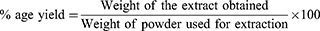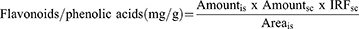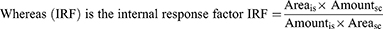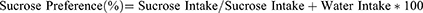Back to Journals » Drug Design, Development and Therapy » Volume 16
Methanolic Extract of Aerva javanica Leaves Prevents LPS-Induced Depressive Like Behavior in Experimental Mice
Authors Arshad HM , Ahmad FUD , Lodhi AH
Received 22 September 2022
Accepted for publication 29 November 2022
Published 7 December 2022 Volume 2022:16 Pages 4179—4204
DOI https://doi.org/10.2147/DDDT.S383054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Hafiza Maida Arshad, Fiaz-ud-Din Ahmad, Arslan Hussain Lodhi
Department of Pharmacology, Faculty of Pharmacy, the Islamia University of Bahawalpur, Bahawalpur, Pakistan
Correspondence: Fiaz-ud-Din Ahmad, Department of Pharmacology, the Islamia University of Bahawalpur, Pakistan Khawaja Fareed Campus, Railway Road, Bahawalpur, 63100, Pakistan, Tel +92-320-8402376, Email [email protected]
Aim: Depression is a chronic recurrent neuropsychiatric disorder associated with inflammation. This study explored the pharmacological activities of Aerva javanica leaves crude extract (Aj.Cr) on lipopolysaccharide (LPS)-induced depressive-like behavior in experimental mice.
Methods: Aj.Cr was evaluated for its phenolic and flavonoid contents, bioactive potential, amino acid profiling and enzyme inhibition assays using different analytical techniques followed by in-silico molecular docking was performed. In addition, three ligands identified in HPLC analysis and standard galantamine were docked to acetyl cholinesterase (AchE) enzyme to assess the ligand interaction along with their binding affinities. In in-vivo analysis, mice were given normal saline (10 mL/kg), imipramine (10 mg/kg) and Aj.Cr (100, 300, and 500 mg/kg) orally for 14-consecutive days. On the 14th day, respective treatment was given 30-minutes before intra-peritoneal administration of (0.83 mg/kg) LPS. Open field, forced swim and tail suspension tests were performed 24-hours after LPS injection, followed by a sucrose preference test 48-hours later. Serum corticosterone levels, as well as levels of nitric oxide (NO), malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), tumor necrosis factor-alpha (TNF-), interleukin-1β (IL-1β), interleukin-6 (IL-6), brain-derived neurotrophic factor (BDNF) and catecholamines were determined in brain tissues.
Results: In-vitro results revealed that crude extract of Aj.Cr possesses anti-depressant agents with solid antioxidant potential. In-vivo analysis showed that LPS significantly increased depressive-like behavior followed by alteration in serum and tissue biomarkers as compared to normal control (p < 0.001). While imipramine and Aj.Cr (100, 300, and 500 mg/kg) treated groups significantly (p< 0.05) improved the depressive-like behavior and biomarkers when compared to the LPS group.
Conclusion: The mitigation of LPS-induced depressive-like behavior by Aj.Cr may be linked to the modulation of oxidative stress, neuro-inflammation and catecholamines due to the presence of potent bioactive compounds exerting anti-depressant effects.
Keywords: Aerva javanica, depression, lipopolysaccharide, oxidative stress
Introduction
Depression, a chronic recurrent illness, progressively affects the quality of life, leading towards increased morbidity and mortality.1 Based on monoaminergic theory, the depletion of amines (serotonin, nor-adrenaline, and dopamine) among brain tissues lead towards pathogenesis of depression.2 The current treatment with anti-depressants focuses on monoamine oxidase inhibitors (MAOIs) as the drug of choice to regulate the monoaminergic activity in the CNS to relieve the symptoms of depression.3 However, literature archives further emphasize that deterioration of the hippocampal region, followed by reduced neurogenesis, is directly correlated to depression. Along with the proven role of the mono-aminergic system, there is increasingly high evidence considering the involvement of ongoing inflammatory processes, neurotrophic factors and oxidative stress as key factors involved in depression.4 Production of cytokines such as IL-1β, IL-6, and TNF-α are initiated in response to tissue injury or psychosocial elements and homeostatic mechanisms that increases their levels.5 However, chronic stress exposure disrupts the existing balance of cytokines,6 leading towards activation of the kynurenine pathway; tryptophan, the principal amino acid constituent of serotonin, is depleted, and neuro-modulators (serotonergic metabolites) are produced which can modify the dopamine and glutamate control via regulating the serotonin receptors.7 Inflammatory mediators strongly affects the cholinergic pathway situated in the basal ganglia and anterior cingulated cortex of the brain, ultimately causing a significant modification of motor function, appetite, anxiety, emotions and distress.8 Exogenous inflammatory agents such as LPS, a substantial element from the cell wall of Escherichia bacteria, effectively induce immune reactions.9 Peripheral systemic single dose of LPS had been shown to produce neuro-inflammation by stimulating the adequate generation of inflammatory mediators (IL-1 β, IL-6 and TNF- α) and antioxidant enzymes10,11 resulting in cognitive deficits.12 Previously, it was claimed that compounds with significant antioxidant capabilities, such as ascorbic acid, beta-carotene, rosmarinic acid and caffeic acid, could have comparable anti-depressant effects with fewer adverse effects than regularly prescribed anti-depressants like fluoxetine and imipramine.13–16 Furthermore, the therapeutic benefits of newly developed synthetic anti-depressants are also accompanied by significant side effects.17 All these factors promote the importance of auxiliary remedies with fewer negative impacts. Aerva javanica (Burm.f.) Juss. Ex-Schult. a member of family Amaranthaceae, is a shallow rooted herb with dioecious flowers, found in Asia, Africa, America, and also scattered in the different areas of the World with 20-species of Genus Aerva are also located in Pakistan and India.18 There have recently been studies of a number of Aerva species having antioxidant, analgesic, anti-bacterial, anti-inflammatory and cytoprotective activities. Traditionally, it also exhibits anti-hyperglycemic, diuretic, and demulcent potential.19 Polyphenols, terpenoids, flavonoids, and alkaloids have been found during the phytochemical analysis. The flowers of A. javanica had generated four novel ecdysteroids, aervecdysteroid A-D and three new acylated flavone glycosides. Furthermore, the primary components were heptacosane (3-allyl-6-methoxyphenol) and pentacosane from javanica seeds oil.20 Using this background information, we employed a experimental model of neuro-inflammation associated depression by intraperitoneal administration of LPS in mice to assess the influence of the crude extract of Aj.Cr measuring parallel biochemical alterations during the study.
Materials and Methods
Chemicals
All the substances utilized during the entire research were of analytical quality. CAT, GSH, MDA, SOD enzyme assay kits, and TNF-α ELISA kits from (Elabsciences Biotechnology, USA) were purchased. In addition, IL-1β (ELISA kit, Bioassay Technology Laboratory), IL-6 (ELISA kit, International Immuno-Diagnostics, USA), LPS harvested from Escherichia coli, strain 055:B5 (Sigma Aldrich, USA), reference standards applied for the determination of serotonin, dopamine, nor-epinephrine (Sigma Aldrich, Purity >99%), imipramine hydrochloride (Sigma Aldrich Corp., St. Louis, MO), xylazine (Prix Pharmaceuticals, Pakistan) and ketamine (Global Pharmaceuticals, Pakistan) were bought and consumed throughout the experimental protocol.
Collection of Plant Material
The leaves of A. javanica were taken from the local market of Bahawalpur, followed by identification from an authentic Botanist, Department of Botany, the Islamia University of Bahawalpur. Finally, a sample of leaves was subjected for shade drying and submitted to the herbarium section of the Pharmacology laboratory with voucher number (AJ-LE-06-14-79) for future reference.
