 subjects with microcalcifications in our study were selected from patients who had been examined using breast mammography. But some other studies selected the patients only by pathology. Maybe that causes the different statistical results.
As the prognosis of IDC and DCIS is very different, we analyzed the relationship between microcalcification and survival, respectively, according to the different pathological types. We analyzed the recurrence rate in patients with DCIS and found that the recurrence rate in patients with microcalcification was higher than that in the control group (P=0.035) and the 5- and 7-year PFS of the two groups were 93.6% vs 96.9% and 86.5% vs 93.6%, respectively. There was no significant difference in OS between the two groups (P=0.257). For the patients with IDC, we found that the recurrence rate in the calcification group was higher than that in the control group (P=0.044) and the 5- and 7-year PFS of the two groups were 82.9% vs 89.4% and 79.2% vs 85.8%, respectively. There was also no significant difference in OS between the two groups of patients with IDC (P=0.183). In multivariate Cox regression analysis, after adjusting for pathological type, LNM, and histological grading, microcalcification was still an independent factor affecting the PFS of the breast cancer patients (HR: 2.263, 95% CI: 1.379–3.717, P=0.032). It has been reported that the 10-year PFS of IDC patients with microcalcification was 76.6%, which was worse than that of patients without microcalcification. The results of our study indicated a 7-year PFS of 79.2%, which was consistent with the previous reports.25 The patients with microcalcification had relatively worse PFS and were more prone to recurrence and metastasis. Another report about the recurrence rate of DCIS, which stated that the recurrence rate in 15 years was 10.3%.20 The results of our study showed that the recurrence rate of DCIS was 6.4% in 7 years and that of patients with microcalcification was 13.5%. It could be seen that microcalcification was also a prognostic factor for DCIS, which could increase the rates of recurrence.
BMP-2 is a strong osteogenic-inducing factor in the BMP family. It can induce mesenchymal cells and smooth muscle cells to differentiate into osteoblasts/odontoblasts in the process of bone formation and tooth formation.26 Runx2 is one of the members of the Runx family and is a key transcription factor for the initiation and regulation of osteoblast differentiation. It is also highly expressed in prostate, lung, and breast cancer cells.27 OPN is a protein that is widely distributed in many tissues and cells and can participate in many functions such as tissue repair and self-metabolism. Osteoblasts, osteocytes, and osteoclasts can secrete OPN, which play an important role in the mineralization and absorption of the bone matrix. OPN has an obvious tendency to promote tumor deterioration, and the expression of OPN in different tumor tissues is different.28–31
To analyze the expression of E-cadherin, Vimentin, BMP-2, Runx2, and OPN in calcification tissue through immunohistochemistry. E-cadherin expression is indicative of an epithelial phenotype, Vimentin expression is indicative of an interstitial phenotype, positive BMP-2 expression is indicated by cytoplasmic/membranous staining, positive Runx2 expression is indicated by nuclear staining, and positive OPN expression is indicated by cytoplasmic/membranous staining. Staining in ≥10% of tumor cells was considered positive. The statistical results showed that the epithelial marker (E-cadherin) of breast cancer with microcalcification was significantly weakened and the interstitial marker (Vimentin) was more highly expressed, suggesting the occurrence of EMT. Irrespective of DCIS or IDC, the proportions of patients who were positive for BMP-2, Runx2, and OPN expression in breast cancer tissues with microcalcifications were significantly higher than those in the control group (P<0.05), indicating that these three key indicators (BMP-2, Runx2, and OPN) of osteogenic pathways may be involved in the formation of microcalcifications in breast cancer.
Several studies have found that the expression of BMP-2 is associated with the prognosis of malignant tumors.21 We divided the patients into BMP-2(+) and BMP-2(−) groups. By analyzing the recurrence rate of DCIS, it was found that the recurrence rate in the BMP-2(+) group was higher than that in the BMP-2(−) group (P=0.044). The 5- and 7-year PFS rates of the two groups were 83.2% vs 94.7% and 75.3% vs 91.5%, respectively. There was no significant difference in OS between the two groups (P=0.264). In the IDC group, we found that the BMP-2(+) group had a higher rate of progression than the BMP-2(−) group (P=0.049). The 5- and 7-year PFS rates were 76.2% vs 94.2% and 72.5% vs 91.2%, respectively, and the mortality of the BMP-2(+) group was relatively higher, although with no statistical significance (P=0.302). The Cox regression model was used to analyze the prognosis of breast cancer. The results showed that the positive expression of BMP-2 was an independent factor affecting the survival rate of breast cancer (HR: 2.234, 95% CI: 1.334–3.766, P=0.012) after adjusting for pathological type and LNM.
