Back to Journals » International Journal of Women's Health » Volume 14
mHealth Phone Intervention to Reduce Maternal Deaths and Morbidity in Cameroon: Protocol for Translational Adaptation
Authors Budhwani H, Enah C, Bond CL, Halle-Ekane G, Wallace E, Turan JM, Szychowski JM, Long DM, Carlo WA, Tih PM, Tita AT
Received 7 January 2022
Accepted for publication 14 April 2022
Published 7 May 2022 Volume 2022:14 Pages 677—686
DOI https://doi.org/10.2147/IJWH.S353919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Marleen van Gelder
Henna Budhwani,1,2 Comfort Enah,3 Christyenne L Bond,1 Gregory Halle-Ekane,4 Eric Wallace,5 Janet M Turan,1,2,6 Jeff M Szychowski,7 Dustin M Long,7 Waldemar A Carlo,8 Pius M Tih,9 Alan TN Tita2,10
1Department of Health Policy and Organization, School of Public Health, University of Alabama at Birmingham, Birmingham, AL, USA; 2Center for Women’s Reproductive Health, University of Alabama at Birmingham, Birmingham, AL, USA; 3School of Nursing, College of Health Sciences, University of Massachusetts Lowell, Lowell, MA, USA; 4Department of Obstetrics and Gynaecology, University of Buea, Buea, Cameroon; 5Depatrment of Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 6School of Medicine, Koç University, Istanbul, Turkey; 7Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, AL, USA; 8Depatrment of Pediatrics, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 9Cameroon Baptist Convention Health Services, Bamenda, North West Region, Cameroon; 10Depatrment of Obstetrics and Gynecology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Correspondence: Henna Budhwani, Department of Health Policy and Organization, School of Public Health, University of Alabama at Birmingham, 330C Ryals Public Health Building, 1720 University Boulevard, Birmingham, AL, 35294, USA, Tel +1 205 975 7613, Fax +1 205 975 7685, Email [email protected]
Purpose: The purpose of this NIH-funded protocol is to adapt (Aim 1) and pilot test (Aim 2) an mHealth intervention to improve maternal and child health in Cameroon. We will adapt the 24/7 University of Alabama at Birmingham Medical Information Service via Telephone (MIST) provider support system to mMIST (mobile MIST) for peripheral providers who provide healthcare to pregnant and postpartum women and newborns in Cameroon.
Methods: In Aim 1, we apply qualitative and participatory methods (in-depth interviews and focus groups with key stakeholders) to inform the adaptation of mMIST for use in Cameroon. We use the sequential phases of the ADAPT-ITT framework to iteratively adapt mMIST incorporating qualitative findings and tailoring for local contexts. In Aim 2, we test the adapted intervention for feasibility and acceptability in Ndop, Cameroon.
Results: This study is ongoing at the time that this protocol is published.
Conclusion: The adaptation, refinement, and pilot testing of mMIST will be used to inform a larger-scale stepped wedged cluster randomized controlled effectiveness trial. If successful, this mHealth intervention could be a powerful tool enabling providers in low-resource settings to deliver improved pregnancy care, thereby reducing maternal and fetal deaths.