Crude Extract Preparation
The coarse powder of crisped leaves of A. javanica weighing 1000 grams was dipped into methanol and water mixture having ratio (70:30) for 72-hours, followed by filtrate collection. This marc obtained after first soaking was processed through further filtrations repeating the procedure mentioned earlier. Then, the filtrate achieved was subjected to the process of evaporation using Heidolph Rotary Evaporator at 37°C until a thick, dense paste of crude extract was obtained.21 The crude extract of leaves of A. javanica (Aj.Cr) was weighed to establish the % age yield:
Phytochemical Analysis
Preliminary Phytochemical Screening
Methanolic crude extract of Aj.Cr was extensively analyzed to confirm the existence of primary as well as secondary phytoconstituents such as alkaloids, saponins, flavonoids, anthraquinones, coumarins, glycosides, tannins, and terpenes by following the previously described protocols.22
Quantification of Total Phenolic and Flavonoid Contents
Using standard calibration curve method plotted against gallic acid employed as a reference standard, the total phenolic contents of Aj.Cr extract was approximated using the previously described Folin-Ciocalteu reagent method with slight adjustments.23 Aliquots of test samples (250 μL) of soluble extract solutions were vigorously fused with reagent (1.25mL). About 1.5 mL of sodium carbonate (Na2CO3) solution (7.5%) was added to the mixture after 5-minutes following 2-hours of incubation at room temperature and absorbance at a wavelength of 765 nm were measured. In terms of gallic acid equivalent per gram of dry crude extract, the total phenolic contents were represented as mg GAE/g. The colorimetric aluminum chloride assay procedure determined the total flavonoid concentrations of the Aj.Cr extract. Each diluted sample received 1.5 mL (2%) of aluminum chloride (AlCl3), which was added, vortexed, and incubated for the following 60-minutes at room temperature. A spectrophotometer (Biolab-310, Biolab Scientific Ltd. Canada) set at 415 nm was used to measure the absorbance. A calibration curve was drawn employing quercetin as a reference standard. The amount of quercetin equivalent per gram of dry extract was used to express the total flavonoid contents (mg QE/g).24
Phenolic Acid and Flavonoid Identification and Quantification by HPLC
The device is composed of a detector (SPD-10AV), column C18 (25 cm × 4.6 mm, 5.0 μM), oven, and SIL −20A auto sampler (Shimadzu Scientific Instruments, Japan). It was employed to calculate the amounts of bioactive components in the crude extract of Aj.Cr. Approximately 0.1g of crude extract was re-dissolved in 1mL of methanol, and 10μL of the mixture was fed into the HPLC apparatus. The acetic acid 1% (v/v) in water marked as (solvent A) and ethanol (solvent B) was employed during the analytical procedure for the linear gradient system. Starting with (15%) for first 15-minutes, (45%) for 15 to 30-minutes and (100%) for 30 to 45-minutes made up the gradient elution. The absorbance was measured at 280 nm, and the flow rate was adjusted to 1 mL per minute. The analyte peaks were achieved by comparing the retention periods and UV-spectra of the analyte peaks to those of the reference standards.25 The concentration of phenolic acids and flavonoid contents were calculated using a calibration graph of mass concentration vs peak area. The correlation co-efficient (R2) for each standard curve and the regression equation of the standard for phenolic acids and flavonoids were determined using the MS Excel 2013 program.26,27 The following equation was employed to calculate the concentrations of polyphenols:
is = internal reference standard and sc = separated polyphenols.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS Agilent, Model 7890-B (Santa Clara, CA, USA) coupled with Mass-Hunter acquisition as the software was used to evaluate an extract of Aj.Cr. An ultra-inert capillary system, non-polar column (HP-5MS), and varied ratios of a film with specifications of 30 mm × 0.25 mm ID × 0.25 m constitutes the instrument. Helium was employed as a carrier gas at a flow rate of 1 mL/minute, while the oven temperature was adjusted at 50°C for 5-minutes which was gradually increased to 250°C at the rate of 100°C/minute later, and eventually to 3000 °C for 10-minutes at a rate of 70°C/minute. The metabolites in the Aj.Cr sample were identified by the library of National Institute of Standards and Technology (NIST).28
Amino Acid Analysis
Aj.Cr crude extract (100 mg) was fused with 1 mL of buffer-1 solution provided by the manufacturer and vortexed for 5-minutes. A volume of (20µL) from the sample vial was picked by an auto-sampler of Biochrom 30+ amino acid analyzer (USA) to evaluate the free amino acids profile among the given sample. 5-buffer lithium-based system (Manufacturer Specific) was incorporated as a solvent following reaction time at 135°C following duration of 2-hours.29
In vitro Biological Assays
Antioxidant Assays
The ability of the methanolic extract of Aj.Cr to reduce and scavenge free radicals was examined using antioxidant assays. In antioxidant experiments, Trolox was used as a reference standard. In DPPH, ABTS, and FRAP experiments, the free radical scavenging and reduction potential activities were shown as mg TE/gram (milligrams of Trolox equivalents per gram of crude extract). All the trials were performed in triplicate and the results were averaged.
DPPH Free Radical Neutralizing Assay
About 4 mL of a (0.004%) methanolic solution of DPPH (1,1-diphenyl-2-picrylhydrazyl) was combined with 1 mL of the crude extract solution of Aj.Cr (1 mg/mL). The mixture solution was vortexed following incubation at room temperature for 30-minutes. After adjusting wavelength at 517 nm, absorbance was measured spectrophotometrically. A blank sample was made and examined in accordance with earlier techniques.30
ABTS Assay
To assess the free radical scavenging potential of Aj.Cr, the ABTS (2, 20-azino-bis (3-ethyl-benzo-thiazoline) 6-sulfonic acid) radical cation de-colorization experiment was conducted. In a nutshell, (7 mM) ABTS in water was reacted with (2.45 mM) potassium per-sulfate in a ratio of (1:1) to produce the ABTS+ cation radical, which was then allowed to settle at room temperature for 12–16 hours in the dark before being utilized. Methanol was employed to dilute the ABTS solution to an absorbance of 0.7 (±0.02) at 734 nm. After mixing the sample solution (1 mg/mL; 1 mL) and ABTS solution (2 mL) for 30-minutes at room temperature, sample absorbance was measured at the same wavelength mentioned earlier. A blank sample was also employed for the calculation of absorbance and appropriate comparison.31
FRAP Assay
The main focus of the ferric reducing antioxidant power (FRAP) protocol was the production of the blue-colored Fe2+-tripyridyltriazine complex as a result of the presence of electron-donating phytoconstituents available in Aj.Cr at a low pH. Acetate buffer (0.3 M:pH=3.6), 2,4,6-tris(2-pyridyl)-S-triazine (TPTZ) (10 mM) dissolved in 40 mM HCl, and ferric chloride (20 mM) were combined to create the FRAP reagent (2 mL), and the mixture was then incubated for 30-minutes at room temperature. At 593 nm, the sample absorbance was calculated. Milligrams of Trolox equivalents per gram of crude extract (mg TE/g extract) were used to measure FRAP activity. According to the prescribed protocol, a blank sample without extract was also employed and assessed for comparison.32
Enzyme Inhibition Assay
About 50 µL extract sample (1 mg/mL) combined with 125µL DTNB (5,5-dithio-bis(2-nitrobenzoic acid)) and (25 µL) of acetyl cholinesterase (AchE) solution already dissolved in Tris-HCl buffer (pH=8) were placed in a 96-well microplate and incubated for 15-minutes at 25°C to assess the AchE inhibition. After adding (25µL) of a substrate, either butyryl thiocholine chloride or acetyl thiocholine iodide, the mixture was incubated for 15-minutes. Following the wavelength of 405 nm, the final absorbance of the sample was assessed. During the protocol, galantamine was employed as a reference standard and inhibition of AchE was measured in milligrams of galantamine equivalent per gram of crude extract of Aj.Cr (mg GALAE/g extract).33
In silico Molecular Docking Studies
The conformational interaction between the chemical and enzymatic entity was investigated by computer-aided molecular modeling. The 3D structure of ligand molecules was downloaded from the drug database. AchE crystal structures can be downloaded from the RCSB-PDB protein data bank at http://www.rcsb.org/pdb. Using Open Babel 2.4.1, the structure file (in XML and PDB format) was translated to PDBQT format. The server Auto Dock Vina was used to obtain the necessary hydrogen atoms. For blind docking, auto grid software and related grid data were utilized. All three parameters starting location, orientation, and torsion were randomized. The conformer stability and energy was then decreased before docking to provide the conformer with the lowest energy. A recent study found that Vander Waals interactions, ionic interactions, and hydrogen bonds account for most protein-ligand binds. So, utilizing Biovia/Discovery Studio 2021, they were mainly targeted in this entire protocol.34
In vivo Studies
Animal Handling
Swiss albino male mice (n=60) weighing (25–30g) were used throughout the experimental protocol. Mice were acclimatized for at least a week in the animal house of research laboratory of Department of Pharmacology, Faculty of Pharmacy, the Islamia University of Bahawalpur. All the animals were kept in polycarbonate cages and given regular feed with free water access. The standard housing conditions for all the animals were temperature (23 ± 2°C), humidity > 66%, and an alternate 12 hour light/dark cycle, respectively.
Animal Model
All the animals were divided into 6-experimental groups (n=10) for the entire study protocol as shown in Table 1. All the assigned groups were given their respective treatments orally for 14-days as a part of pre-treatment paradigm. Then, LPS (0.83 mg/kg) was intraperitoneally administered to all groups on the 14th day except for the normal control following 30-minutes after the complementary therapy.35 Analysis of the mice behavior and biochemical markers were assessed following 24–hours of LPS administration. The approval for animal protocol was given by the Pharmacy Animal Ethics Committee with approval number (PAEC/20/10) from the department of Pharmacology, Faculty of Pharmacy; the Islamia University of Bahawalpur. The Care and Use of Laboratory Animals Guide were also consulted and followed strictly for the implementation of every step of this experimental design.
 |
Table 1 Study Design Showing Diet and Treatment Plan for Various Groups |
Acute Toxicity Study
Following the recommendations of Organization for Economic Co-operation and Development (OECD), an acute toxicity analysis was designed with five mice in each group were placed in the control and test groups. Following a 12-hour fasting, the methanolic extract of Aj.Cr was given orally to several groups with graded doses (300, 1000, and 3000 mg/kg). On the first day, all animals were monitored for an hour and continuously observed for the next 14-days. The mortality and behavioral alterations of every experimental animal were tracked. The parameters such as alertness, convulsions, grooming, lacrimation, hyperactivity, sweating, touch response, urination, gripping strength, writhing reflex and corneal reflex were noted at a regular time interval of 0th, 1st, 2nd, 3rd, 4th, 6th, 12th, 24th, 48-hours and at 3rd, 5th, 7th, and 14th days, respectively.36
Behavioral Assays
Behavioral assays mainly constituted the initial experimental procedure. Then, using distinct batches of mice to prevent interference, open field, forced swim, tail suspension and sucrose preference tests were conducted to assess the behavioral changes among several animal groups before and following treatments.
Open Field Test
The open field test was performed to determine whether the methanolic extract of Aj.Cr affects the locomotor activity in mice. Locomotor activity was tracked for 5-minutes by observing the number of line crossings and rearings in the enclosure, separated into 12-virtual quadrants. Following each animal test, the gadget was washed with the 10% alcohol solution and dried to remove any olfactory hints from the preceding animal.37
Forced Swim Test
The apparatus consisted of a transparent Plexiglas cylinder (25cm × 12cm × 25cm) for measuring the desperate behavior of mice using the previously reported method with slight adjustments.38 It was filled with water (24 ± 1°C) at 15cm depth. After administering LPS for 24-hours, each animal was individually placed in a cylinder for a final swimming session lasting for 10-minutes. Following treatments for each group, an expert observer un-aware of the experimental setup, timed each animal’s immobility, swimming, and climbing tendencies for six out of ten minutes. When mice did not attempt to escape other than the movements required to maintain their heads above the water, they were declared as stationary.39
Tail Suspension Test
A tail suspension test was designed with slight modifications as described previously using a tail suspension test device. Separate animals were hung on the hook of tail suspension test box in the darkroom at 60 cm above the table surface, with adhesive tape applied 1 cm above from the tip of each animal following 6-minute immobility period for each animal was noted.40
Sucrose Preference Test
The sucrose preference test was designed to determine the presence of anhedonia in mice. Before the administration of LPS, all animals were adapted to drinking water and a 2% sucrose solution for 5-days to create a baseline sucrose preference among each animal. Sucrose solution was placed in an animal cage in drinking bottle with a stopper valve, and the relative position of the bottles was changed regularly to avoid developing the place preference. Before testing, all experimental animals underwent a minimum 2-hour fasting from food and water. After LPS administration, fluid content was observed critically for next 48-hours, and after the test, sucrose preference was calculated using the formula:41
Estimation of Biochemical Parameters
The second protocol was adapted to confirm the biochemical alterations among various groups. Mice were sacrificed, and blood samples were taken via a retro-orbital puncture after 24-hours of LPS injection. The serum samples were divided to assess the corticosterone levels, and the brains were dissected to isolate the hippocampus region and stored at −80°C for additional neurochemical research. Because one of the central regions of the adult brain that retains active neurogenesis and participates in the anti-depressant response is the hippocampus.42
Determination of Oxidative Enzyme Activities
In order to evaluate various biochemical parameters, the tissue samples were homogenized at 3000-rpm in ice chilled phosphate buffer saline having pH=7.4 at a concentration of 10% w/v. Measurements of thiobarbituric acid reactive compounds were used to analyze the level of lipid peroxidation. Using a spectrophotometer, the absorbance of clear supernatant was determined at 535 nm.43,44 Similarly, the colorimetric Griess method was adapted to determine the quantity of nitric oxide, and absorbance was attained at a wavelength of 550 nm.45,46
Determination of Antioxidant Enzyme Activities
Following the assigned protocol of manufacturer, the previously mentioned methods were slightly modified to test antioxidant potential. The tetrazolium nitro-blue reduction caused by riboflavin as a superoxide producer was inhibited in the SOD assay. A (50 mM) phosphate buffer solution containing (0.025% triton X-100), (75 mM) nitro blue tetrazolium chloride NBT, (0.1 mM) EDTA with (pH=8), and (12 mM) L-methionine was added to the homogenate (20 µL). At room temperature, (2µM) riboflavin was added to initiate the reaction and at a wavelength of 560 nm, absorbance was measured.47 By mixing (10 µL) of the homogenate with (30µL) of H2O2 (7.3 mM solution) and incubating it on ice for five minutes, the catalase enzyme assay was carried out. After that, the reaction slowed by adding (20 µL) of sulfuric acid (H2SO4, 6N). A titration with 140 µL of (2 mM) potassium permanganate was used to assess how much H2O2 was still present in the reaction mixture after five minutes of catalase action (KMnO4). Using spectrophotometer at 480 nm, the rate of H2O2 oxidation was determined.48 The Flohé and Günzler method was used to evaluate the antioxidant activity of the glutathione peroxidase enzyme.49 Briefly, (300 µL) of homogenate was added to a reaction mixture containing (300 µL) of potassium phosphate buffer (0.1 M, pH=7), 200 µL of reduced glutathione (2 mM), 100 µL of H2O2 and (100 µL) of sodium azide (1 mM). The reaction mixture was then incubated for 15-minutes at 37°C before being stopped with 0.5 mL of TCA (5%). Next, 100 µL of the supernatant was added into the 200 µL phosphate buffer (50 mM, pH =7), and in the 0.7 mL (5, 5-dithiobis (2-nitrobenzoic acid; DTNB) buffer (0.4 mg/mL) then the absorbance was measured at 420 nm after 5-minutes of centrifugation.
Inflammatory Biomarkers and BDNF
ELISA kits were used to measure inflammatory biomarkers such as IL-1, IL-6, TNF-α,50 and BDNF.51 The concentration of the cytokines and BDNF in brain tissue samples were also assessed as per technique specified by the manufacturer.
Estimation of Catecholamines
The levels of norepinephrine (NE), dopamine (DA), and 5-hydroxytryptamine (5-HT) were estimated using HPLC with a slight modifications to the method previously described (Shimadzu HPLC apparatus paired with an electrochemical detector, N2000 HPLC workstation software, and column C18 (25 cm 4.6 mm, 5.0) Tokyo, Japan). After being sonicated in an aqueous solution of 0.1 M NaH2PO4 containing (0.85 mM) octanesulfonic acid (OSA) and (0.5 mM) ethylenediaminetetraacetic acid disodium (Na2 EDTA), the brain tissues were centrifuged at 13,000 g for 15-minutes at 4°C. A 0.45 M pore size filter was used to filter the mobile phase, which contained 0.1 M NaH2PO4 in water, (0.85 mM) OSA, (0.5 mM) Na2 EDTA, and (11%) methanol that had been adjusted to a (pH=3.4) using phosphate acid. The test had a detection limit of 20 pg/g samples, and the injection volume was set at (20µL). The quantities of NE, DA, and 5-HT in each sample were determined using the area under the curve technique in the hippocampus of the brain region utilizing HPLC and external standard curve.52
Serum Corticosterone Levels
After 24-hours of LPS administration, animals were anesthetized following intraperitoneal administration of (0.075%) ketamine and (0.01%) xylazine, respectively. Through retro-orbital puncture, 5mL of blood sample was collected via micro-hematocrit capillary tubes. All blood samples were centrifuged at 2000 g for 5-minutes at room temperature to collect serum for sub-sequent analysis. Serum corticosterone concentrations were measured by using the kit method.53
Statistical Analysis
Three independent behavioral and biochemical assays were carried out, and the findings were reported as (Mean ± SEM) and processed by Graph Pad Prism 8.0 software (Graph Pad, SanDiego, CA) using one-way analysis of variance (ANOVA). The difference across groups was regarded as significant, accompanied by post hoc Tukey Kramer’s multiple comparison test at (p<0.05).
Results
Percentage Yield of Plant Extract
The % age yield obtained from the crude extract of was 11.32%. It was the actual yield of crude extract of Aj.Cr obtained after the extraction.
Phytochemical Analysis
Preliminary Phytochemical Assays
The qualitative phytochemical screening of crude extract of Aj.Cr proved the presence of essential bioactive phytoconstituents such as flavonoids, phenols, terpenoids, and saponins in higher percentages, whereas glycosides, quinones, and tannins were detected at lesser levels, as shown in Table 2.
 |
Table 2 Preliminary Phytochemical Screening of the Methanolic Extract of Aj.Cr |
Phenolic Acid and Flavonoid Identification and Quantification by HPLC
Crude extract of Aj.Cr displayed the highest phenolic acid and flavonoid levels as indicated in Figure 1. Kaempferol (9.68 mg/g) was found in the highest concentration, followed by quercetin (4.15 mg/g), m-coumaric acid (4.73 mg/g), gallic acid (3.11mg/g), benzoic acid (3.43 mg/g), and kaempferol-3-o-rutinoside (1.04mg/g) were also found while sinapic acid (0.39 mg/g) was found in the lowest concentration of dry material of crude extract of Aj.Cr as shown in Table 3.
 |
Table 3 List of Polyphenolic Compounds Identified in Crude Extract of Aj.Cr by HPLC |
 |
Figure 1 Chromatogram representing different phenolic acids and flavonoids in methanolic extract of Aj.Cr. |
GC-MS Analysis for Identification of Metabolites
Twenty non-polar molecules relating to different chemical classes at different retention times (RT) were identified during GC-MS, as shown in Table 4, along with total ion chromatogram (TIC), as shown in Figure 2
 |
Table 4 Metabolic Profile of Methanolic Extract of Aj.Cr by GC-MS Analysis |
 |
Figure 2 GC-MS Chromatogram of methanolic extract of Aj.Cr. |
Amino Acid Profiling
As shown in Figure 3, free amino acids were identified and quantified in Table 5 from the methanolic extract of Aj.Cr. Among nineteen identified amino acids, asparagine, β-alanine, ammonia, valine, and serine were the most abundant compounds, while gamma amino butyric acid (GABA) and histidine were also identified in the lowest quantities.
 |
Table 5 Amino Acid Profile of Methanolic Extract of Aj.Cr |
 |
Figure 3 Spectra showing various amino acids profiling of methanolic extract of Aj.Cr. |
Antioxidant, Total Bioactive Contents and Enzyme Inhibition Assays
Antioxidant potential was estimated by DPPH, ABTS, and FRAP assays. Crude extract of Aj.Cr showed significant-free radical scavenging (DPPH=77.98±0.96; ABTS=96.57±0.71) and reducing potential in FRAP (119.6±0.67) respectively. Aj.Cr showed total phenolic contents (91.39± 5.1) and total flavonoid contents (63.5±2.7).Also showed maximum enzyme inhibition against cholinesterase was (4.37±0.59) as compared to butyryl cholinesterase (3.83±0.7) as shown in Table 6.
 |
Table 6 Antioxidant, Total Bioactive Contents and Enzyme Inhibition Assays |
Molecular Docking for Acetyl Cholinesterase
Molecular docking of three significant compounds kaempferol, m-coumaric acid, and quercetin identified in HPLC analysis was docked with AChE enzyme. The respective binding affinities with 2D, 3D, and H-bonding connections are depicted in Table 7 and Figures 4 and 5 respectively.
 |
Table 7 Binding Affinities and Interactions of the Selected Ligands from Aj.Cr Crude Extract by HPLC Analysis Against AChE Enzyme |
Acute Toxicity Analysis
Acute toxicity analysis showed that mortality, toxic response, and morbidity were non-significantly found (p>0.05) among different groups based upon the progressive treatment of crude extract of Aj.Cr at 100, 300 and 500 mg/kg dose and was safe up to 3000 mg/kg dose administered orally during the 14-day protocol.
Behavioral Assessment
Effects of Aj.Cr on Open Field Test
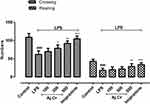
Mice treated with LPS showed a significant reduction (p˂0.001) in the crossings (51.30± 6.22) and rearing (19.70 ± 2.1) movement as compared to crossings (111.1±4.99) and rearing (34.80±2.53) among the normal control group. Imipramine treated mice exhibited considerably significant (p<0.001) number of crossings (105.6±3.85) and rearing (31.50 ±3.3) as compared to LPS group as shown in Figure 6. Aj.Cr at a dose of 100 mg/kg had non-significant effect (p>0.05) on the locomotor activity, while 300 mg/kg (82.90±1.464) and 500 mg/kg (98.9±1.67) substantially increased (p< 0.01) the number of crossings and only 500 mg/kg (28.10±1.28) showed significant (p<0.01) increased in the number of rearing as compared to the LPS-treated group as shown in Figure 6.
Effects of Aj.Cr on Forced Swim Test
The duration of mobility in the forced swim test was significantly reduced (p˂0.001) in the LPS-treated group (96.2 ± 11.7 seconds) as compared to the normal control group (203.4 ± 10.3) seconds as shown in Figure 7. In contrast, the length of immobility was significantly prolonged (p˂0.001) in the LPS-treated group (251.8 ± 13.7 seconds) than in the normal control group (55.3±7.9 seconds). Imipramine (243.67 ± 9.62 seconds) and Aj.Cr at a dosage of (100 mg/kg, 300 mg/kg, and 500 mg/kg) showed significantly (p<0.05) improvement in mobility time (ie, 168.7 ± 13.91, 207.2 ± 15.01, 229.7 ± 10.12 seconds) and decreased immobility time in imipramine (39.4 ± 5.16) and (ie, 103.91± 11.3, 76.83± 10.75, 46.73± 6.3 seconds, respectively) compared to the LPS-treated group. However, at a dose of 500 mg/kg and imipramine-treated groups had shown significantly (p<0.05) greater mobility and decreased immobility time than the normal control group, as illustrated in Figure 7.
Effects of Aj.Cr on Tail Suspension Test
In the tail suspension test, the LPS-treated group mobility time (54.50±9.14 seconds) was substantially (p˂0.001) shorter than that of the normal control group (248.3±9.1 seconds). In contrast, the duration of immobility was significantly increased (p˂0.001) in the LPS-treated group (242.9 ± 15.14 seconds) than the normal control group (59.5 ± 7.7 seconds). The duration of mobility was significantly increased in all Aj. Cr treated groups in a dose-related manner, with 100 mg/kg (103.33 ± 8.1 seconds), 300 mg/kg (191.5±16.76 seconds) and 500 mg/kg (207.5 ±13.15 seconds) in comparison to the LPS-treated group (54.50 ± 9.14) and imipramine-treated group (241.2 ±15.58 seconds) also showed significant effects in improving the mobility in mice. However, the period of immobility was considerably reduced (p <0.05) in 500 mg/kg (79.73 ± 15.73 seconds) and 300 mg/kg (93.5 ± 17.15 seconds) of -treated groups and imipramine-treated group (64.83 ± 9.58 seconds) as compared to the LPS-treated group as shown in Figure 8.
Effects of Aj.Cr on Sucrose Preference Test
Anhedonia was defined as a decrease in sucrose preference. Sucrose preference was considerably (p<0.001) lower in LPS-treated mice (33.84±5.19%) than in the normal control group (83.94±4.1%). In comparison, anhedonic behavior was prevented in mice pre-treated with imipramine (79.47±6.7%) and treatment with Aj.Cr also significantly improved the sucrose preference in 100 mg/kg (51.45±3.57%), 300 mg/kg (63.41±3.26%) and 500 mg/kg (72.04±3.21%), respectively, as demonstrated in Figure 9.
Effect of Aj.Cr on Biochemical Parameters
Estimation of Oxidative and Antioxidant Enzymes Biomarkers
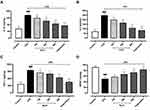
Thiobarbituric acid reactive substances (TBARS) is measured in ng/mg in the hippocampal tissue were considerably elevated (p˂0.001) in the LPS-treated mice group (58.873± 3.9 ng/mg) as compared to the normal control (8.790±3.3 ng/mg). TBARS level was considerably decreased (p˂0.01) in imipramine (13.760± 1.2 ng/mg) and by Aj.Cr at a dose of 300 mg (39.491± 1.7 ng/mg), 500 mg (25.79± 1.3ng/mg) than the LPS-treated group but remained non-significant (p˃0.05) at 100 mg/kg dose, as shown in Figure 10A. Nitrite levels (Mean±SEM µmol/g) in the hippocampus of mice were observed to be substantially higher (p˂0.001) in the LPS group (73.39±6.87 µmol/g) in contrast to the normal control group (29.44±4.23 µmol/g). Nitrite levels were significantly lowered (p˂0.001) in imipramine (41.28± 5.30 µmol/mg) and Aj.Cr at a dose of 300 mg (54.83±9.76µmol/mg), 500 mg (47.36±6.13µmol/mg) as compared to the LPS treated group but showed non-significant effect (p<0.05) at the dose of 100 mg (60.95 ±8.76µmol/mg) as shown in Figure 10B. GPX-1 levels (Mean±SEM pg/mg) in the hippocampus of mice were substantially reduced (p˂0.001) in the LPS-treated group (164.67±7.6 pg/mg) as compared to the normal control group (317.57±7.9pg/mg). GPX-1 levels were significantly elevated (p˂0.001) in imipramine (309.76±5.9 pg/mg) with Aj.Cr at a dose of 100 mg (239.53±7.8pg/mg),300 mg (246.38±9.60pg/mg) and 500 mg (267.86±5.6 pg/mg) showed a significant increase in GPX-1 level as indicated in Figure 10C, compared to the LPS-treated mice group. SOD levels (Mean±SEM U/mg) in the hippocampus of mice were significantly lowered (p˂0.001) in the LPS-induced group (3.63± 1.45 U/mg) in comparison with the control group (10.591±0.97 U/mg). Whereas dose of Aj.Cr at 100 mg (5.971±0.87 U/mg), 300 mg (7.270±1.2 U/mg), 500 mg (8.56±0.75 U/mg) and imipramine dose 10mg/kg (9.36±0.88 U/mg) increased SOD level significantly (p<0.001) as compared to the LPS group as shown in Figure 10D. Catalase levels (Mean±SEM nmol/min/g) were considerably lowered (p˂0.001) in the LPS group (7.98± 3.2 nmol/min/g) than the control group (17.530± 2.9 nmol/min/g), while Aj.Cr at a dose of 500 mg/kg group (9.97± 2.71 nmol/min/g) and imipramine (13.17± 2.30 nmol/min/g) significantly (p<0.01) prevented the LPS-induced reduction in CAT level as shown in Figure 10E.
Proinflammatory Cytokines and BDNF Levels
The pro-inflammatory cytokines (Mean±SEM pg/mg) IL-1β levels (147.25±13.6 pg/mg) among LPS-treated mice groups were considerably (p˂0.001) elevated in mice hippocampus following 24-hours of LPS-stimulation as compared to the normal control group (59.75±9.3 pg/mg) while IL-1β levels were substantially (p<0.01) reduced in imipramine group (71.69 ±14.3pg/mg) and Aj.Cr at a dose of 500 mg/kg (83.87 ± 8.19 pg/mg), 300 mg/kg of (97.2 ± 11.6 pg/mg) and 100 mg/kg of (113.78±10.0 pg/mg) group in contrast to LPS-treated mice as illustrated in Figure 11A. IL-6 levels (243.87 ± 11.8 pg/mg) in the LPS group were considerably (p˂0.001) higher in mice hippocampus after 24-hours of LPS treatment than in the control group (73.56±17.5 pg/mg). IL-6 levels reduced considerably (p<0.01) in Aj.Cr at 500 mg/kg (107.30±13.2 pg/mg), 300 mg/kg (165.82 ±10.8 pg/mg) and 100 mg/kg (197.35±11.06 pg/mg) showed dose-related pattern and imipramine group (85.83 ± 18.3 pg/mg) also showed significantly (p<0.001) reduced levels than the LPS group as seen in Figure 11B. TNF-α levels (971.93±17.8 pg/mg) in LPS-treated groups were considerably (p˂0.001) rise in mice hippocampus after 24 hours of LPS administration in contrast to the normal control group (367.46±19.1pg/mg) while, imipramine group (439.37±14.9 pg/mg) effective (p˂0.001) prevented the LPS triggered TNF-α. TNF-α levels were also substantially decreased (p<0.01) with Aj.Cr at a dose of 500 mg/kg (607.51±11.5 pg/mg), 300 mg/kg (789.55±15.6 pg/mg) and 100 mg/kg (833.57±18.1pg/mg) group than the LPS-treated group, as demonstrated in Figure 11C. BDNF (Mean±SEM ng/mg) level (47.97±11.6ng/mg) in the LPS group was significantly (p˂0.001) lowered in mice hippocampus as compared to the control group (91.53±6.61ng/mg). BDNF levels were considerably increased (p<0.01) with Aj.Cr at a dose of 500 mg/kg (75.57 ± 13.5 ng/mg), 300 mg/kg (67.45±15.16 ng/mg), while 100 mg/kg (59.37±11.5ng/mg) group showed insignificant (p>0.05) increase in BDNF levels as compared to the LPS-treated group. BDNF level was also significantly elevated (p<0.001) in the imipramine-treated group (83.37±7.9 ng/mg) as compared to the LPS group as presented in Figure 11D.
Effect on the Catecholamine Level
The catecholamine (Mean±SEM) mainly nor-epinephrine (33.86± 17.6ng/g), dopamine (41.93 ± 9.89 ng/g) and 5-HT (39.59 ± 17.56 ng/g) concentrations in mice hippocampus of LPS-treated mice group was significantly (p < 0.001) reduced than the control group nor-epinephrine (137.85± 20.3ng/g), dopamine (211.59±13.19 ng/g) and 5-HT (181.87±11.89 ng/g) levels respectively. Pre-treatment with imipramine (p < 0.001) reverted LPS-induced decline in NE (134.45± 17.3 ng/g), DA (184.73± 15.7 ng/g) and 5-HT (172.90±11.63 ng/g), respectively. Similarly, Aj, Cr at dosages of (100, 300, and 500 mg/kg) also significantly (p< 0.05) prevented the LPS-induced decrease in NE (ie, 98.73± 15.3, 109.69± 19.6, 121.37± 11.9ng/g) levels as shown in Figure 12A, DA (ie, 127.36± 9.8, 151.73 ± 14.7, 168.79± 10.7 ng/g) levels as shown in Figure 12B and 5-HT (ie, 105.59±19.12, 124.96 ± 17.19, 147.87± 7.89 ng/g) levels in the hippocampus as shown in Figure 12C.