The results of this study suggested that the expression of BMP-2 in patients with microcalcification was significantly higher. It may play a similar function in bone tissue, that is, the tumor tissue produces BMP-2 locally through autocrine or paracrine ways, which combines with related receptors and enters the nucleus, acts at the target gene, and then regulates Runx2, OPN, and other bone matrix proteins to form a microenvironment of calcium and phosphorus deposits in the tumor tissue, resulting in the formation of microcalcifications.31–34 In this study, immunohistochemical staining showed that the epithelial markers of the microcalcification of breast cancer were obviously weakened, and the interstitial marker Vimentin was expressed, suggesting that the EMT phenomenon had occurred, which may be one of the mechanisms of microcalcification, that is, the epithelial tumors have mesenchymal properties after the occurrence of EMT and have the ability to form calcifications.35,36 Due to the high recurrence rate in breast cancer patients with microcalcification, it was presumed that the high expression of these proteins such as BMP-2 and Runx2 may promote the invasion and metastasis of tumor cells. It was reported that BMP-2 participates in the regulation of EMT, which can promote the migration and metastasis of breast cancer, gastric cancer, and pancreatic cancer.37 The specific mechanism is not very clear. This study continued to explore the effect of BMP-2 on the proliferation and metastasis of tumor cells.
Conclusion
Through the analysis of this study, it was found that the prognosis of the patients with microcalcification was relatively poor. BMP-2 was highly expressed in the breast cancer with microcalcification and was associated with poor prognosis. The mechanisms that induce the formation of microcalcifications in breast cancer are still not clear. Future study will focus on mechanism research.
Data sharing statement
The datasets used during the present study are available from the corresponding author upon reasonable request.
Acknowledgment
This research was supported by grants from the National Natural Science Foundation of China (Grant No 81702636), National Natural Science Foundation of China (Grant No 81472183), Key Task Project of Tianjin Health and Family Planning Commission (16KG128), and Anticancer Key Technologies R&D Program of Tianjin (12ZCDZSY16200).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. | ||
Ferranti C, Coopmans de Yoldi G, Biganzoli E, et al. Relationships between age, mammographic features and pathological tumour characteristics in non-palpable breast cancer. Br J Radiol. 2000;73(871):698–705. | ||
Gülsün M, Demirkazık FB, Ariyürek M. Evaluation of breast microcalcifications according to breast imaging reporting and data system criteria and Le Gal’s classification. Eur J Radiol. 2003;47(3):227–231. | ||
Radi MJ. Calcium oxalate crystals in breast biopsies. An overlooked form of microcalcification associated with benign breast disease. Arch Pathol Lab Med. 1989;113(12):1367–1369. | ||
Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002;62(18):5375–5380. | ||
Frappart L, Boudeulle M, Boumendil J, et al. Structure and composition of microcalcifications in benign and malignant lesions of the breast: study by light microscopy, transmission and scanning electron microscopy, microprobe analysis, and X-ray diffraction. Hum Pathol. 1984;15(9):880–889. | ||
Ferreira LB, Eloy C, Pestana A, et al. Osteopontin expression is correlated with differentiation and good prognosis in medullary thyroid carcinoma. Eur J Endocrinol. 2016;174(4):551–561. | ||
Zhang J, Yamada O, Kida S, et al. Down-regulation of osteopontin mediates a novel mechanism underlying the cytostatic activity of TGF-beta. Cell Oncol. 2016;39(2):119–128. | ||
Shi X, Yang X, Chen D, Chang Z, Cao X. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem. 1999;274(19):13711–13717. | ||
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. | ||