Keywords: pregnancy, mobile applications, mHealth, intervention, maternal health
Introduction
Reducing maternal and perinatal deaths is highlighted in the global millennium and sustainable development goals as a priority for both high- (HICs) and low-income countries (LICs).1,2 Over 300,000 women die annually from pregnancy-related complications, and millions more become ill or disabled.3,4 Ninety-nine percent of maternal mortality occurs in LICs, most in sub-Saharan Africa (SSA), where the average lifetime risk of maternal death is reported to be as high as 1 in 13 in some settings (vs 1 in 4085 in HICs).3–5 Adverse maternal health leads to poor infant outcomes, and 98% of neonatal deaths globally occur in LICs.5,6 Maternal and child health outcomes represent the largest disparity in health status between LICs and HICs,3–5 with neonatal deaths accounting for over 50% of infant mortality.7,8
The peripartum period is a critical time when most maternal and perinatal deaths and severe morbidities occur.9–11 As access to maternity care increases in LICs and more births occur in health facilities, improving the quality of care at these facilities is critical to addressing negative maternal and child health outcomes. According to the global strategy12 to improve peripartum care quality, promising interventions should be scientifically adapted for local contexts and their implementation rigorously evaluated for effectiveness.13 Evidence-based approaches are vital to support regional scale-up of interventions and maximize reduction of maternal and perinatal morbidity using limited financial and personnel resources.14
MIST™ (Medical Information Service via Telephone) has been operational at the University of Alabama at Birmingham (UAB) since 1969 and was the first provider-to-provider consultation hotline.15–17 MIST was established in response to the need for health professionals in rural Alabama to have rapid communication with experts at the state’s only tertiary facility (UAB) to optimize patient care and/or initiate transfers.15–17 The service provides toll-free 24/7 access to UAB faculty experts and clinical specialists within minutes. This simple idea was so successful that demand and usage of MIST grew from 2413 calls in the 1st year to 11,370 in the 2nd. Currently, over 100,000 calls are received from providers annually and are recorded for reference and quality improvement. Surveys of users demonstrate high satisfaction (>95%). While large healthcare centers in the US have been quick to adopt MIST, this expansion has not occurred in LICs, in part due to structural limitations. The proliferation of mobile infrastructure and mobile phone personal ownership in LICs presents a timely opportunity to adapt, implement, and evaluate this type of intervention in LICs that struggle with high rates of maternal and perinatal deaths, warranting an urgent response.13
There has been rapid proliferation of a variety of types of mHealth interventions in both HICs and LICs with some evidence of effectiveness in improving patient behaviors (eg, clinic visits, illness monitoring, and medication compliance).13,18,19 A recent review spotlighted four digital health interventions that not only made notable impact on their intended outcomes but were also adapted multiple times to address new outcomes across new settings successfully.20 In contrast, a systematic review of mHealth interventions to address maternal, newborn and child health in LICs (and middle income) found a lack of rigor in assessing impact.21 And, in 2019, the World Health Organization (WHO) released a set of evidence-based guidelines for digital health, providing recommendations for the use of a mobile phone technology (as well as implementation consideration).22 Our study builds upon the evidence that mHealth is an acceptable modality for research in LICs; mHealth interventions work well when scientifically adapted with attention to rigor and contexts, and addresses three of the WHO’s nine guidelines, namely provider-to-provider telemedicine, health worker decision support via mobile devices, and provision of training to health workers via mobile devices.12,17,18,20,22
While provider hotlines are in routine use in HICs, there has been insufficient use and evaluation of their effectiveness in LICs.19,23 In LICs, opportunities for providers to keep abreast of evidence-based information and to have point-of-care access to actionable information are limited.24 This is supported by prior studies in Cameroon by our group25–37 and others.38–42 mHealth interventions to promote timely access to evidenced-based information and clinical guidelines, may improve the quality of care in LICs; however, this hypothesis must be tested in order to ensure rational use of limited resources. Mobile technology provides an opportunity to overcome longstanding barriers posed by lack of access to fixed land lines in LICs and increases ability of peripheral or rural providers to access expert providers and electronic point of care protocols.