Effect on Serum Corticosterone Level
In serum analysis, corticosterone level (Mean±SEM) in the LPS-treated group (2.78±0.34 ng/mL) was considerably increased (p<0.001) than the normal control group (1.18±0.34 ng/mL). Conversely, the corticosterone level was substantially reduced (p<0.01) in Aj.Cr at a dose of 500 mg/kg (1.53±0.07 ng/mL), 300 mg/kg (1.79±0.19 ng/mL) and 100 mg/kg (2.49±0.2 ng/mL) produced statistically non-significant results (p< 0.05) as compared to LPS-treated groups. Imipramine group (1.384±0.09 ng/mL) prevented the LPS-induced elevation of corticosterone level (p<0.001) significantly as shown in Figure 13.
Discussion
The study explored the in-vitro and in-vivo therapeutic potential of crude extract of Aj.Cr against neuro-inflammatory depression. In-vitro phytochemical and chromatographic screening demonstrated the higher proportions of flavonoids, phenols, terpenes, and saponins. In contrast, glycosides, quinones, and tannins were discovered in lesser quantities, which can be linked to their antioxidant potential.19 HPLC detected more benzoic acid, m-coumaric acid, quercetin, and Kaempferol. Studies suggest these chemicals have anti-inflammatory, antioxidant, and neuroprotective properties.55 GC-MS found 20 non-polar chemicals, ie, camphene, 1-chloro-4-phenoxy- benzene,2-methyl- benzothiazole, and 1,2-benzenedicarboxylic acid, bis(2-methylpropyl), which possess anti-depressant properties. Asparagine, alanine, ammonia\, valine, and serine were the most prevalent amino acids, while GABA and histidine were rare. Aj.Cr extract had stronger scavenging and reducing capacity, phenolic, flavonoid content, and cholinesterase inhibitory action. Quercetin had the highest binding interactions (−9.8 kJ/mol) for the AChE enzyme, followed by kaempferol and m-coumaric acid. The binding affinity of galantamine was −8.6 kJ/mol. Van der Waals forces and other weak intermolecular forces have higher binding affinities than hydrogen bonds. In 2D docking data, Van der Waals force interactions are more abundant than conventional hydrogen bonds between amino acids, indicating our ligands have larger enzyme binding affinities that support in-vitro enzyme inhibitory efficacy. The in-vitro study demonstrated that Aj. Cr extract has strong antioxidant, anti-depressant, and nutritional qualities,81,82 which can benefit depression. Depression is a chronic neuropsychiatric disorder that is further exacerbated by inflammation. Psycho physiological stress can activate the immune system and rapidly generate cytokines, which may modify the central nervous system and affect neurogenesis, neurotransmitter levels, neuro-endocrine function, neuroplasticity, and behavioral neural circuits.83,84 A single dose of LPS had been shown to produce neuro-inflammation by stimulating the adequate release of proinflammatory cytokines (IL-1 β, IL-6, and TNF- α) and oxidative stress resulting in a significant alteration in antioxidant enzyme levels, accompanied by the development of depressive behavior among rodents.35 The systemic treatment of LPS in mice resulted in prolonged periods of immobility in the forced swim and the tail suspension test, decreased sucrose preference as well as a significant increase in the overall immobility time and a decrease in the total distance and time spent in the central region, therefore revealing depressive behavior in a rodent model that can be treated with molecules possessing anti-depressant potential.1,85,86 We found that in open field forced swim and tail suspension tests, LPS-injected mice were treated with methanolic extract of Aj.Cr had pronounced locomotor activity and shorter periods of immobility than LPS-injected mice treated with saline. Aj.Cr on LPS-induced depressive-like behavior was investigated based on mice sucrose preference. Behaviors depicted a diminished susceptibility to re-inforces like sucrose are observed in LPS-treated mice1,87,88 and results from this study correlated to previous research showing that LPS treatment in mice reduces their desire for sucrose consumption. While, Aj.Cr treated mice clearly preferred sucrose and decreased anhedonia in behavior was observed. Previous research established a link between elevated hippocampus acetyl cholinesterase activity and a decline in self-care and motivating behavior, which may indicate anhedonic-like behavior patterns.89 BChE activity was also implicated in the pathology of depression, which was thought to be impacted by genetic differences in BChE.90 Clinical study suggests a relationship between depression and cytokines because depressed persons frequently have increased inflammatory activation levels.91,92 Following LPS treatment, high levels of IL-1, IL-6, and TNF-alpha in the brain might cause sleep difficulties, loss of pleasure, and cognitive inadequacy.93 In the current investigation, LPS injection elevated these three cytokines and depressed behaviors such as anhedonia and decreased locomotion and imipramine attenuated the LPS-induced elevation in inflammatory mediators and behavioral abnormalities. Inflammation badly affects brain BDNF expression, based on various research activities. LPS, a cytokine inducer, lowered mature BDNF in the hippocampus and cerebral cortex.94 A decline in BDNF, especially in the hippocampus, is a direct marker of depression. It involves the BDNF-activated PI3K/Akt signaling pathway, which affects neuronal survival and anti-apoptotic glial cell activities.95 Depression reduces BDNF-PI3K/Akt pathway activation96,97 while LPS-treated mice showed reduced hippocampus BDNF levels. Aj.Cr therapy increased BDNF owing to quercetin and kaempferol, which have an anti-depressant impact through the BDNF-TrkB-PI3K/Akt regulation pathway.98,99 A prior research on bioactive components of Aj.Cr found kaempferol and its glycoside derivatives, such as kaempferol3-(2G-glucosylrutinoside), kaempferol 3-(2,3-diacetyl –4“-(Z)-p-coumaryl-6”-(E)-p-coumarylglucoside), kaempferol 3-neohesperidoside100 Kaempferol and kaempferol-3-O-glucoside exhibit anti-depressant effects due to their antioxidant activity, pro-inflammatory cytokine reduction, AKT/-catenin cascade enhancement, and imipramine tolerance reversed.101,102 Chronic exposure to high amounts of inflammatory cytokines and continuous variations in central neurotransmitters may enhance oxidative stress and injure microglial cells103 by activating NF-kB and boosting the production of the i-NOS gene. LPO, elevated nitrite levels, and decreased antioxidant enzymes pre-dispose to neuro progression in depression (ie, GSH, SOD).4 LPS-induced oxido-nitrosative stress affected mice hippocampus. MDA, a lipid peroxidation marker, was higher in LPS-treated mice hippocampus than in the vehicle-treated group. LPS-treated mice exhibit lower GSH levels, indicating reduced antioxidant status. Aj.Cr dramatically reduced MDA and nitrite levels, while GSH, SOD, and CAT levels increased, produced reduction in oxido-nitrosative stress in the hippocampus of LPS-treated mice which can be linked to its antioxidant potential. Free radicals and oxidative stress are involved in the pathophysiology of inflammation, degenerative disease, and other chronic diseases like depression. Plant-based antioxidants are directly involved in the neutralization of free radicals to avoid substrate oxidation19,104,105 NO, and ROS decrease monoamines and BDNF levels in the brain, leading to neurotoxic effects41,106 via activating indolamine 2,3-dioxygenase (IDO). It affects tryptophan metabolism by increasing TRP metabolism-kynurenine level, reducing 5-HT and ultimately increasing the risk of depression.107 Aj.Cr also substantially increased norepinephrine (NE), dopamine (DA), and serotonin (5-HT) levels in the hippocampus of LPS-challenged mice. Some investigations have shown the adaptogenic impact of the plant extract by normalizing stress parameters and monoaminergic levels, which may imply that the extract may have an anti-depressant effect by restoring normal monoaminergic neuromodulation.108,109 Corticosterone levels were lower in -treated animals than in LPS-treated animals. Aj.Cr may have adjusted glucocorticoids by reducing IL-1 as corticosterone levels fell. Interleukin-1 peripherally stimulates the HPA axis and hypothalamic norepinephrine metabolism.110 Corticosterone increase is associated to reduced HPA axis response regulation, likely due to altered glucocorticoids receptor function, and severe depression.111 Imipramine suppressed inflammatory cytokines, oxidative stress, and BDNF expression, confirming a prior research.112 In the current study, methanolic extract of A. javanica leaves contains 19 essential and non-essential free amino acids that operate as a reservoir, precursor, and regenerator of injured neural components. Many studies have found that amino acids assist cure depression.113 Kaempferol, caffeic acid, ferulic acid, and benzoic acid have anti-inflammatory and neuroprotective action, whereas Camphene, 1-chloro-4-phenoxy- Benzene,2-methyl- Benzothiazole, and 1.2-Benzenedicarboxylic acid, bis(2-methylpropyl) have anti-depressant properties. Long-term studies are needed to evaluate if and its bioactive components have an anti-depressant-like effect on an LPS-induced depressed behavior model.
