Back to Journals » Infection and Drug Resistance » Volume 15
Microbiological Profiles of Ocular Fungal Infection at an Ophthalmic Referral Hospital in Southern China: A Ten-Year Retrospective Study
Authors Pei Y, Chen X, Tan Y, Liu X, Duan F, Wu K
Received 19 March 2022
Accepted for publication 16 June 2022
Published 22 June 2022 Volume 2022:15 Pages 3267—3276
DOI https://doi.org/10.2147/IDR.S367083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yinhui Pei, Xiaoling Chen, Yiwei Tan, Xiuping Liu, Fang Duan, Kaili Wu
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, People’s Republic of China
Correspondence: Kaili Wu, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yan-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, People’s Republic of China, Email [email protected] Fang Duan, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yan-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, People’s Republic of China, Email [email protected]
Purpose: This study aims to assess the testing methods used to detect fungal pathogens as well as the pathogenic profiles and drug susceptibility among fungal isolates from ocular sources collected in a tertiary eye hospital.
Methods: The laboratory test records of patients with suspected ocular fungal infection from January 2010 to December 2019 at a tertiary eye care center in southern China were retrospectively evaluated. The isolated pathogens identified by smear examination and culture combined with microscopy were analyzed. For 2017– 2019 fungal isolates, their antifungal susceptibilities to four antifungal agents were evaluated.
Results: A total of 22,233 specimens (16,315 for culture and 5918 for direct smear examination) from 16,209 individuals were assessed, and 3317 specimens (2169 for culture and 1148 for smear examination) from 2395 patients had confirmed fungal infection. The mean age of patients was 50.6± 14.2 years and 63.4% were male. The positive rate of smear examination was 19.4% and significantly higher than 13.3% of fungal culture (p< 0.001). Of 5812 patients who were simultaneously examined for culture and smear staining, 897 (15.43%) had positive findings for fungi. Among the 2420 positive findings, 2138 (88.3%) were from the cornea, and 133 (5.5%) were from intraocular samples. Fusarium spp. (40.5%) and Aspergillus spp. (22.0%) were the major fungal genera among the molds, whereas Candida spp. (4.6%) constituted the major yeast. Fusarium spp. had a lower antifungal susceptibility than Aspergillus spp. against voriconazole and amphotericin. And yeast spp. had the highest susceptibility to caspofungin.
Conclusion: This study provided a 10-year assessment of fungi in ocular infections in southern China, giving support to epidemiological understanding and guiding empiric antimicrobial therapy.
Keywords: fungal infection, eye, corneal scrape, fungal culture, keratomycosis, antifungal susceptibility, endophthalmitis
Introduction
Oculomycosis is a severe and refractory infection that may result in the permanent loss of vision, due to the rapid progression of fungal infections and limited availability of antifungal agents. The epidemiological patterns of causative agents of ophthalmic mycosis vary significantly from region to region. Mycotic ophthalmitis is relatively uncommon in temperate, developed countries such as the UK,1 USA2,3 and Switzerland.4 A two-year (2003–2005) national surveillance study in the UK showed the average incidence of fungal keratitis at 0.32% cases per million individuals per year.5 In contrast, fungal ophthalmitis has a relatively high incidence in tropical or subtropical, low-income countries, accounting for up to 37.6–72.0% of keratitis in Ghana, India and China.6–10 The distribution of fungi not only varies in different countries but also changes with time. Ong et al concluded that the constituent ratio of organismal agents of fungal keratitis switched between filamentous fungi and yeast spp. since 2007.1 Durand pointed out that yeast spp. were the most common cause of endogenous fungal endophthalmitis, while Fusarium spp. and Aspergillus spp. were the most frequent fungal etiologies in exogenous endophthalmitis.11 Thus, for the early application of appropriate antifungal agents in the empiric management of potentially blinding ocular fungal infections, updating our knowledge of specific fungi and drug susceptibility profiles is very important.