Study Setting
The countries with the highest maternal and perinatal mortality ratios and rapidly growing mobile technology infrastructure are located in SSA.43 Cameroon has a high maternal mortality ratio of 600/100,000 births, and a perinatal mortality rate of about 50–60/1000 births, among the highest worldwide.8,19,44 Common causes of maternal death (hemorrhage, eclampsia, sepsis, obstructed labor, preexisting complications) and perinatal death (sepsis, malformations, fetal growth restriction, preexisting complications) death are similar to those in other LICs and in Alabama.44 Eighty five percent of pregnant women receive some antenatal care43 and up to 85% of pregnant women have a skilled attendant at delivery. Cameroon has expanding mobile phone coverage making it an ideal LIC in which to evaluate mMIST to improve pregnancy outcomes. Mobile subscriptions have risen exponentially over the past decade in Cameroon to over 19.7 million in 2017 (population 23 million, about 85%).43
As in many SSA countries, the Cameroon pregnancy care system is predominantly led by midwives and nurses in accordance with World Health Organization (WHO) recommendations.25,26,44 The health system in Cameroon is a mix of public and private institutions under the oversight of the Ministry of Health. There are ten administrative health regions which are further divided into districts. Cameroon is classified by the WHO as having a critical shortage of healthcare personnel and equipment, exacerbated in rural areas (where most of the population reside).45,46
Protocol Objectives
Aim 1 will focus on the adaptation and development of a 24/7 mHealth support system, mMIST, for primary providers of pregnant women in Cameroon built upon stakeholder feedback and the existing UAB schematics. Aim 2 will test mMIST’s feasibility and acceptability in the Ndop health district in Cameroon.
Materials and Methods
Ethics Statement
Ethics approval was provided by the Cameroon Baptist Convention Health Board Institutional Review Board, under IRB study number: IRB2020-49 and the UAB Institutional Review Board under number: IRB-300006254. Informed consent will be obtained from human study participants, and this protocol is in compliance with all guidelines outlined in the Declaration of Helsinki.
Community Engagement
We will establish community and stakeholder committees and working groups, with members from both the United States and Cameroon, to inform all steps of this study. The Local Advisory Committee (LAC) will meet monthly to provide local perspectives and troubleshoot any arising issues, as needed. The LAC will include 10–12 stakeholders who will provide insight to all aspects of the mMIST adaptation. An Expert Providers Committee (EPC) will include 12–15 expert clinical providers in Cameroon; all EPC members will be practicing OB/GYNs, pediatricians, nurses, and midwives. The EPC will be convened about every two months. A Technology Working Group (TWG) will be established to provide guidance on the mMIST prototype. The TWG will include US and Cameroon-based technology experts and will assist the principal investigators to troubleshoot barriers related to infrastructure and prototyping. The TWG will include no more than 10 members. Feedback from these groups will enhance the rigor, replicability, and scalability of the adapted mMIST intervention.
Partner in Cameroon
Our key partner, the Cameroon Baptist Convention Health System (CBCHS), is a major healthcare provider responsible for over 90 health facilities (7 hospitals and 85 health centers) in six of the ten administrative regions of Cameroon.
Formative Qualitative Assessment
The first step in adapting MIST is to conduct interviews and focus groups with key stakeholders to inform the adaptation of UAB’s MIST to the local context of Cameroon. In order to do so, participants will be recruited from six different categories: the Ministry of Health, primary providers, currently pregnant women, previously pregnant women, and mobile service provider leadership. We estimate that in-depth interviews will be conducted with around 12 maternity providers, 10 previously pregnant women who suffered an adverse outcome, 10 health system administrators and clinical staff, 8 mobile service providers, and 6 representatives with the Ministry of Health. Three focus groups are planned with currently pregnant women. Although estimates are presented data collection will continue until the team achieves data saturation. Qualitative data collection will occur in English and Pidgin English, depending on the preference of the study participant.
In-depth interviews and focus groups will be audio-recorded using digital recorders; audio files will be uploaded to a protected UAB server. Audio files will first be transcribed into Microsoft Word by an expert transcriptionist. Transcribed files in Pidgin will then be translated to American English. Qualitative coding and analysis will be conducted using a modified Grounded Theory47 approach in which key conceptual domains are inductively derived from the data. NVivo software will be used for coding and analysis. A preliminary coding scheme will be developed based on the topics in the interview guide and relevant literature. The coding scheme will be appended during review based on emerging themes and topics, resulting in a refined qualitative coding scheme. Transcripts will be re-reviewed for more detailed, second-level fine coding. Attention to trustworthiness (credibility, dependability and transferability) will be given though inclusivity of participant invitation, recruitment being conducted by a known an trusted local research team member, and during data analysis, seeking agreement and consensus of meaning among co-researchers, experts, and research participants.48 Results will inform the adaptation of MIST to mMIST.
Intervention Adaptation Process
We will use the sequential phases of the ADAPT-ITT framework to iteratively adapt MIST to mMIST to incorporate the qualitative findings and adapt for local contexts. ADAPT-ITT is a pragmatic 8-step model developed for the adaptation and tailoring on HIV interventions that has been extended to other areas of research.49 Adaptation is the process of modifying an intervention without contradicting its core elements or internal logic.20 Attention to culture and contexts (both inner and outer) of a new environment or group promote relevance and acceptability of interventions.20,49 ADAPT-ITT phases and tasks for mMIST are listed in Table 1.
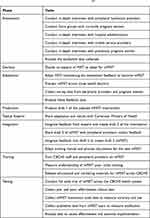 |
Table 1 Steps of ADAPT-ITT for the mMIST Adaptation51 |
System and Pre-Pilot Testing
After a draft of mMIST is developed (technical design in Figure 1), we will share process documents and demonstrate mMIST to members of our community groups. We will collect verbal feedback using a standardized question set to assess the quality and acceptability of the adaptation. After evaluating responses, we will make additional refinements to mMIST to test for feasibility and acceptability.
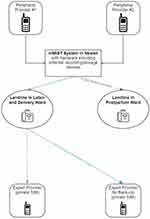 |
Figure 1 mMIST system structure with call flow process. |
Feasibility and Acceptability Testing
The goal in conducting a small-scale implementation of mMIST is to refine the adaptation using constructs from Bowen’s model of feasibility and acceptability, see Table 2, prior to large-scale implementation and evaluation of mMIST. Feasibility of mMIST in one health district (Ndop) will be assessed with a primary focus on demand and acceptability.50 Demand will be assessed using an inventory system of the number of times providers access the hotline and electronic files. Acceptability will be assessed through satisfaction surveys and medical record reviews to determine adherence to advice received. The satisfaction survey will include structured and semi-structured sections; the structured section will consist of five Likert scale items that will be analyzed descriptively to determine measures of central tendency. The open-ended section will consist of four questions on recommended changes that will be analyzed through content analyses. The secondary outcome is feasibility, while demand and acceptability will be primary.
 |
Table 2 Bowen’s Model of Feasibility and Assessment Measures |
Training
In order to test mMIST on a smaller scale in the pilot, all maternity providers at a minimum of five maternity centers in one district in Cameroon will be selected to utilize the system. This will include approximately 25–30 providers who will be trained via Zoom on study protocols and intervention functionality. Once mMIST is launched, we expect, on average, a minimum of 1 call every 2 days as an acceptable demand level. We expect that in the survey, at least 70% of providers will find mMIST acceptable.
Training will also be provided to support staff who will answer the mMIST line, primary maternity and pediatric providers, and administrators (who will be involved in supporting timely transfer of high-risk patients). Maternity healthcare workers who answer the mMIST line, called first line responders, and maternity service heads (responsible for the target health units) will attend a 1-day workshop on mMIST. Other providers will be trained by their maternity service leads (train-the-trainer model). Trained maternity service leads will be provided with detailed training and marketing materials (eg cards to be kept by providers, clinic posters, etc.) to train their maternity providers (eg physicians, nurses, skilled birth attendants, etc.) and monitored by the research team.
Data Collection
Electronic logs from mMIST will be assessed for number, type, and location of providers using the system and number of calls made in total, regardless of which provider initiates the call. mMIST first line responders will keep a paper record of the reason for each call, advice or referral given to the patient post-call, and assessment of whether the patient followed the advice or referral. An oversight team consisting of investigators and providers will monitor accuracy of advice given through auditing of a random sample of taped calls quarterly. Qualtrics surveys, record reviews guided by an assessment matrix, and follow-up in-depth interviews using a standardized guide will be used to assess acceptability, demand, implementation, practicality, adaptation, integration, and expansion. Administrative data collection on maternal and perinatal deaths will be optimized at start of project and collected on a rolling basis through the project.
A database will be developed to log provider call information. Data collected through a log or auditing of taped calls will include date, time, call duration, caller type (eg, nurse, midwife, physician, etc.) location, patient type (eg, pregnant, postpartum, or baby), call reason, respondent (eg, maternity worker, specialist expert, both), recommendation, and follow-up on advice. Provider demand will be quantified by the number of calls by location. Satisfaction survey and follow up interview data will be analyzed to gauge acceptability and feasibility.
Once all providers have been trained, the research team will supplement the existing data collection processes as needed to ensure that all components of feasibility can be evaluated as planned. Implementation will be on a rolling basis with lessons learned from each center informing the roll out in the next center. After the implementation cycle, structured surveys on feasibility domains will be shared with providers (peripheral and experts). Findings from survey data, chart reviews, and interviews with experts and related stakeholders will be used to inform updates to mMIST prior to testing through a subsequent cluster stepped wedge randomized controlled trial.
Results
Data collection processes and results will be reported in late 2022.
Discussion
Cameroon is, in several ways, similar to rural Alabama where MIST has been highly utilized and valued. Both have relatively high maternal and perinatal mortality, a large underserved rural population, and regionalization with urban concentration of higher-level care and equipment. There are 65,000 births annually in both settings. The findings of our study could inform mHealth intervention development and scale-up into the poorest nations with the greatest needs related to maternal and newborn health.
Potential Impact
If our pilot is successful, we will conduct a full-scale stepped wedge cluster randomized controlled trial of mMIST in which we will evaluate the intervention’s ability to reduce a composite of maternal or perinatal deaths or severe morbidities in Northwest Cameroon.
Challenges and Limitations
In Aim 1, pregnant women could be uncomfortable speaking openly in a focus group about their health. If so, one-on-one in-depth interviews will be used to collect data from this group. Although in-depth interviews with previously pregnant women who experienced a complication will likely yield rich data, these women are also at high-risk for having an adverse emotional reaction. To prepare for this, an experienced female interviewer will conduct all interviews and will be trained on when to pause or stop data collection, offer counseling, and link to care for supportive services. After the initial adaptation, it could be difficult to re-engage the same peripheral providers to solicit feedback. The nature of the ADAPT-ITT framework allows feedback from new providers for each new version of mMIST.
Conclusion
This pragmatic mHealth intervention could be a powerful tool enabling providers in LIC settings to deliver improved pregnancy, postpartum and newborn care. The mMIST program could be scalable to other regions in Cameroon and other low income countries in sub-Saharan Africa, as well as to other health medical departments (eg internal medicine or surgery), leading to improved maternal and newborn outcomes and ultimately to improved population health.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Fogarty International Center (FIC) of the National Institutes of Health (NIH) under Award Number R21HD103061, as well as by the National Institute of Mental Health (NIMH) of the NIH under Award Number K01MH116737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. We would also like to thank our Cameroon study staff who support this work under particularly difficult sociopolitical circumstances.
Disclosure
Dr. Henna Budhwani reports grants from NIH, CDC, and Merck Sharp and Dohme during the conduct of the study. Dr. Eric Wallace reports grants from Baxter Healthcare, personal fees from SANOFI Genzyme, grants, personal fees from Idorsia, grants, personal fees from Chiesi, grants, personal fees from Protalix, grants from 4dmt, personal fees from Natera, outside the submitted work. Dr. Waldemar A Carlo is board member in Mednax, during the conduct of the study. Dr. Alan TN Tita reports grants from NICHD/NIH, during the conduct of the study; grants from Pfizer, grants from CDC, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Hogan MC, Foreman KJ, Naghavi M, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards millennium development goal 5. Lancet. 2010;375(9726):1609–1623. doi:10.1016/S0140-6736(10)60518-1
2. Murray CJL. Shifting to sustainable development goals - implications for global health. N Engl J Med. 2015;373(15):1390–1393. doi:10.1056/NEJMp1510082
3. World Health Organization. The World Health Report 2005: Make Every Mother and Child Count. World Health Organization; 2005.
4. GBD Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1775–1812. doi:10.1016/S0140-6736(16)31470-2
5. Bhutta ZA, Black RE. Global maternal, newborn, and child health–so near and yet so far. N Engl J Med. 2013;369(23):2226–2235. doi:10.1056/NEJMra1111853
6. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. doi:10.1016/S2214-109X(14)70227-X
7. Yu VYH. Global, regional and national perinatal and neonatal mortality. J Perinat Med. 2003;31(5):376–379. doi:10.1515/Jpm.2003.057
8. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–3035. doi:10.1016/S0140-6736(16)31593-8
9. Tuncalp O, Were WM, MacLennan C, et al. Quality of care for pregnant women and newborns-the WHO vision. Bjog Int J Obstet Gy. 2015;122(8):1045–1049. doi:10.1111/1471-0528.13451
10. Helova A, Hearld KR, Budhwani H. Associates of neonatal, infant and child mortality in the Islamic Republic of Pakistan: a multilevel analysis using the 2012–2013 demographic and health surveys. Matern Child Health J. 2017;21(2):367–375. doi:10.1007/s10995-016-2121-y
11. Budhwani H, Hearld KR, Harbison H. Individual and area level factors associated with prenatal, delivery, and postnatal care in Pakistan. Matern Child Hlth J. 2015;19(10):2138–2146. doi:10.1007/s10995-015-1726-x
12. Task Force Hlth Syst Res. Informed choices for attaining the millennium development goals: towards an international cooperative agenda for health-systems research. (vol 364, pg 997, 2004). Lancet. 2004;364(9447):1756.
13. Ciapponi A, Lewin S, Herrera CA, et al. Delivery arrangements for health systems in low‐income countries: an overview of systematic reviews. Cochrane Database Syst Rev. 2017;2017(9). doi:10.1002/14651858.CD011083.pub2
14. Garner P, Kale R, Dickson R, Dans T, Salinas R. Getting research findings into practice: implementing research findings in developing countries. BMJ. 1998;317(7157):531–535. doi:10.1136/bmj.317.7157.531
15. Holt N, Crawford MA. Medical information service via telephone. The pioneer of physician consultation services. Ann NY Acad Sci. 1992;670:155–162. doi:10.1111/j.1749-6632.1992.tb26086.x
16. Fisher J. Continuing ED when you need it most. Med Econ. 1984;1984:140–148.
17. TIME. Medicine: epidemics, diagnosis (MIST in Alabama). 1969.
18. Pantoja T, Opiyo N, Lewin S, et al. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev. 2017;2017(9). doi:10.1002/14651858.CD011086.pub2
19. Karari C, Tittle R, Penner J, et al. Evaluating the uptake, acceptability, and effectiveness of Uliza! Clinicians’ HIV hotline: a telephone consultation service in Kenya. Telemed JE Health. 2011;17(6):420–426. doi:10.1089/tmj.2010.0220
20. Budhwani H, Kiszla BM, Hightow-Weidman LB. Adapting digital health interventions for the evolving HIV landscape: examples to support prevention and treatment research. Curr Opin HIV AIDS. 2022;17(2):112–118. doi:10.1097/coh.0000000000000721
21. Lee SH, Nurmatov UB, Nwaru BI, Mukherjee M, Grant L, Pagliari C. Effectiveness of mHealth interventions for maternal, newborn and child health in low- and middle-income countries: systematic review and meta-analysis. J Glob Health. 2016;6(1):010401. doi:10.7189/jogh.06.010401
22. Labrique A, Agarwal S, Tamrat T, Mehl G. WHO digital health guidelines: a milestone for global health. Npj Digit Med. 2020;3(1):120. doi:10.1038/s41746-020-00330-2
23. Ndlovu K, Littman-Quinn R, Park E, Dikai Z, Kovarik CL. Scaling up a mobile telemedicine solution in Botswana: keys to sustainability. Front Public Health. 2014;2:275. doi:10.3389/fpubh.2014.00275
24. Tesfalul M, Littman-Quinn R, Antwi C, et al. Evaluating the potential impact of a mobile telemedicine system on coordination of specialty care for patients with complicated oral lesions in Botswana. J Am Med Inform Assoc. 2016;23(e1):e142–5. doi:10.1093/jamia/ocv140
25. Geyoushi BE, Matthews Z, Stones RW. Pathways to evidence-based reproductive healthcare in developing countries. BJOG. 2003;110(5):500–507. doi:10.1046/j.1471-0528.2003.02381.x
26. Tita ATN, Selwyn BJ, Waller DK, Kapadia AS, Dongmo S. Evidence-based reproductive health care in Cameroon: population-based study of awareness, use and barriers. Bull World Health Organ. 2005;83(12):895–903.
27. Tita ATN, Selwyn BJ, Waller DK, Kapadia AS, Dongmo S. Factors associated with the awareness and practice of evidence-based obstetric care in an African setting. Bjog Int J Obstet Gynaecol. 2006;113(9):1060–1066. doi:10.1111/j.1471-0528.2006.01042.x
28. Anderson S, Harper LM, Dionne-Odom J, Halle-Ekane G, Tita ATN. A decision analytic model for prevention of hepatitis B virus infection in Sub-Saharan Africa using birth-dose vaccination. Int J Gynecol Obstet. 2018;141(1):126–132. doi:10.1002/ijgo.12434
29. Dionne-Odom J, Westfall AO, Nzuobontane D, et al. Predictors of infant hepatitis B immunization in Cameroon: data to inform implementation of a hepatitis B birth dose. Pediatr Infect Dis J. 2018;37(1):103–107. doi:10.1097/Inf.0000000000001728
30. Dionne-Odom J, Westfall AO, Apinjoh TO, Anchang-Kimbi J, Achidi EA, Tita ATN. Predictors of the use of interventions to prevent malaria in pregnancy in Cameroon. Malar J. 2017;16. doi:10.1186/s12936-017-1786-z
31. Dionne-Odom J, Welty TK, Westfall AO, et al. Factors associated with PMTCT cascade completion in four African Countries. AIDS Res Treat. 2016;2016:1–9. doi:10.1155/2016/2403936
32. Dionne-Odom J, Mbah R, Rembert NJ, et al. Hepatitis B, HIV, and syphilis seroprevalence in pregnant women and blood donors in Cameroon. Infect Dis Obstet Gynecol. 2016;2016:4359401. doi:10.1155/2016/4359401
33. Pasko DN, Blanchard CT, Szychowski JM, et al. Use of a novel device (Moyo) for intrapartum fetal monitoring in 1000 consecutive pregnancies in Cameroon, Africa. Am J Obstet Gynecol. 2018;218(1):S524–S524. doi:10.1016/j.ajog.2017.11.417
34. Subramaniam A, Blanchard CT, Ngek S, et al. Prevalence, perinatal outcomes, and maternal risk factors for group B streptococcus colonization in a low-income country. Am J Obstet Gynecol. 2018;218(1):S552–S553. doi:10.1016/j.ajog.2017.11.521
35. Budhwani H, Hearld KR, Dionne-Odom J, et al. HIV status and contraceptive utilization among women in Cameroon. J Int Assoc Provid AIDS Care. 2019;18:2325958219826596. doi:10.1177/2325958219826596
36. Budhwani H, Shivkumar P, Purandare CN, et al. Examining the use of magnesium sulfate to treat pregnant women with preeclampsia and eclampsia: results of a program assessment of Emergency Obstetric Care (EmOC) training in India. J Obstet Gynecol India. 2017;67(5):330–336. doi:10.1007/s13224-017-0964-9
37. Hearld KR, Anderson JL, Budhwani H. Examining the relationship between individual characteristics, community-level traits, multidimensional empowerment, and maternal health care utilization in the Islamic Republic of Pakistan. Matern Child Hlth J. 2018;22(9):1319–1326. doi:10.1007/s10995-018-2512-3
38. Hearld KR, Budhwani H. Maternal health care use by pregnant women living with HIV: factors associated with prenatal, delivery, and postnatal care in Haiti. J Health Care Poor Underserved. 2017;28(4):1452–1461. doi:10.1353/hpu.2017.0126
39. Kouo-Ngamby M, Dissak-Delon FN, Feldhaus I, Juillard C, Stevens KA, Ekeke-Monono M. A cross-sectional survey of emergency and essential surgical care capacity among hospitals with high trauma burden in a Central African country. BMC Health Serv Res. 2015;15. doi:10.1186/s12913-015-1147-y
40. Nkwabong E, Nguel RM, Kamgaing N, Jippe ASK. Knowledge, attitudes and practices of health personnel of maternities in the prevention of mother-to-child transmission of HIV in a sub-Saharan African region with high transmission rate: some solutions proposed. BMC Pregnancy Childbirth. 2018;18. doi:10.1186/s12884-018-1876-0
41. Tebeu PM, Fezeu LY, Ekono MR, Fosso GK, Ymele FF, Fomulu JN. Postpartum hemorrhage at Yaounde University Hospital, Cameroon. Int J Gynecol Obstet. 2013;121(3):283–284. doi:10.1016/j.ijgo.2013.01.010
42. Goldenberg RL, Nathan RO, Swanson D, et al. Routine antenatal ultrasound in low- and middle-income countries: first look - a cluster randomised trial. Bjog Int J Obstet Gynaecol. 2018;125(12):1591–1599. doi:10.1111/1471-0528.15287
43. Demographic and Health Surveys. Cameroon demographic and health survey; 2011. Available from: https://www.measuredhs.com.
44. Chomba E, Carlo WA, Goudar SS, et al. Effects of essential newborn care training on fresh stillbirths and early neonatal deaths by maternal education. Neonatology. 2017;111(1):61–67. doi:10.1159/000447421
45. Mobile cellular subscriptions 2000-2020. Available from: https://data.worldbank.org/indicator/IT.CEL.SETS. Accessed February 26, 2019.
46. Kingue S, Rosskam E, Bela AC, Adjidja A, Codjia L. Strengthening human resources for health through multisectoral approaches and leadership: the case of Cameroon. Bull World Health Organ. 2013;91(11):864–867. doi:10.2471/BLT.13.127829
47. Nyblade L, Stangl A, Weiss E, Ashburn K. Combating HIV stigma in health care settings: what works? J Int AIDS Soc. 2009;12:15. doi:10.1186/1758-2652-12-15
48. Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–112. doi:10.1016/j.nedt.2003.10.001
49. Wingood GM, DiClemente RJ. The ADAPT-ITT model: a novel method of adapting evidence-based HIV Interventions. J Acquir Immune Defic Syndr. 2008;47(Suppl 1):S40–6. doi:10.1097/QAI.0b013e3181605df1
50. iSurvey harvest your data. Available from: https://www.harvestyourdata.com.
51. Dresang LT, Gonzalez MMA, Beasley J, et al. The impact of Advanced Life Support in Obstetrics (ALSO) training in low-resource countries. Int J Gynecol Obstet. 2015;131(2):209–215. doi:10.1016/j.ijgo.2015.05.015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