Conclusion
The study showed that Aj.Cr partially reduced LPS-induced depressive-like behavior via modulation of neuroinflammation and monoaminergic pathways. The presence of phytoconstituents that exerted anti-depressant, antioxidant and nutritional potential due to the presence of free amino acids suggested that crude extract of Aj. Cr can be useful in the treatment of depression associated with neuropsychiatric disorders. The anti-depressant effects can be seen throughout the study which may be due to the normalization of BDNF levels, oxidative stress markers, suppression of pro-inflammatory cytokines (IL-1, IL-6, and TNF-a) followed by reduction in corticosterone. Further studies are also required to explore the components of the Aj.Cr crude extract and identify their precise functions.
Provision of Data
All the data is provided within the manuscript submitted.
Author’s Consent
All the authors mutually consented to publish this study in an esteemed journal.
Acknowledgments
The authors would like to thank the Department of Pharmacology, the Faculty of Pharmacy, the Islamia University of Bahawalpur for laboratory access and un-interrupted research facilities.
Author Contributions
All authors made substantial contributions in the design, conception, acquisition of data, analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors disclosed that there is no competing interests in this work.
References
1. Cordeiro RC, Chaves Filho AJM, Gomes NS, et al. Leptin prevents lipopolysaccharide-induced depressive-like behaviors in mice: involvement of dopamine receptors. Front Psychiatry. 2019;10:125.
2. Cowen PJ. Neuroendocrine and neurochemical processes in depression. Psychopathol Rev. 2016;3(1):3–15. doi:10.5127/pr.034513
3. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855–871. doi:10.1017/S1092852900016965
4. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–692. doi:10.1016/j.pnpbp.2010.05.004
5. Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro Psychopharmacol Biol Psychiatry. 2005;29(2):201–217. doi:10.1016/j.pnpbp.2004.11.003
6. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495–502. doi:10.1136/jnnp-2011-301779
7. O’Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–522. doi:10.1038/sj.mp.4002148
8. Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. doi:10.1002/da.22084
9. Yu H-Y, Cai Y-B LZ. Activation of AMPK improves lipopolysaccharide-induced dysfunction of the blood–brain barrier in mice. Brain Injury. 2015;29(6):777–784. doi:10.3109/02699052.2015.1004746
10. Ho Y-H, Lin Y-T, Wu C-WJ, Chao Y-M, Chang AY, Chan JY. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci. 2015;22(1):1–14. doi:10.1186/s12929-015-0157-8
11. Li R, Zhao D, Qu R, Fu Q, Ma S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci Lett. 2015;594:17–22. doi:10.1016/j.neulet.2015.03.040
12. Sharma N, Nehru B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: role of oxidative stress and neuroinflammation. Neurochem Int. 2015;87:92–105. doi:10.1016/j.neuint.2015.06.004
13. Takeda H, Tsuji M, Inazu M, Egashira T, Matsumiya T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur J Pharmacol. 2002;449(3):261–267. doi:10.1016/S0014-2999(02)02037-X
14. Zafir A, Ara A, Banu N. In vivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):220–228. doi:10.1016/j.pnpbp.2008.11.010
15. Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228–237. doi:10.4161/oxim.3.4.12858
16. Dhingra D, Bansal Y. Antidepressant-like activity of beta-carotene in unstressed and chronic unpredictable mild stressed mice. J Funct Foods. 2014;7:425–434. doi:10.1016/j.jff.2014.01.015
17. Berk A, Yılmaz İ, Abacıoğlu N, Kaymaz MB, Karaaslan MG, Kuyumcu Savan E. Antidepressant effect of Gentiana olivieri Griseb. in male rats exposed to chronic mild stress. Libyan J Med. 2020;15(1):1725991. doi:10.1080/19932820.2020.1725991
18. Khan AW, Jan S, Parveen S, et al. Phytochemical analysis and enzyme inhibition assay of Aerva javanica for Ulcer. Chem Cent J. 2012;6(1):76. doi:10.1186/1752-153X-6-76
19. Pandey A, Kaushik A, Wanjari M, Dey YN, Jaiswal BS, Dhodi A. Antioxidant and anti-inflammatory activities of Aerva pseudotomentosa leaves. Pharm Biol. 2017;55(1):1688–1697. doi:10.1080/13880209.2017.1321022
20. Samejo MQ, Memon S, Bhanger MI, Khan KM. Chemical compositions of the essential oil of Aerva javanica leaves and stems. Pak J Analyt Envir Chem. 2012;13(1):5.
21. Javed F, Jabeen Q, Aslam N, Awan AM. Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. f. J Ethnopharmacol. 2020;259:112966. doi:10.1016/j.jep.2020.112966
22. Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12(1):221. doi:10.1186/1472-6882-12-221
23. Belkacem N, Djaziri R, Lahfa F, El-Haci I, Boucherit Z. Phytochemical screening and in vitro antioxidant activity of various Punica granatum l. Peel extracts from Algeria: a comparative study. Phytothérapie. 2014;12(6):372–379. doi:10.1007/s10298-014-0850-x
24. Benariba N, Djaziri R, Bellakhdar W, et al. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extracts. Asian Pac J Trop Biomed. 2013;3(1):35–40. doi:10.1016/S2221-1691(13)60020-9
25. George VC, Kumar DR, Suresh PK, Kumar RA. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J Food Sci Technol. 2015;52(4):2328–2335. doi:10.1007/s13197-014-1289-7
26. Inbaraj BS, Lu H, Kao T, Chen B. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J Pharm Biomed Anal. 2010;51(3):549–556. doi:10.1016/j.jpba.2009.09.006
27. Kuppusamy P, Lee KD, Song CE, et al. Quantification of major phenolic and flavonoid markers in forage crop Lolium multiflorum using HPLC-DAD. Rev Bras Farmacogn. 2018;28(3):282–288. doi:10.1016/j.bjp.2018.03.006
28. Hites RA. Development of gas chromatographic mass spectrometry. Anal Chem. 2016;88(14):6955–6961. doi:10.1021/acs.analchem.6b01628
29. Reeds PJ. Dispensable and indispensable amino acids for humans. J Nutr. 2000;130(7):1835s–1840s. doi:10.1093/jn/130.7.1835S
30. Dezsi Ș, Bădărău AS, Bischin C, et al. Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) KD Hill & LAS Johnson leaves. Molecules. 2015;20(3):4720–4734. doi:10.3390/molecules20034720
31. Mocan A, Schafberg M, Crișan G, Rohn S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J Funct Foods. 2016;24:579–594. doi:10.1016/j.jff.2016.05.007
32. Zengin G, Uysal A, Gunes E, Aktumsek A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): a potential source for functional food ingredients and drug formulations. PLoS One. 2014;9(11):e113527. doi:10.1371/journal.pone.0113527
33. Mocan A, Zengin G, Crişan G, Mollica A. Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J Enzyme Inhib Med Chem. 2016;31(sup4):200–210. doi:10.1080/14756366.2016.1222585
34. Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 2011;7(2):146–157. doi:10.2174/157340911795677602
35. Mello BS, Monte AS, McIntyre RS, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res. 2013;47(10):1521–1529. doi:10.1016/j.jpsychires.2013.06.008
36. Bektas N, Arslan R, Goger F, Kirimer N, Ozturk Y. Investigation for anti-inflammatory and anti-thrombotic activities of methanol extract of Capparis ovata buds and fruits. J Ethnopharmacol. 2012;142(1):48–52. doi:10.1016/j.jep.2012.04.011
37. Kraeuter AK, Guest PC, Sarnyai Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Meth Mol BioL. 2019;1916:99–103.
38. Porsolt R, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336.
39. Yousuf S, Marifatul Haq S, Rasool A, et al. Evaluation of antidepressant activity of methanolic and hydroalcoholic extracts of Acorus calamus L. rhizome through tail suspension test and forced swimming test of mice. J Tradit Chin Med. 2020;7(3):301–307. doi:10.1016/j.jtcms.2020.07.002
40. Liu L, Dong Y, Shan X, Li L, Xia B, Wang H. Anti-depressive effectiveness of baicalin in vitro and in vivo. Molecules. 2019;24(2):326.