Methods of sampling and testing are crucial for early diagnosis. During recent years, MALDI-TOF-MS, PCR and DNA sequencing have been used to identify fungi in ocular infections.12–17 However, in the clinical laboratory setting, microscopic identification of fungi is still the most common technique in the diagnosis of eye disorders, especially for quickly identifying pathogens in keratitis and endophthalmitis.18,19 Thus, in the process of smearing and culturing of specimens followed by microscopic identification to the genus or species in clinical laboratories, the accuracy of fungal identification depends on having a sufficient amount of scraped material, the staining dye (KOH, Calcofluor white, Gram and Giemsa stain), the size of the corneal ulcer and the clinical laboratory physician’s experience.18–20
Moreover, the development of antifungal drug resistance highlights the need for in vitro susceptibility testing.21 There have been various methods for in vitro antifungal susceptibility testing used, including macro- and micro-dilution, E-tests, and disk diffusion, based on the standards of the Clinical and Laboratory Standards Institute (CLSI) or The European Committee on Antimicrobial Susceptibility Testing (EUCAST).18,22,23 Different fungal isolates have different susceptibilities for antifungal agents. Although clinical therapeutic approaches cannot be routinely based on the results of antifungal susceptibility testing, in vitro determination of susceptibility profiles could provide helpful information in cases of clinically resistant fungal infection and uncommon pathogens as well as for long-term antifungal therapy.14,24
Thus, the understanding of the etiological and epidemiological characteristics of fungal ocular infection in different geographical regions, social economic statuses, climate statuses, ocular anatomical positions, etc. are significant for better prevention and treatment of this disease.13,18 This study was undertaken to collect comprehensive information about ocular specimens for laboratory-proven positive fungal infection in the last ten years at a tertiary eye hospital in Guangzhou, South China. The previous studies reported the fungal isolates of keratitis and endophthalmitis, respectively, in same institution,25,26 however, the susceptibility of antifungal medicines were not available. Our study updated the spectra of ocular fungal isolates and analyzed the susceptibility of common fungal pathogens against voriconazole amphotericin B, caspofungin and fluconazole, with the goal to provide a reference to clinicians.
Subjects and Methods
This study was designed as a retrospective review of microbiological records of all samples with suspected fungal infection based on patients’ clinical manifestations. All tests were undertaken for microbiological evaluation at the Zhongshan Ophthalmic Center between January 2010 and December 2019. The data of antifungal susceptibility by the E-test was reviewed for two years (2017–2019). This study was performed in compliance with the principles of the Helsinki agreement and was approved by the Institutional Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-Sen University. The requirement for patient consent was waived given the retrospective nature of the study. In this article, we analyzed the legal components without disclosing confidential patients’ private information.
Organismic Isolation and Identification
Specimens were taken in the microbiological lab in our hospital under topical anesthesia (0.5%, proparacaine hydrochloride) or during operation. Protocols were conducted following our established methods published previously.27 Secretions of the conjunctival sac were collected by sterile cotton swabs; cornea specimens were sampled by scraping the base and edges of the pathological lesions; and anterior chamber fluids and vitreous tissue were obtained by syringe aspiration or vitrectomy in an operating room. Most specimens were simultaneously tested for bacteria and fungi. This study only evaluated the ocular fungal infection over ten years.
The collected specimens were tested by direct smears and culturing by established methods.27,28 Fungal culture was conducted using potato dextrose agar medium for 7 days or more at 28°C. Positive fungal findings were evaluated based on colony morphology, growth characteristics and microscopic features with staining following a lactophenol cotton blue mount preparation.29,30
Bacterial cultures were made in nutrient broth for up to 7 days at 37°C and after in sheep blood agar for 24 hours at 37°C.31 Then, bacterial isolates were identified by an automated microbiology system (Vitek 2 Compact, BioMerieux, Inc. 100 Rodolphe Street, Durham, USA) or by a MALDI-TOF-MS system (BioMerieux).
Antifungal Susceptibility Testing
Data from the antifungal susceptibility testing from January 2017 to December 2019 were available and retrospectively evaluated. After the identification of fungal isolates using conventional techniques, E-test susceptibility testing for voriconazole, amphotericin B, caspofungin and fluconazole against fungal isolates was performed according to the guidelines of the CLSI method using commercial kits (Autobio Diagnostics Co., Ltd, Zhengzhou, China) certificated by the China Food and Drug Administration. Pure colonies were selected for susceptibility testing. Plates were inoculated in three directions with a nontoxic cotton swab dipped in the diluted stock inoculum suspensions. Then, the E-test strips were applied to the plate. The plates were incubated at 28°C, and MICs were read around 48 h at the lowest drug concentration.