Jung JW, Shim SY, Lee DK, Kwiatkowski W, Choe S. An activin A/BMP2 chimera, AB215, blocks estrogen signaling via induction of Id proteins in breast cancer cells. BMC Cancer. 2014;14(1):549. | ||
Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–e72. | ||
Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. JCO. 2013;31(31):3997–4013. | ||
Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. | ||
Altaf FJ, Mokhtar GA, Emam E, et al. Metaplastic carcinoma of the breast: an immunohistochemical study. Diagn Pathol. 2014;9(1):139. | ||
Wilkinson L, Thomas V, Sharma N. Microcalcification on mammography: approaches to interpretation and biopsy. Br J Radiol. 2017;90(1069):20160594. | ||
Heine JJ, Malhotra P. Mammographic tissue, breast cancer risk, serial image analysis, and digital mammography. Part 1. Tissue and related risk factors. Acad Radiol. 2002;9(3):298–316. | ||
Heine JJ, Malhotra P. Mammographic tissue, breast cancer risk, serial image analysis, and digital mammography. Part 2. Serial breast tissue change and related temporal influences. Acad Radiol. 2002;9(3):317–335. | ||
Baré M, Torà N, Salas D, et al. Mammographic and clinical characteristics of different phenotypes of screen-detected and interval breast cancers in a nationwide screening program. Breast Cancer Res Treat. 2015;154(2):403–415. | ||
Sharma T, Radosevich JA, Pachori G, Mandal CC. A molecular view of pathological Microcalcification in breast cancer. J Mammary Gland Biol Neoplasia. 2016;21(1–2):25–40. | ||
Liu F, Bloch N, Bhushan KR, et al. Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. Mol Imaging. 2008;7(4):175–186. | ||
Bellahcène A, Castronovo V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer. 1997;84(1):17–24. | ||
Bellahcène A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146(1):95–100. | ||
Durhan G, Öztekin PS, Ünverdi H, et al. Do histopathological features and microcalcification affect the elasticity of breast cancer? J Ultrasound Med. 2017;36(6):1101–1108. | ||
Qi X, Chen A, Zhang P, Zhang W, Cao X, Xiao C. Mammographic calcification can predict outcome in women with breast cancer treated with breast-conserving surgery. Oncol Lett. 2017;14(1):79–88. | ||
Castronovo V, Bellahcene A. Evidence that breast cancer associated microcalcifications are mineralized malignant cells. Int J Oncol. 1998;12(2):305–308. | ||
Vishal M, Swetha R, Thejaswini G, Arumugam B, Selvamurugan N. Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol. 2017;99:608–614. | ||
Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine. 2016;122(333):147–153. | ||
Castronovo V, Bellahcene A. Evidence that breast cancer associated microcalcifications are mineralized malignant cells. Int J Oncol. 1998;12(2):305–308. | ||
Bellahcène A, Castronovo V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer. 1997;84(1):17–24. | ||
Langenfeld EM, Bojnowski J, Perone J, Langenfeld J. Expression of bone morphogenetic proteins in human lung carcinomas. Ann Thorac Surg. 2005;80(3):1028–1032. | ||
Arnold SF, Tims E, McGrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of Bmp2. Cytokine. 1999;11(12):1031–1037. | ||
Gámez B, Rodríguez-Carballo E, Bartrons R, Rosa JL, Ventura F. MicroRNA-322 (miR-322) and its target protein Tob2 modulate Osterix (Osx) mRNA stability. J Biol Chem. 2013;288(20):14264–14275. | ||
Wang Y, Wu B, Chamberlain AA, et al. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLoS One. 2013;8(4):e60244. | ||
Piotrowski-Daspit AS, Tien J, Nelson CM. Interstitial fluid pressure regulates collective invasion in engineered human breast tumors via Snail, vimentin, and E-cadherin. Integr Biol (Camb). 2016;8(3):319–331. | ||
Duncan SA, Watt AJ. BMPs on the road to hepatogenesis. Genes Dev. 2001;15(15):1879–1884. | ||
Tan CC, Li GX, Tan LD, et al. Breast cancer cells obtain an osteomimetic feature via epithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget. 2016;7(48):79688–79705. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.