41. Barua CC, Haloi P, Saikia B, et al. Zanthoxylum alatum abrogates lipopolysaccharide-induced depression-like behaviours in mice by modulating neuroinflammation and monoamine neurotransmitters in the hippocampus. Pharm Biol. 2018;56(1):245–252. doi:10.1080/13880209.2017.1391298
42. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–297. doi:10.1002/dneu.20758
43. Niehaus WG, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6(1):126–130. doi:10.1111/j.1432-1033.1968.tb00428.x
44. Aguilar Diaz De LJ, CR Borges. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J Vis Exp. 2020;159:e61122.
45. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analyt Biochem. 1982;126(1):131–138. doi:10.1016/0003-2697(82)90118-X
46. Zembron-Lacny A, Tylutka A. Intermittent hypoxic exposure reduces endothelial dysfunction. BioMed Res Int. 2020;2020:6479630. doi:10.1155/2020/6479630
47. Beyer JWF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analyt Biochem. 1987;161(2):559–566. doi:10.1016/0003-2697(87)90489-1
48. Ni J, Sasaki Y, Tokuyama S, Sogabe A, Tahara Y. Conversion of a typical catalase from Bacillus sp. TE124 to a catalase-peroxidase by directed evolution. J Biosci Bioeng. 2002;93(1):31–36. doi:10.1016/S1389-1723(02)80050-0
49. Flohé L, Günzler WA. [12] assays of glutathione peroxidase. In: Methods Enzymol. Vol. 105. Elsevier; 1984:114–120.
50. Xue J, Li H, Deng X, Ma Z, Fu Q, Ma S. L-Menthone confers antidepressant-like effects in an unpredictable chronic mild stress mouse model via NLRP3 inflammasome-mediated inflammatory cytokines and central neurotransmitters. Pharmacol Biochem Behav. 2015;134:42–48. doi:10.1016/j.pbb.2015.04.014
51. Elfving B, Plougmann PH, Wegener G. Detection of brain-derived neurotrophic factor (BDNF) in rat blood and brain preparations using ELISA: pitfalls and solutions. J Neurosci Methods. 2010;187(1):73–77. doi:10.1016/j.jneumeth.2009.12.017
52. Zhao D, Zheng L, Qi L, et al. Structural Features and potent antidepressant effects of total sterols and β-sitosterol extracted from sargassum horneri. Mar Drugs. 2016;14(7):123. doi:10.3390/md14070123
53. Adebesin A, Adeoluwa OA, Eduviere AT, Umukoro S. Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. J Psychiatr Res. 2017;94:29–35. doi:10.1016/j.jpsychires.2017.06.007
54. Dixon Clarke SE, Ramsay RR. Dietary inhibitors of monoamine oxidase A. J Neural Transm. 2011;118(7):1031–1041. doi:10.1007/s00702-010-0537-x
55. Silvestro S, Bramanti P, Mazzon E. Role of quercetin in depressive-like behaviors: findings from animal models. Applied Sci. 2021;11(15):7116. doi:10.3390/app11157116
56. Wu J, Chen H, Li H, et al. Antidepressant potential of chlorogenic acid-enriched extract from eucommia ulmoides Oliver bark with neuron protection and promotion of serotonin release through enhancing synapsin I expression. Molecules. 2016;21(3):260. doi:10.3390/molecules21030260
57. Yan S-X, Lang J-L, Song -Y-Y, et al. Studies on anti-depressant activity of four flavonoids isolated from Apocynum venetum Linn (Apocynaceae) leaf in mice. Trop J Pharm Res. 2015;14(12):2269–2277. doi:10.4314/tjpr.v14i12.17
58. Diniz LRL, Souza MTS, Barboza JN, Almeida RN, Sousa DP. Antidepressant potential of cinnamic acids: mechanisms of action and perspectives in drug development. Molecules. 2019;24(24):4469. doi:10.3390/molecules24244469
59. Zeni ALB, Zomkowski ADE, Maraschin M, Rodrigues ALS, Tasca CI. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: evidence for the involvement of the serotonergic system. Eur J Pharmacol. 2012;679(1–3):68–74. doi:10.1016/j.ejphar.2011.12.041
60. Chuang H-W, Wei IH, Lin F-Y, et al. Roles of Akt and ERK in mTOR-dependent antidepressant effects of vanillic acid. ACS Omega. 2020;5(7):3709–3716. doi:10.1021/acsomega.9b04271
61. Dalmagro AP, Camargo A, Zeni ALB. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab Brain Dis. 2017;32(6):1963–1973. doi:10.1007/s11011-017-0089-y
62. Oliveira C, Bagetta D, Cagide-Fagín F, et al. Benzoic acid-derived nitrones: a new class of potential acetylcholinesterase inhibitors and neuroprotective agents. Eur J Med Chem. 2019;174:116–129. doi:10.1016/j.ejmech.2019.04.026
63. Lim DW, Park J, Han D, Lee J, Kim YT, Lee C. Anti-inflammatory effects of asian fawn lily (Erythronium japonicum) extract on lipopolysaccharide-induced depressive-like behavior in mice. Nutrients. 2020;12(12):3809. doi:10.3390/nu12123809
64. Patel D, Witt SN. Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxid Med Cell Longev. 2017;2017:4829180. doi:10.1155/2017/4829180
65. Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino Acid. J Biol Chem. 2012;287(24):19786–19791. doi:10.1074/jbc.R112.357194
66. Gao J, Zhang W, Chai X, Tan X, Yang Z. Asparagine endopeptidase deletion ameliorates cognitive impairments by inhibiting proinflammatory microglial activation in MPTP mouse model of Parkinson disease. Brain Res Bull. 2022;178:120–130. doi:10.1016/j.brainresbull.2021.11.011
67. Holten AT, Gundersen V. Glutamine as a precursor for transmitter glutamate, aspartate and GABA in the cerebellum: a role for phosphate-activated glutaminase. J Neurochem. 2008;104(4):1032–1042. doi:10.1111/j.1471-4159.2007.05065.x
68. Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr. 2008;138(10):2003S–2007S. doi:10.1093/jn/138.10.2003S
69. Wang TJ, Ngo D, Psychogios N, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123(10):4309–4317. doi:10.1172/JCI64801
70. Osowska S, Moinard C, Neveux N, Loï C, Cynober L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut. 2004;53(12):1781–1786. doi:10.1136/gut.2004.042317
71. Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura R, Miura S. Regulation of skeletal muscle function by amino acids. Nutrients. 2020;12(1):261. doi:10.3390/nu12010261
72. Yu X, Long YC. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci Rep. 2016;6(1):30033. doi:10.1038/srep30033
73. Zhu H, Blake S, Chan KT, Pearson RB, Kang J. Cystathionine β-synthase in physiology and cancer. Biomed Res Int. 2018;2018:3205125. doi:10.1155/2018/3205125
74. Liu S, Sun Y, Zhao R, Wang Y, Zhang W, Pang W. Isoleucine increases muscle mass through promoting myogenesis and intramyocellular fat deposition. Food Funct. 2021;12(1):144–153. doi:10.1039/D0FO02156C
75. Radchenko DS, Kattge S, Kara S, Ulrich AS, Afonin S. Does a methionine-to-norleucine substitution in PGLa influence peptide-membrane interactions? Biochim Biophys Acta. 2016;1858(9):2019–2027. doi:10.1016/j.bbamem.2016.06.002
76. Mena Gomez MA, Carlsson A, Garcia de Yebenes J. The effect of beta-alanine on motor behaviour, body temperature and cerebral monoamine metabolism in rat. J Neural Transm. 1978;43(1):1–9. doi:10.1007/BF02029014
77. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi:10.1186/1475-2891-14-6
78. Pedrón VT, Varani AP, Bettler B, Balerio GN. GABA(B) receptors modulate morphine antinociception: pharmacological and genetic approaches. Pharmacol Biochem Behav. 2019;180:11–21. doi:10.1016/j.pbb.2019.02.015
79. Krebs HA, Hems R, Lund P, Halliday D, Read WW. Sources of ammonia for mammalian urea synthesis. Biochem J. 1978;176(3):733–737. doi:10.1042/bj1760733
80. Szende B. The effect of amino acids and amino acid derivatives on cell proliferation. