Back to Journals » International Journal of Nanomedicine » Volume 12
Methodological assessment of the reduction of the content of impurities in nimodipine emulsion via the use of 21 amino acid protection
Authors Xie YQ, Zhuang ZQ, Zhang S, Xia ZH, Chen D, Fan KY, Ren JL, Lin CC, Chen YZ, Yang F
Received 15 December 2016
Accepted for publication 16 March 2017
Published 27 April 2017 Volume 2017:12 Pages 3407—3419
DOI https://doi.org/10.2147/IJN.S130348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Yiqiao Xie,1,* Zhiquan Zhuang,2,* Shu Zhang,1 Zihua Xia,1 De Chen,1 Kaiyan Fan,1 Jialin Ren,1 CuiCui Lin,1 Yanzhong Chen,1 Fan Yang1
1Department of Pharmaceutics, Guangdong Pharmaceutical University, 2Department of Pharmacy, First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Purpose: The present study examined the factors affecting the content of impurities of nimodipine (NMP) emulsion and the associated methods of compound protection.
Methods: Destructive testing of NMP emulsion and its active pharmaceutical ingredient (API) were conducted, and ultracentrifugation was used to study the content of impurities in two phases. The impurity of NMP was measured under different potential of hydrogen (pH) conditions, antioxidants and pH-adjusting agents.
Results: Following destruction, the degradation of NMP notably occurred in the basic environment. The consumption of the pH-adjusting agent NaOH was proportional to the production of impurities since the inorganic base and/or acid promoted the degradation of NMP. The organic antioxidants, notably amino acids with an appropriate length of intermediate chain and electron-donating side group, exhibited improved antioxidant effects compared with inorganic antioxidants. The minimal amount of impurities was produced following addition of 0.04% lysine and 0.06% leucine in the aqueous phase and adjustment of the pH to a range of 7.5–8.0 in the presence of acetic acid solution.
Conclusion: NMP was more prone to degradation in an oxidative environment, in an aqueous phase and/or in the presence of inorganic pH-adjusting agents and antioxidants. The appropriate antioxidant and pH-adjusting agent should be selected according to the chemical structure, while destructive testing of the drug is considered to play the optimal protective effect.
Keywords: nimodipine emulsion, impurity, destructive testing, ultracentrifugation, antioxidation, pH
Introduction
Nimodipine (NMP) is a second-generation molecule of 1,4-dihydropyridine calcium channel antagonists that effectively reduces blood pressure via the expansion of cerebral blood vessels. NMP exerts a significant effect on the reduction of spasticity of cerebral blood vessels and the treatment of ischemic and hemorrhagic cerebrovascular diseases.1,2 Currently, the commercial formulations of NMP notably consist of tablets, capsules, sustained release agents and injectable formulations. Oral administration of NMP can be rapidly absorbed, although the absolute bioavailability is estimated from 3% to 28%3 that seriously affects the treatment. As a result, the use of injectable formulations is more extensive in the clinical application. Although NMP is an insoluble drug, ethanol, polyethylene glycol 400 and water are used as solvents in an effort to increase its solubility. The addition of physiological saline and/or glucose solution with NMP can cause the precipitation of the drug in the form of crystals and induce vein inflammation associated with irreversible damage to the limbs.4,5 This formulation should be avoided for intravenous injection of NMP.
Recently, several new technologies and methods, such as emulsion, liposome, nanoparticle suspension and polymer micelle, have been widely used as a putative carrier of the insoluble form of NMP.6 Emulsion has been shown to increase the solubility of the drug, reduce the adverse reactions and enhance the effects of targeted and sustained release of the drug compared with liposomal, microencapsulated and other particle formulations.7,8 It can significantly improve the clinical efficacy of NMP.7,8 More importantly, several intravenous emulsion products are present in the commercial market, namely, lipid injectable emulsion, phytonadione injectable emulsion and propofol injectable emulsion, demonstrating that this formulation satisfies the technical conditions for large-scale production and clinical application.
In the present study, emulsion was selected as the vehicle for NMP, which could effectively avoid the disadvantages of commercial injection. Since emulsion is considered thermodynamically unstable, the concomitant physical and chemical stability of the emulsion must be considered in the research and development of NMP emulsion. The optimal formulation and technology of NMP emulsion has been established on the basis of its physical stability, via specific univariate and orthogonal tests.9 The chemical stability notably depends on whether the active pharmaceutical ingredient (API) degrades in the process of manufacture, transportation and storage,10 resulting in the generation of impurities.