Statistical Analysis
The statistical analysis was performed with SPSS 16.0 (Chicago, IL, USA). The Chi-squared test was applied for the comparison of categorical variables. To determine the temporal trends in fungal proportions, the Pearson correlation coefficient was used. Differences were considered to be significant at P<0.05.
Results
Demographics and Epidemiological Data
A total of 16,209 individuals who were suspected to have ocular bacteria or fungal infection over a ten-year-period (2010–2019) were reviewed. A total of 22,233 specimens were analyzed, including 16,315 specimens for fugal culture and 5918 specimens for smear examination, and specimens from 5812 patients were tested with both culture and the smear tests. Of them, there were 2420 specimens (2395 patients, including 25 patients examined twice on different days) with positive fungal findings detected through different methods. Among 2395 patients, males accounted for 63.3%, and the 50-year-old group had the highest incidence of fungal infection, followed by the 40- and 60-year-old groups (Figure 1A). The number of yearly positive findings of the 2395 patients were similar among the ten years reviewed in this study (Figure 1B). The fungal infections were unequally distributed in different months of the whole year (Figure 1C). October to December had a higher incidence than others, especially compared to the months of April and May.
Features of Sampling and Testing
Overall, there were 3317 (2169 for culture and 1148 for smear examination) positive fungal findings among 22,233 (16,315 for culture and 5918 for smear examination) specimens (14.9%), including 897 positive findings (15.4%) in 5812 patients who were sampled by two examination methods (Table 1). The positive rate was 19.4% (1148/5918) for smear examination, while it is 13.3% (2169/16,315) for culturing. Of 2420 specimens with positive fungal findings, 89.6% (2169 specimens) of fungal isolates were found through culture assays, and 47.4% (1148 specimens) were found through smear staining.
 |
Table 1 Fungal Examination Results by Two Methods Over 10 Years |
Among the 2420 positive specimens from 2395 individuals, 2138 (1887 from culturing and 251 from smear examination) were from keratitis, and 133 (5.5%) were from intraocular samples. The majority of corneal sampling, accounting for 88.3% of our positive findings, was from outpatients. Among 133 intraocular specimens of endophthalmitis as the result of fungal infection, 26 cases were detected from aqueous fluid, 93 cases from vitreous body, 11 cases from intraocular tissues, 2 cases from intraocular foreign bodies and 1 case from an intraocular lens. In addition, there were 40 specimens (1.65%) obtained from the conjunctiva and 22 cases (0.91%) from the lacrimal duct and its secretions.
Fungal Assay Results
The cumulative distribution of various isolated fungi is shown in Table 2. Among 2169 positive findings from culturing, fungi were identified to the genus level. Of them, Fusarium spp. were the most common fungi isolated (879, 40.5%), followed by Aspergillus spp. (477, 22.0%) and Mucor spp. (219, 10.1%). The other two listed genera, Penicillium spp. and Curvularia spp. account for 4.2% and 5.5%, respectively. There were 133 (6.1%) isolates belonging to yeast, including 99 Candida spp. (4.6%). Of the Fusarium spp. isolates, F. solani, found in 278 cases (31.6%), was the most common. Among 477 isolates of Aspergillus spp., A. fumigatus (167 cases, 35.0%), A. flavus (149, 31.2%) and A. niger (101, 21.2%) were the most common.
 |
Table 2 Isolated Organisms from Ocular Fungal Infections, 2010–2019 |
The temporal trends for three major fungal isolates from 2010 to 2019 are shown in Figure 2. There was a significant increasing trend in the isolates of Fusarium spp. (r=0.785, p=0.007). Similarly, Candida spp. (r=0.887, p=0.001), also presented a significant increasing trend. Meanwhile, the proportion of Aspergillus spp. presented a decreasing trend (r=−0.614, p=0.059).
 |
Figure 2 Temporal trends of fungal isolates from 2010 to 2019. There were significant increasing trends in the isolates of Fusarium and Candida (p=0.007 and p=0.001 respectively). |
In this study, among 2169 culturing positive fungal isolates, 1887 (87.0%) isolates were identified from keratitis. There were 133 fungal-positive findings from patients with endophthalmitis (Table 3). In intraocular ocular mycosis, the predominant genus was Aspergillus spp. and Candida spp., followed by Fusarium spp., while the predominant pathogenic genus in corneal mycosis was Fusarium spp., followed by Aspergillus spp.. Although the major categories of fungi resulting in both keratitis and endophthalmitis were the same, the proportions of the fungal genera revealed striking differences. Fusarium spp. accounted for 44.8% in mycotic keratitis and 14.3% in fungal endophthalmitis (P<0.001); Candida spp. accounted for 23.3% in internal ophthalmitis and 2.8% in keratomycosis samples (P<0.001). In addition, Aspergillus spp. exhibited similar a prevalence in endophthalmitis and keratitis.
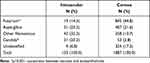 |
Table 3 Comparison of the Major Fungal Categories Between Corneal and Intraocular Isolates |
There were 136 fungal-positive specimens that appeared with co-infections of bacteria, of which, 95 (69.85%) were Gram-positive bacteria, including 89 Staphylococcus spp. (with 44 specimens with S. epidermidis). Of these specimens, 115 were from corneal ulcers, 10 were from patients with endophthalmitis and 11 were from other samples.
Antifungal Susceptibility Testing Results
Table 4 summarizes the in vitro activities of voriconazole, amphotericin B, caspofungin and fluconazole against 559 fungal isolates that were identified during 2017 and 2019, with only 42 from intraocular specimens. A total of 341 Fusarium spp., 131 Aspergillus spp., 55 Yeast spp., 17 penicillum spp. and 15 Mucor spp. isolates were included to determine the MIC50 and MIC90. The isolates of Fusarium spp. required higher concentrations of voriconazole than Aspergillus spp.. Most of the Aspergillus spp. isolates were inhibited by voriconazole at a concentration of ≥0.5 μg/mL, while most Fusarium spp. isolates were inhibited at a concentration of ≥4 μg/mL. The MIC90 of amphotericin-B was ≥32 μg/mL for all fungi, while the MIC50 was 32 μg/mL for Fusarium spp., 16 μg/mL for Penicillum spp., 4 ug/mL for Aspergillus spp., 0.75 μg/mL for Yeast spp. and Mucor spp. The MIC50 and MIC90 of caspofungin were ≥32 μg/mL for Aspergillus spp., Penicillum spp. and Mucor spp., while the MIC50 was 0.19 μg/mL for Yeast spp. Fluconazole were inactive against Yeast spp., showing MICs ≥ 256 μg/mL.
 |
Table 4 Minimun Inhibitory Concentration (μg/Ml) of Antifungal Agents in 2017–2019 |
Discussion
Fungal infective disorders of the eye are sight-threatening diseases and cases of ocular mycosis have soared worldwide during the past few decades, particularly in developing countries and tropical and subtropical regions.13 Several studies have reported the spectrum of ocular mycosis in the central and northern parts of the Chinese mainland.10,32–34 However, limited information of antifungal drug sensitivity is available. In the present study, we reviewed records of 2395 patients with 2420 positive fungal findings among 16,209 patients with 22,233 specimens over 10 years in the tertiary eye hospital in Guangzhou [Northern latitude 22°12”, East longitude 113°15”] in the southern part of China. We found that Fusarium and Aspergillus were the major fungal genera among molds, whereas Candida was the major genus among the yeast group. In addition, the isolates of filamentous fungi, especially Fusarium spp. and Candida spp. had increasing trends over time. Meanwhile, Aspergillus spp. presented a decreasing trend. Like previous reports,12,14,35 men aged between 40 and 60 suffered more ocular fungal infections than other people; and a higher incidence of fungal infection occurred from October to January in contrast to other months. Additionally, the results of antibiotic resistance of these pathogens provide reference information for use in clinical practice. We found that molds had the highest sensitivity to voriconazole, followed by amphotericin B, and yeast spp. were most sensitive to caspofungin, followed by amphotericin B, which provides reference information for use in clinical practice.
Currently, the clinical diagnosis of ocular fungal infection is dependent on fungal culturing that takes a long time (often 1 to 2 weeks or more) and often delays early diagnosis and treatment.18,20 Thus, direct microscopic examination of ocular specimens has been recommended as a rapid and effective measure of finding fungal infection.10,19,36 The demonstration of fungal elements in microscopic examination besides the isolation of fungi in culture is the gold standard for laboratory diagnosis.18,37 In our present study, the overall rate of positive fungal findings was 14.9% (excluding bacterial findings), with 19.4% for smear examination and 13.3% for culturing. Since there were 88.3% positive specimens from fungal keratitis, the diagnosis of the fungal keratitis by microscopy with smearing of specimens from corneal ulcers is a truly valuable method. In addition, the positive rate of culture and smear testing for fungi in identified infected patients is up to 70% or more in some published observations.1,34 However, fungal culture has a low sensitivity of less than 50%,18,38 and it usually takes weeks for fungal organisms to grow on culture media.14,39 Our institute is a tertiary center, and thus, a high number of patients with a drug treatment history are referred/transferred to our hospital. In addition, the shift from empirical treatment to evidence-based treatment has increased the prevalence of sampling. Both may be responsible for the relatively lower fungal detection rates in our hospital.
Generally, Aspergillus spp. and Fusarium spp. are common in tropical and subtropical regions, and Candida spp. are dominant in temperate areas.18 In our research, Fusarium spp. were the leading isolate, followed by Aspergillus spp. This was comparable to the findings reported in Tunisia,40,41 North India,42 Addis Ababa,39 Ghana,9 South India,9 Saudi Arabia,12 and different parts of China.6,10,32,34,43 Xie et al reported that two dominant pathogenic groups were the genus Fusarium (77.6%) and Aspergillus (10.8%) in 549 positive cultures of fungal keratitis.6 In contrast, Candida spp. were predominant in South Korea and the USA.44,45 In addition, our results revealed that, different from other fungi, Fusarium spp. increased during the last ten years, which was not found in several previous studies. Thus, reviewing local cases periodically will provide novel information for clinical treatment.
Drug sensitivity testing was performed against four drugs, ie, voriconazole, amphotericin B, caspofungin and fluconazole, which are frequently used in ophthalmic clinics in China. Although antifungal drug resistance was examined for isolates from specimens collected over two years, these isolates might reflect the latest native biological variability. Filamentous fungi, which account for the majority of fungal isolates, were the most sensitive to voriconazole, followed by amphotericin B, which is consistent with previous reports that suggested that voriconazole is the first choice for treatment of ocular mycosis caused by Fusarium and Aspergillus isolates.46–48 Voriconazole, with a broad spectrum of activity against Candida spp., Aspergillus spp. and Fusarium spp., was reported to have broad-spectrum antifungal activity, greater bioavailability, and higher aqueous and vitreous levels in the eye.49–51 Thus, it has become the first-line antifungal therapy against ocular infection, especially in unknown cases, during the last decade.13,47,52 However, there are studies that revealed that voriconazole was less effective for the in vitro inhibition of Fusarium spp. than AMB15,53 and more effective than natamycin for in vivo clinical outcomes of filamentous fungal keratitis,54 although the addition of oral voriconazole to topical natamycin could be beneficial for Fusarium keratitis.55 In the case AMB, Xie et al6 and Alastruey-Izquierdo et al56 reported that AMB was more effective against Fusaria spp. than Aspergillus spp. In our study, both Fusaria spp. and Aspergillus spp. had less sensitivity to AMB than to voriconazole. Different from molds, yeast spp. had the highest susceptibility to caspofungin, followed by amphotericin B (Table 4). That is, for ocular mycosis caused by yeast spp., we prefer caspofungin to amphotericin B, followed by voriconazole. Overall, the variation in the activity of the antifungal drugs depends on the fungal species and the nature and concentration of the vitreous antifungal agent.
Our study had some limitations, including the retrospective nature of the review of records from a microbiological lab, in which we could not reference the detailed medical history, and the study being performed at a tertiary hospital, where a significant number of patients are referrals after initial treatment. In addition, as the majority of specimens were from the cornea (1887 specimens with culturing positive findings), the relatively lower number of intraocular specimens make the findings to be less comparable to data for the ocular surface and to be hard to evaluate the resistant prevalence of each antifungal drug. When using our data as a reference for treating patients with endophthalmitis, especially for empiric antimicrobial therapy, clinicians should be cautious. Additionally, we also note that that previous studies suggested that the susceptibility levels of fungi to various antifungal compounds may be variable among different species.53,57,58 Further evaluation of the susceptibility for filamentous fungi at the species level, even to specific strains, is required in the future.
Conclusion
Above all, our study revealed the etiological and demographic characteristics of ocular fungal infection in a tertiary hospital in southern China from 2010 to 2019 by smear and culture examination. Since the majority of ocular mycosis was fungal keratitis, microscopy with smearing of specimens is a very valuable method for diagnosis. In addition, the isolates of filamentous fungi, especially Fusarium spp. and Candida spp. had increasing trends over time. Meanwhile, Aspergillus spp. presented a decreasing trend. Filamentous fungi, accounting for 90% or more of fungal pathogens, had the highest susceptibility to voriconazole, and yeast spp. had the highest susceptibility to caspofungin in the in vitro drug susceptibility testing. In short, we hope our study will be helpful in generating management strategies for ocular fungal infections and for future studies.
Funding
This work was supported by grants from the Guangzhou Science Technology and Innovation Commission (201607020011) and the National Natural Science Foundation of China (81770896, 81970848).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ong HS, Fung SS, Macleod D, et al. Altered patterns of fungal keratitis at a London ophthalmic referral hospital: an eight-year retrospective observational study. Am J Ophthalmol. 2016;168:227–236. doi:10.1016/j.ajo.2016.05.021
2. Tanure MA, Cohen EJ, Sudesh S, et al. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. doi:10.1097/00003226-200005000-00010
3. Ritterband DC, Seedor JA, Shah MK, et al. Fungal keratitis at the New York eye and ear infirmary. Cornea. 2006;25:264–267. doi:10.1097/01.ico.0000177423.77648.8d
4. Iselin KC, Baenninger PB, Schmittinger-Zirm A, et al. Fungal keratitis: a six-year review at a tertiary referral centre. Klin Monbl Augenheilkd. 2017;234:419–425. doi:10.1055/s-0042-123233
5. Tuft SJ, Tullo AB. Fungal keratitis in the United Kingdom 2003–2005. Eye. 2009;23:1308–1313. doi:10.1038/eye.2008.298
6. Xie L, Zhai H, Zhao J, et al. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. Am J Ophthalmol. 2008;146:260–265. doi:10.1016/j.ajo.2008.04.019
7. Sharma S, Taneja M, Gupta R, et al. Comparison of clinical and microbiological profiles in smear-positive and smear-negative cases of suspected microbial keratitis. Indian J Ophthalmol. 2007;55:21–25. doi:10.4103/0301-4738.29490
8. Shah A, Sachdev A, Coggon D, et al. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95:762–767. doi:10.1136/bjo.2009.169607
9. Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi:10.1136/bjo.86.11.1211
10. Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. doi:10.1016/j.ophtha.2006.05.035
11. Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30:597–613. doi:10.1128/CMR.00113-16
12. Manikandan P, Abdel-Hadi A, Randhir Babu Singh Y, et al. Fungal keratitis: epidemiology, rapid detection, and antifungal susceptibilities of fusarium and aspergillus isolates from corneal scrapings. Biomed Res Int. 2019;2019:6395840. doi:10.1155/2019/6395840
13. Kredics L, Narendran V, Shobana CS, et al. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses. 2015;58:243–260. doi:10.1111/myc.12306
14. Garg P, Roy A, Roy S. Update on fungal keratitis. Curr Opin Ophthalmol. 2016;27:333–339. doi:10.1097/ICU.0000000000000272
15. Homa M, Shobana CS, Singh YR, et al. Fusarium keratitis in South India: causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses. 2013;56:501–511. doi:10.1111/myc.12062
16. Miqueleiz Zapatero A, Hernando C, Barba J, et al. [First report of a case of fungal keratitis due to Curvularia hominis in Spain]. Rev Iberoam Micol. 2018;35:155–158. Spanish. doi:10.1016/j.riam.2018.03.004
17. Li Z, Breitwieser FP, Lu J, et al. Identifying corneal infections in formalin-fixed specimens using next generation sequencing. Invest Ophthalmol Vis Sci. 2018;59:280–288. doi:10.1167/iovs.17-21617
18. Mahmoudi S, Masoomi A, Ahmadikia K, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61:916–930. doi:10.1111/myc.12822
19. Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbiological diagnosis of infective keratitis: comparative evaluation of direct microscopy and culture results. Br J Ophthalmol. 2006;90:1271–1276. doi:10.1136/bjo.2006.096230
20. Zhang W, Yang H, Jiang L, et al. Use of potassium hydroxide, Giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J Int Med Res. 2010;38:1961–1967. doi:10.1177/147323001003800609
21. Heuer C, Bahnemann J, Scheper T, et al. Paving the way to overcome antifungal drug resistance: current practices and novel developments for rapid and reliable antifungal susceptibility testing. Small Methods. 2021;5:e2100713. doi:10.1002/smtd.202100713
22. Al-Hatmi AM, Normand AC, Ranque S, et al. Comparative evaluation of Etest, EUCAST, and CLSI methods for amphotericin B, voriconazole, and posaconazole against clinically relevant fusarium species. Antimicrob Agents Chemother. 2016;61. doi:10.1128/AAC.01671-16
23. Meletiadis J, Geertsen E, Curfs-Breuker I, et al. Intra- and interlaboratory agreement in assessing the in vitro activity of micafungin against common and rare candida species with the EUCAST, CLSI, and Etest methods. Antimicrob Agents Chemother. 2016;60:6173–6178. doi:10.1128/AAC.01027-16
24. Behrens-Baumann WJ, Hofmuller W, Tammer I, et al. Keratomycosis due to Tintelnotia destructans refractory to common therapy treated successfully with systemic and local terbinafine in combination with polyhexamethylene biguanide. Int Ophthalmol. 2018;39:1379–1385. doi:10.1007/s10792-018-0930-2
25. Lin L, Duan F, Yang Y, et al. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infect Drug Resist. 2019;12:1295–1302. doi:10.2147/IDR.S206831
26. Lin L, Mei F, Liao J, et al. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of infectious endophthalmitis from a large referral eye center in Southern China. Infect Drug Resist. 2020;13:493–500. doi:10.2147/IDR.S235954
27. Wang N, Yang Q, Tan Y, et al. Bacterial spectrum and antibiotic resistance patterns of ocular infection: differences between external and intraocular diseases. J Ophthalmol. 2015;2015:813979. doi:10.1155/2015/813979
28. Hua GO, Yiwei TA, Xiangming GO, Jiahui LI, Zhiwei CA. Spectrum of fungal keratitis change in South China. Chin J Exp Ophthalmol. 2017;2017:161–164.
29. Kumar A, Pandya S, Kavathia G, et al. Microbial keratitis in Gujarat, Western India: findings from 200 cases. Pan Afr Med J. 2011;10:48.
30. Keshav BR, Zacheria G, Ideculla T, Bhat V, Joseph M. Epidemiological characteristics of corneal ulcers in South Sharqiya region. Oman Med J. 2008;23:34–39.
31. Zhu M, Cheng C, Yi H, et al. Quantitative analysis of the bacteria in blepharitis with demodex infestation. Front Microbiol. 2018;9:1719. doi:10.3389/fmicb.2018.01719
32. Wang L, Sun S, Jing Y, et al. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37:763–771. doi:10.1111/j.1442-9071.2009.02155.x
33. Sun RL, Jones DB, Wilhelmus KR. Clinical characteristics and outcome of Candida keratitis. Am J Ophthalmol. 2007;143:1043–1045. doi:10.1016/j.ajo.2007.02.016
34. He D, Hao J, Gao S, et al. Etiological analysis of fungal keratitis and rapid identification of predominant fungal pathogens. Mycopathologia. 2016;181:75–82. doi:10.1007/s11046-015-9950-x
35. Ghosh AK, Gupta A, Rudramurthy SM, et al. Fungal keratitis in North India: spectrum of agents, risk factors and treatment. Mycopathologia. 2016;181:843–850. doi:10.1007/s11046-016-0042-3
36. Sharma S, Kunimoto DY, Gopinathan U, et al. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21:643–647. doi:10.1097/00003226-200210000-00002
37. Watson SL, Cabrera-Aguas M, Keay L, et al. The clinical and microbiological features and outcomes of fungal keratitis over 9 years in Sydney, Australia. Mycoses. 2019;63:43–51. doi:10.1111/myc.13009
38. Gopinathan U, Sharma S, Garg P, et al. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57:273–279. doi:10.4103/0301-4738.53051
39. Kibret T, Bitew A. Fungal keratitis in patients with corneal ulcer attending Minilik II Memorial Hospital, Addis Ababa, Ethiopia. BMC Ophthalmol. 2016;16:148. doi:10.1186/s12886-016-0330-1
40. Zbiba W, Baba A, Bouayed E, et al. A 5-year retrospective review of fungal keratitis in the region of Cap Bon. J Francais d’ophtalmologie. 2016;39:843–848. doi:10.1016/j.jfo.2016.09.006
41. Cheikhrouhou F, Makni F, Neji S, et al. Epidemiological profile of fungal keratitis in Sfax (Tunisia). J Mycol Med. 2014;24:308–312. doi:10.1016/j.mycmed.2014.06.047
42. Verma S, Sharma V, Kanga A, et al. Current spectrum of oculomycosis in North India: a 5-year retrospective evaluation of clinical and microbiological profile. Indian J Med Microbiol. 2016;34:72–75. doi:10.4103/0255-0857.174104
43. Zhang Y, Wang ZQ, Sun XG. [Analysis of etiology and in vitro drug susceptibility of fungal keratitis in northern China]. Zhonghua Yan Ke Za Zhi. 2018;54:432–436. Chinese. doi:10.3760/cma.j.issn.0412-4081.2018.06.009
44. Ho JW, Fernandez MM, Rebong RA, et al. Microbiological profiles of fungal keratitis: a 10-year study at a tertiary referral center. J Ophthalmic Inflamm Infect. 2016;6:5. doi:10.1186/s12348-016-0071-6
45. Cho CH, Lee SB. Clinical analysis of microbiologically proven fungal keratitis according to prior topical steroid use: a retrospective study in South Korea. BMC Ophthalmol. 2019;19:207. doi:10.1186/s12886-019-1212-0
46. Bunya VY, Hammersmith KM, Rapuano CJ, et al. Topical and oral voriconazole in the treatment of fungal keratitis. Am J Ophthalmol. 2007;143:151–153. doi:10.1016/j.ajo.2006.07.033
47. Al-Badriyeh D, Leung L, Davies GE, et al. Successful use of topical voriconazole 1% alone as first-line antifungal therapy against Candida albicans keratitis. Ann Pharmacother. 2009;43:2103–2107. doi:10.1345/aph.1M318
48. Wang LY, Xu ZZ, Zhang JJ, et al. [Topical voriconazole as an effective treatment for fungal keratitis]. Zhonghua Yan Ke Za Zhi. 2016;52:657–662. Chinese. doi:10.3760/cma.j.issn.0412-4081.2016.09.005
49. Marangon FB, Miller D, Giaconi JA, et al. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–825. doi:10.1016/j.ajo.2003.11.078
50. Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122:42–47. doi:10.1001/archopht.122.1.42
51. Al-Badriyeh D, Neoh CF, Stewart K, et al. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin Ophthalmol. 2010;4:391–405. doi:10.2147/opth.s6374
52. Tuli SS. Fungal keratitis. Clin Ophthalmol. 2011;5:275–279. doi:10.2147/OPTH.S10819
53. Oliveira Dos Santos C, Kolwijck E, van der Lee HA, et al. In vitro activity of chlorhexidine compared with seven antifungal agents against 98 fusarium isolates recovered from fungal keratitis patients. Antimicrob Agents Chemother. 2019;63. doi:10.1128/AAC.02669-18
54. Prajna NV, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128:672–678. doi:10.1001/archophthalmol.2010.102
55. Prajna NV, Krishnan T, Rajaraman R, et al. Adjunctive oral voriconazole treatment of fusarium keratitis: a secondary analysis from the mycotic ulcer treatment trial II. JAMA Ophthalmol. 2017;135:520–525. doi:10.1001/jamaophthalmol.2017.0616
56. Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, et al. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61:805–809. doi:10.1093/jac/dkn022
57. Nayak N, Satpathy G, Prasad S, et al. Molecular characterization of drug-resistant and drug-sensitive Aspergillus isolates causing infectious keratitis. Indian J Ophthalmol. 2011;59:373–377. doi:10.4103/0301-4738.83614
58. Wagner L, de Hoog S, Alastruey-Izquierdo A, et al. A revised species concept for opportunistic mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob Agents Chemother. 2019;63. doi:10.1128/AAC.00653-19
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