Impurities are referred to the products that exhibit homologous structure with API with different pharmacological properties and effects. In certain cases, impurities can further exert a toxic effect. The types of impurities of different NMP formulations are numerous due to the synthetic process of API, prescription and technology of the formulation as well as the conditions for storage and transport.11 In contrast to these observations, the content of impurities is used as a marker to further optimize the prescription and technology of NMP emulsion. This in turn reduces the generation of impurities and decreases the adverse reactions associated with the clinical application.12,13
According to the European Pharmacopoeia, three impurities of NMP have been recorded, including impurities A, B and C, whose molecular structures are shown in Figure 1.14 Impurity A has demonstrated liver and kidney toxicity that should be strictly controlled by limiting the percentage of this compound to 0.5% (c/c).15 To date, previous studies on the impurity of NMP have investigated the stage of quantitative detection.16,17 The studies that have been conducted lack investigation of the factors that result in an increase in the impurities of NMP and the examination of valuable methodologies for its protection.
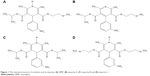  | Figure 1 The chemical structure of emulsion and its impurity: (A) NMP, (B) impurity A, (C) impurity B and (D) impurity C. |
The chemical structures of NMP and impurity A were analyzed, and it was reported that the difference between the two substances was the transformation of the 1,4-dihydropyridine ring of the NMP into the pyridine ring of impurity A.18 This caused an oxidation reaction. Destructive testing of NMP emulsion and NMP API were conducted, including acid–base treatment, oxidation and temperature and light damage in order to investigate the nature of the conditions that could promote the degradation of NMP.19,20 The protection of NMP usually involves the following two aspects: the reduction of the exposure to adverse environments, such as the adjustment of the potential of hydrogen (pH) value, and the stability exhibited in adverse environments such as in the presence of protective reagents.
Prior to the investigation of the type and dosage of the protective agents, the content of impurities in oil and/or aqueous phases should be initially determined by ultrafiltration centrifugation,21 since the encapsulation efficiency of the drug in the emulsion cannot reach the levels of 100%.22,23 This determines the addition of protective agents and narrows the investigation scope of the study. The factors that promote the generation of impurities of NMP can be identified, and the effective ways to protect NMP in emulsion can be implemented by comprehensive analysis of the chemical structure of NMP and impurity A, as well as the results of the destructive testing. The protective methods provide guidance for all NMP preparations and for the control of impurities in the case of oxidizable drugs.
Materials and methods
Materials
Materials included NMP (purity was ≥98.9%); NMP emulsion (in-house production); sodium bisulfite, sodium thiosulfate and sodium metabisulfite (Guangzhou Chemical Reagent Factory); amino acids (Shanghai McLean Biochemical Technology Co., Ltd.); sodium hydroxide solution (0.1 mol/L; laboratory made); hydrochloric acid solution (0.1 mol/L; laboratory made); acetic acid solution (0.1 mol/L; laboratory made); ethylenediamine solution (0.1 mol/L; laboratory made); potassium hydroxide solution (0.1 mol/L; laboratory made); distilled water (Guangzhou Watsons Food & Beverage Co., Ltd.) and methanol and acetonitrile (German Merck Company). Methanol and acetonitrile were of high-performance liquid chromatography (HPLC) grade. All other chemicals were of analytical grade.
Methods
Destructive testing of NMP emulsion and NMP API
A total of 1 mL of NMP emulsion was treated with 1 mL of hydrochloric acid solution (1 mol/L), 1 mL of sodium hydroxide solution (1 mol/L) and 1 mL hydrogen peroxide solution (3%) at 90°C for 10, 15, 20, 25, 30 and 35 min, respectively, in order to identify the degradation rate of NMP emulsion under different destructive conditions. A total of 1 mL of NMP emulsion was respectively destroyed under temperature conditions of 120°C at the time periods of 1, 2, 3, 4, 5 and 6 h and under 4500 Lx (LS-3000 light tester; Beijing Star Tech Co., Ltd.) at the time periods of 6, 6.5, 7, 7.5, 8 and 8.5 h.
Following destruction, the samples that were treated under acid and base stress conditions were neutralized with additions of the same volume and concentration of base and acid prior to the HPLC-ultraviolet (UV; Agilent 1260, G1311C quaternary pump; G1329B standard autosampler; G1314F variable wavelength UV detector) analysis. Subsequently, the destructive conditions at which the peak area of NMP decreased by 10% were applied to the NMP API. The chromatographic conditions that were set up in the preliminary study for the determination of impurities are as follows. The chromatographic column used in the present study was a ZORBAX SB-C18 (4.6×150 mm, 5 μm; Agilent). Water, acetonitrile and methanol were used as mobile phase constituents at a ratio of 40:45:15. All separations were conducted at a column temperature of 40°C using a 1.0 mL/min flow rate and a 10 μL injection volume. The data were acquired at 227 nm, and the total run time was 30 min.
Study of pH value
According to the instructions of the manufacturer’s product specifications in the “Destructive testing of NMP emulsion and NMP API” section, NMP could be destroyed in basic conditions; thus, the adjustment of the pH value was a prerequisite for the reduction of the exposure to a basic environment. The NMP emulsion was acidic during preparation, and sodium hydroxide solution was notably used in order to adjust the pH to basic levels, since the pH value of the emulsion would decrease by 1–2 units following sterilization, whereas a low pH value would result in stratification of emulsion. Consequently, the pH values of the aqueous phase, coarse emulsion and final emulsion were adjusted to 7.0, 7.2, 7.5 and 8.0, with the addition of sodium hydroxide solution (0.1 mol/L) using a pH meter (pHS-2F; ShanghaiRex-Chongyi Instrument Co., Ltd.). The amount of impurities of emulsion was measured in order to determine the optimum pH value of these three stages.
Ultracentrifugation of NMP emulsion
A total of 0.5 mL of NMP emulsion prior to sterilization was added into ultrafiltration tubes (molecular weight cutoff of 10 K) and centrifuged at 4,000 rpm/min for 10 min in a high-speed centrifuge (3–30 K; German Sigma Corporation). Following centrifugation, an equal volume of distilled water was added in the filter tube according to the volume of the aqueous phase produced in the centrifugal casing. The centrifugation process was repeated four times. The aqueous phase comprised the supernatant in the centrifugal casing, and the oil phase was retained in the filter tube. Finally, the same volume of distilled water was added in the oil phase and mixed well to ensure that the concentration of NMP in the oil phase did not change. The oil and aqueous phases were sealed in ampoules under high temperature and pressure in order to remain sterile. The NMP emulsion following sterilization was also further centrifuged to separate the oil and water phases. The contents of impurities of the oil phase, aqueous phase and emulsion prior to and following sterilization were sequentially measured by HPLC-UV.24
Study of antioxidants
According to the “The Latest National Standards Handbook of Pharmaceutical Excipients”, the commonly used antioxidants include sodium bisulfite, sodium metabisulfite, sodium thiosulfate and amino acids. Their corresponding dosages are usually in the range of 0.10%–0.20% (m/v%). The method was conducted according to the “Ultracentrifugation of NMP emulsion” section, and each antioxidant was successively added into the aqueous phase at a dosage of 0.1% (m/v%) in order to prepare the NMP emulsions. The corresponding impurities were measured in order to select the most suitable antioxidant.
The pH value required for the emulsion process that contained sodium bisulfite, sodium metabisulfite or sodium thiosulfate was adjusted according to the “Study of pH value” section. Since amino acids possess –NH2 and –COOH groups and exhibit a cushioning effect, the method provided in the “Study of pH value” section may not be fully applicable. The fluctuation in the pH value from the aqueous phase to the coarse emulsion and to the final emulsion was determined based on the emulsion containing methionine. This measurement aimed to elucidate how emulsions containing amino acids can modify the pH value. Subsequently, the pH of the aqueous phase was adjusted with additions of sodium hydroxide solution (0.1 mol/L) and/or hydrochloric acid solution (0.1 mol/L) according to the acidic and/or basic characteristics of the amino acid used.
Study of pH-adjusting agent and pH value
pH-adjusting agent
Based on the reference to “Recent National Standards Handbook of Pharmaceutical Excipients”, the pH-adjusting agents are separated into two types, including inorganic and organic compounds. The compounds sodium and potassium hydroxide were selected as inorganic bases, whereas ethylenediamine was selected as an organic base. In addition, hydrochloric acid was selected as an inorganic acid and acetic acid as an organic acid. The aforementioned compounds were used to adjust the pH value of NMP emulsion, since they fulfilled the following requirements: miscibility with water, withstand of high temperature and pressure, steam sterilization and applicability in injection.25
Cysteine (acidic amino acid) was used as an example, and three identical NMP emulsions were prepared by pH adjustment with sodium hydroxide, potassium hydroxide and ethylenediamine solutions, respectively. A second methodology utilized histidine (basic amino acid), and two identical NMP emulsions were prepared by pH adjustment with hydrochloric acid and acetic acid solutions. All the solutions of the pH-adjusting agents were used at the concentration of 0.1 mol/L. The content of impurities in the emulsions was measured in order to determine the suitability of the pH-adjusting agents.
pH value
Based on the results presented in the “Study of antioxidants” section, leucine, serine, asparagine, proline and lysine exhibited optimal antioxidative effect among the antioxidants investigated. According to the acidic and/or basic characteristics of these five amino acids, the pH of the aqueous phases was adjusted to 7.0, 7.2, 7.5, 8.0 and 8.5, respectively, by addition of acetic acid (1 mol/L) and/or ethylenediamine solutions (1 mol/L). The optimum pH range was determined via the measurement of the impurities of emulsions.
Study of compound antioxidants
Based on the aforementioned studies, the selection of the optimal antioxidants required for impurity reduction in emulsions was focused on lysine and leucine.
The amino acids were added into the NMP emulsion at a ratio of 1:1 and at a total dosage of 0.05%, 0.1% and 0.2%, respectively. According to the amount of impurities in the NMP emulsion, the optimal total dosage was used in order to further study the antioxidative effects of the two amino acids under nine different ratios.
Determination of content of impurities in NMP emulsion
Following a series of research, the best method to reduce the content of impurities in the NMP emulsion was as follows: the addition of 0.04% lysine and 0.06% leucine in the aqueous phase and the adjustment of the pH value to the range of 7.5–8.0 with acetic acid solution (0.1 mol/L). In order to demonstrate the reproducibility and efficiency of this protective method, six NMP emulsions were prepared using the aforementioned method and their content of impurities was measured. The relative standard deviation (RSD) values of the content of impurities A and C were calculated.
Results
Destructive testing of NMP emulsion and NMP API
Following a series of destructive testing, the destructive principle of NMP emulsion under each condition was obtained and is summarized in Figure 1. The data indicated that the degradation rate of NMP in the emulsion reached a plateau following a decrease by 10% of the peak area of the NMP, in response to basic conditions. The conditions that could cause a decrease by 10% in the peak area of NMP prior to and following the destruction were as follows: 1) acid: 1 mL hydrochloric acid solution (1 mol/L) was used for destruction at 90°C at a time duration of 25 min; 2) base: 1 mL sodium hydroxide solution (1 mol/L) was used for destruction at 90°C at a time duration of 20 min; 3) oxidation: 1 mL hydrogen peroxide solution (3%) was used for destruction at 90°C at a time duration of 25 min; 4) temperature: destruction at 120°C for 4 h and 5) light: destruction at 4500 Lx for 8 h (Figure 2). The aforementioned destructive characteristics of acidic and basic conditions, oxidation, temperature and light were applied to NMP API, and its degradation rate was 15.31%, 51.5%, 15.51%, 11.14% and 12.89%, respectively. The degradation rates observed were considerably greater than those noted during NMP emulsion, notably in the presence of a basic environment. Therefore, a preliminary estimate could be drawn regarding the basic condition that would accelerate the degradation of NMP and exert a significant influence on the generation of the impurities.
  | Figure 2 Destructive principle of NMP emulsion: (A) acid, (B) base, (C) oxidation, (D) temperature and (E) light. |
Study of pH value
With the change in the pH value of the aqueous phase, coarse emulsion and final emulsion, the contents of impurity A and impurity C in emulsion varied. The data indicated that the generation of impurities could be effectively controlled when the pH value was adjusted to 7.5, 7.2 and 7.0 for the aqueous phase, coarse emulsion and final emulsion, respectively. Following statistical analysis, the consumption of sodium hydroxide solution (1 mol/L) was estimated to be 1 mL (Figure 3).
  | Figure 3 The effect of pH value of aqueous phase/coarse/final emulsions on the impurities A and C: (A) impurity A and (B) impurity C. |
Ultracentrifugation of NMP emulsion
The increased percentage of impurity A in the aqueous phase was greater compared with that noted in the oil phase, as demonstrated by the increased percentage of impurities of oil and aqueous phases prior to and following sterilization. Consequently, the degradation of NMP notably occurred in the aqueous phase, and it was suggested that the antioxidants should be added in the aqueous phase in order to facilitate the protection of NMP (Table 1).
  | Table 1 The increased percentage of impurities of oil phase/aqueous phase/emulsion prior to and following sterilization |
Study of antioxidants
Sodium bisulfite, sodium metabisulfite and sodium thiosulfate
Following sterilization, the emulsion with sodium thiosulfate as an antioxidant was stratified since the latter was the only soluble compound in the basic condition. The pH value of NMP emulsion following sterilization reduced to a value of ~5, whereas the acidic environment caused precipitation of the antioxidant that resulted in the stratification of emulsion. Thus, an unexpected result was observed regarding the content of impurity A that was greater than the emulsion in the absence of antioxidant compared with the antioxidative effect of sodium bisulfite and sodium metabisulfite. The consumption of sodium hydroxide solution (1 mol/L) was estimated to range between 4 and 5 mL (Table 2).
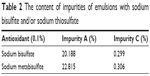  | Table 2 The content of impurities of emulsions with sodium bisulfite and/or sodium thiosulfate |
Amino acids
The pH value of the emulsion with methionine was reduced to 0.2, following sterilization compared with that noted in the aqueous phase that demonstrated that the amino acid acted as a buffer. Taken collectively, the findings suggested that the minimum amount of impurity was produced when the pH value of the final emulsion was adjusted to 7.0. As a result, the emulsion with methionine was solely required to adjust the pH value of the aqueous phase to 7.2, which ensured that the emulsion retained a stable state during the preparation (Table 3).
  | Table 3 The fluctuation of pH value of emulsion in the presence of methionine and in the absence of amino acids |
Preliminary experiments indicated that the content of impurities of emulsions containing different amino acids was calculated at the fixed amount of 0.1%. Statistical analysis revealed that the consumption of sodium hydroxide solution was ~0.2–0.5 mL, while five out of 21 amino acids exhibited favorable antioxidant effects, including leucine, serine, asparagine, proline and lysine (Table 4).
  | Table 4 The amount of impurities in emulsions containing different amino acids |
Study of pH-adjusting agent and pH value
pH-adjusting agent
The content of impurities of the emulsion that contained cysteine depended on the different types of pH-adjusting agents that were used. The results indicated that the content of impurities of emulsion using sodium hydroxide and/or potassium hydroxide solutions as pH-adjusting agents was similar, but this effect was greater compared with that noted with ethylenediamine solution. The content of impurities of the emulsion that contained histidine and hydrochloric acid solution as pH-adjusting agents was greater compared with that noted using acetic acid solution. The hydroxyl group in the molecule of sodium hydroxide and/or potassium hydroxide promoted the degradation of NMP, while the combination of the organic base and the acid was more suitable for the adjustment of the pH value compared with the combination of the inorganic base and the acid (Table 5).
  | Table 5 The content of impurities of emulsions in the presence of different pH-adjusting agents |
pH value
The content of impurities of emulsions was affected by the antioxidants and the pH value. The use of lysine and/or leucine as antioxidants reduced the content of the impurities of emulsion to minimal levels when the pH value of the aqueous phase was adjusted to a range of 7.5–8.0 (Figure 4).
  | Figure 4 The content of impurities of emulsion containing different amino acids under different pH values: (A) impurity A and (B) impurity C. |
Study of compound antioxidants
The effects of the total dosage of antioxidants and the proportion of lysine and leucine in the impurities of the NMP emulsion were investigated under a pH range of 7.5–8.0. The contents of impurity A and impurity C were <0.5% when the total dosage of lysine and leucine was 0.1%. The proportion of lysine and leucine was investigated at the dosage of 0.1%, and the results indicated that the content of impurity A was different, whereas the content of impurity C was approximately constant. The contents of impurity A and impurity C exhibited the lowest percentages at a 4:6 proportion of lysine and leucine that was in compliance with the regulation regarding single impurities of the “Technical guidelines to research impurities of chemicals”26 (Table 6).
  | Table 6 The content of impurities of emulsions using lysine and leucine as antioxidants |
Determination of content of impurities in NMP emulsion
As shown in Table 7, the contents of impurities A and C in six NMP emulsions are <0.5%, which proved that this method could play an excellent role in the protection of NMP emulsion. The RSD values of the contents of impurities A and C were both <2%, which demonstrated that the reproducibility of this protective method was good.
  | Table 7 The content of impurities A and C in six NMP emulsions |
Discussion
NMP emulsion exhibits a broad commercial application with regard to the high incidence of cerebrovascular disease, due to the optimal clinical benefit by NMP and the advantages conferred by the emulsion process. The control of the impurities of the NMP emulsion is considered a critical and laborious task. In contrast to previous studies that examined the quantitative determination of NMP impurities, the present study investigated the factors that are associated with the production of impurities and highlighted protective methods for their control.
The differences between NMP and impurity A were analyzed with regard to the chemical structures of these compounds, and the results indicated that the production of impurity A by NMP was based on an oxidation reaction.
In order to identify the conditions that would facilitate the degradation of NMP, the destructive testing of NMP emulsion and NMP API were conducted. The degradation rate of NMP in the emulsion was proportional to the destructive time under the basic conditions (Figure 2). The degradation rate of NMP API was >10%, notably under the basic conditions that resulted in a decrease in emulsion by 10%. This indicated that the emulsion played a protective role for NMP via the retention of the latter in the oil phase. Thus, it could be concluded that the degradation of NMP was accelerated by oxidative and basic conditions.
Based on the aforementioned findings, the protection of NMP was conducted by two methods. First, the pH values of the aqueous phase, coarse emulsion and final emulsion were adjusted in order to reduce the explosion of NMP emulsion to an adverse pH environment.
The minimum content of impurities was obtained when the pH values of the aqueous phase, coarse emulsion and final emulsions were adjusted to 7.5, 7.2 and 7.0, respectively, which indicated that when the environment was stabilized at or close to a neutral state, the emulsion would protect NMP from degradation in the presence of sodium hydroxide solution (Figure 3).
Second, antioxidants were added for the reduction of the extent of the oxidative environment. NMP was degraded in the aqueous phase of emulsion, as demonstrated by ultracentrifugation of the NMP emulsion, whereas antioxidants were soluble in the aqueous phase. Sodium bisulfite, sodium thiosulfate, sodium metabisulfite and amino acids are water-soluble antioxidants and have been taken into consideration in this research as stated in the The Latest National Standards Handbook of Pharmaceutical Excipients. The content of impurities of emulsion with sodium bisulfite and/or sodium metabisulfite was considerably greater compared with the emulsions that contained amino acids as antioxidants (Tables 2 and 4). A possible explanation for this result could be that an approximate volume of 4–5 mL (0.1 mol/L) of sodium hydroxide solution was required for the emulsion containing sodium bisulfite and/or sodium metabisulfite when the pH values of the aqueous phase, coarse emulsion and final emulsion were adjusted to 7.5, 7.2 and 7.0, respectively. With regard to the emulsions containing amino acids, the volume of sodium hydroxide solution used to adjust the pH was approximately estimated at the range between 0.2 and 0.5 mL, since amino acids exhibited buffering action.
The antioxidative effect of the amino acids was attributed to the amino group (–NH2),27 yet the effect noted was uneven, and among the various amino acids tested, leucine, serine, asparagine, proline and lysine demonstrated the best antioxidative effect. Analysis of the content of impurities in NMP emulsion in the presence of different amino acids demonstrated that the difference in electron density of the amino group (–NH2) could result to various antioxidative effects. The higher the density, the better the effect and the less the production of impurities in the NMP emulsion.
With regard to the electron density analysis of the amino group (–NH2), the 21 amino acids were classified into two categories according to the properties of the side groups, namely, electron-donating and electron-withdrawing groups. The side groups of aspartate and glutamate were electron-withdrawing group due to the presence of the carboxyl group, and the electron density of the amino group (–NH2) was low, resulting in a weak antioxidative effect. However, the effect of glutamate was greater compared with aspartate, since the length of the intermediate chain between the chiral carbon and the side group was different. The electron-withdrawing effect of carboxyl group was weakened, due to the length of the intermediate chain of glutamate that was longer compared with that of aspartate.
The remaining amino acids could be further classified into four subcategories based on the length of the intermediate chain. First, with regard to glycine, alanine, serine and threonine, no connecting links were present, but the hydroxyl group was more potent than the alkyl group and the side groups of serine and threonine were branched. As a result, the antioxidative effect of the amino acids could be categorized in terms of potency as follows: threonine > serine > alanine > glycine. Second, the intermediate chain of the amino acids methionine and glutamine exhibited two carbon atoms and the strength of the electron-donating groups was similar, resulting in a similar potency of antioxidative effect. Furthermore, the intermediate chain of the amino acids arginine, lysine and proline exhibited three or more carbon atoms, and although the electron-donating potency of the lysine amino group was greater than that of the arginine guanidine group, the intermediate chain of lysine was longer than that of arginine, which resulted in a similar antioxidative effect. With regard to proline, the intermediate chain formed a ring with the amino group that facilitated the delivery of electrons. In addition, the intermediate chain of the remaining amino acids contained one carbon atom and their antioxidative effect could be analyzed in a similar way.
The volume of the side group of tryptophan, phenylalanine, histidine and tyrosine was considerably large that blocked the transmission of electron clouds, whereas cysteine exhibited the lowest antioxidative effect compared with the other amino acids due to its instability.
Following the aforementioned analysis, four major factors that may affect the electron density of the amino group (–NH2) were confirmed and summarized as follows:
- The properties of the side groups: the electron density of the amino group (–NH2) increased in the presence of an electron-donating side group. The potency of the common electron-donating groups was as follows: amino, hydroxy, alkoxy > ester group and aminoacyl, guanidino > alkyl group. The electron density of the amino group (–NH2) decreased in the presence of an electron-withdrawing group, such as carboxyl, aldehyde and acyl groups.
- The length of the intermediate chain between the chiral carbon and the side group: the electron-donating effect was weakened, as the length of the chain increased. In the case of no connecting link (the shortest), the effect was substantially reduced.
- The branch of the side group: the electron density around the amino group (–NH2) increased when the side group was branched.
- The volume of the side group: the larger volumes of the side group exhibited greater steric hindrance, which could in turn block the transmission of the electron cloud.
The dosage of sodium hydroxide solution in the NMP emulsion appeared to correlate with the content of impurities, as demonstrated by the analysis of the two protective methods (Table 8). The amount of impurities may be affected by the addition of sodium hydroxide solution, but the use of organic lysine may control the production of impurity A by increasing the pH value of the emulsion. As a result, the basic environment influenced the production of impurities, while it depended on the increase in the pH value of the emulsion.
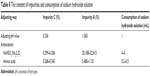  | Table 8 The content of impurities and consumption of sodium hydroxide solution |
The ethylenediamine compound in combination with potassium hydroxide was selected as a pH-adjusting agent for the comparison with sodium hydroxide. Both compounds produced base by their corresponding –NH2 and –OH functional groups. The basic conditions produced by sodium hydroxide and/or potassium hydroxide promoted the generation of impurities, while this effect was not observed in the presence of ethylenediamine. The data further demonstrated that the destructive effect was associated with the free hydroxyl group (Table 5). These findings indicated that the preliminary estimation regarding the accelerated degradation of NMP by the basic conditions was invalid, since the basic reagent used in the destructive testing was the sodium hydroxide solution. This conclusion suggested that other type of reagents should be used for verification during acidic, basic or oxidative destruction in order to establish a complete understanding of the factors that affect the drug degradation.
The organic base was shown to possess greater efficacy in stabilizing the NMP compared with the inorganic base, as demonstrated by the results obtained from the experiments with sodium hydroxide, potassium hydroxide and ethylenediamine. In order to confirm this hypothesis, the comparison between hydrochloric acid (inorganic acid) and acetic acid (organic acid) as pH-adjusting agents was conducted, and the data demonstrated that the organic acid was more efficacious for the stability of NMP compared with the inorganic acid. Consequently, the use of organic compounds as pH-adjusting agents was more suitable for NMP emulsion.
The five amino acids that exhibited the greatest antioxidant efficacy, namely, leucine, serine, asparagine, proline and lysine, as demonstrated by the follow-up study, were selected in the presence of ethylenediamine (0.1 mol/L) and/or acetic acid solutions (0.1 mol/L) in order to adjust the pH value according to the acidic and/or basic characteristics of the amino acids. The emulsion with leucine or lysine exhibited the least amount of impurities under the pH range of 7.5–8.0, which demonstrated that the basic condition was incapable of promoting the degradation of NMP (Figure 4).
Following the selection of lysine and leucine as compound antioxidants for the NMP emulsion, studies were conducted in order to determine the effective dose and proportion required for the emulsion process. The content of impurities was the lowest when the total amount of antioxidants was used at a concentration of 0.1% (m/v%) and the proportion of lysine and leucine was 4:6 (Table 6).
An appropriate antioxidant and pH-adjusting agent should be selected according to the chemical structure and destructive testing of the drug in order to exert the optimal protective effect.
Conclusion
The degradation of NMP in emulsion was inherently connected to the oxidative conditions, notably in the aqueous phase and/or occurred in the presence of an inorganic pH-adjusting agent and antioxidants. The impurities of NMP emulsion could be controlled by the addition of suitable antioxidants in the aqueous phase and the adjustment of the pH value with organic base (ethylenediamine) and/or acid (acetic acid). Organic antioxidants, notably the amino acids with appropriate length of intermediate chain and electron-donating side groups, revealed optimal antioxidant effects compared with inorganic antioxidants. Consequently, the addition of 0.04% lysine and 0.06% leucine in the aqueous phase and the adjustment of the pH value to the range of 7.5–8.0 with acetic acid solution (0.1 mol/L) were highlighted as the best protective method for the NMP emulsion. The aforementioned findings suggest an important guidance for all NMP preparations, as well as other oxidizable drugs, to control the production of impurities.
Acknowledgments
The project was supported by the Major Scientific and Technological Project of Guangzhou Technology Bureau (Item number: 201300000046), Natural Sciences Fund of the First Affiliated Hospital of Guangdong Pharmaceutical University (Item number: GYFYLH201310) and two scientific and technological projects of Guangdong province (Item numbers: 2013B021800091 and 201508010036).
Disclosure
The authors report no conflicts of interest in this work.
References
Wang Y, Xu Y, Zhang X, et al. Calcium channel blockers of clinical application and adverse reactions. Chin J Mod Appl Pharm. 2015;32(3):385–390. | ||
Chen W. How patients with cerebrovascular disease choose antihypertensive drugs. Fam Med. 2015;3:44–45. | ||
Li S, Wang W, Li C, et al. Progress in quality of nimodipine and its preparations. Central South Pharm. 2012;10(9):689–691. | ||
Huang C, Su L. Common causes of adverse reaction to intravenous drip of nimodipine injection in patients and its nursing countermeasures. Nurs J Chin PLA. 2010;27(7):519–520. | ||
Wei X, Tan Q, Li H. The test for compatibility for nimodipine and its adsorption on infusion sets. Chin J New Drug. 2002;11(1):80–81. | ||
Chingunpituk J. Nanosuspension technology for drug delivery. Walailak J Sci Tech. 2007;4(2):139–153. | ||
Chen T, Hui M, Fu J, et al. Progress in fat emulsion of pharmaceutical preparations. Digest World Core Med J Ophthalmol. 2004;3(5):1295–1297. | ||
Liang X, Zhao G, Liu H, et al. Application and recent research of lipid emulsion injection. World Chin Med. 2015;10(3):327–330. | ||
Yang F, Lu L, Qin L, Inventors. Guangdong Pharmaceutical University, Assignee. One kind of nimodipine fat emulsion injection and its preparation method. China Patent Office: CN103893119A, 20140702. | ||
Alsante KM, Huynh-Ba KC, Baertschi SW, et al. Recent trends in product development and regulatory issues on impurities in active pharmaceutical ingredient (API) and drug products. Part 2: safety considerations of impurities in pharmaceutical products and surveying the impurity landscape. AAPS Pharm Sci Tech. 2014;15(1):237–251. | ||
Xie M. Some opinions on the studies of impurities in generic drugs. Chin J Pharm. 2013;44(11):1174–1183. | ||
Lei Y, Song L, Jiang Q. Recent development in the application of liquid chromatography-mass spectrometry in analysis of drug-related impurities. Modern Instrum. 2011;4(11):9–13. | ||
Yu Q, Ni K, Wang Z. Determination of drug-related impurities by chromatographic method. Prog Pharm Sci. 2000;24(5):270–274. | ||
European Directorate for the Quality Control of Medicines. European Pharmacopoeia. 7th ed. European Directorate for the Quality of Medicines. France. 2006:2578–2579. | ||
Luo J, Liu H, He L, et al. HPLC determination of impurities of nimodipine and its preparation. Chin J Pharm Anal. 2007;27(7):1039–1042. | ||
Peng D, Huang K, Liu Y, et al. Determination of nimodipine content and impurities in nimodipine for injection by RP-HPLC. West China J Pharm Sci. 2006;21(2):194–195. | ||
Dong L, Qiu Y, Hu R. Determination of nimodipine tablet and impurities by HPLC. Chin Pharm Affairs. 2006;20(1):48–50. | ||
Zhang B. The Synthesis of Nimodipine Impurity in EP and the Process Control of Impurity. Jinan: Shandong University; 2012. | ||
Xie M. Some opinions on the studies of impurities in generic drugs. Chin J Pharm. 2013;44(11):1174–1183. | ||
Cheng H. Effects of stressing test for determination of impurities during validation of the analytical procedure. Chin J New Drug. 2008;17(17):1548–1549. | ||
Tian L, Tang X, He H, et al. Preparation and in vitro and in vivo evaluation of etoposide submicro-emulsion for intravenous injection. Acta Pharm Sin. 2007;42(8):892–897. | ||
Zhang G. Preparation and In Vivo Studies of Ibuprofen Fat Emulsion. Shanghai: Second Military Medical University; 2014. | ||
Zhang X. Study of Silibinin Fat Emulsion. Chengdu: Chengdu University of Traditional Chinese Medicine; 2011. | ||
Chen JP, Rong R, Li J, et al. Formulation optimization and characterization of nimodipine submicron emulsion for intravenous injection. Chin J Pharm. 2008;7(12):7770–7780. | ||
Xiao S. The Latest National Standards Handbook of Pharmaceutical Excipients. Beijing: Chinese Medical Science and Technology Electronic Press; 2006:47. | ||
GPH3-1. Technical Guidelines to Research Impurities of Chemicals. Beijing: State Food and Drug Administration; 2005. | ||
Lu Y, Liu J. Organic Chemistry. Beijing: People’s Health Publishing House; 2013:112. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
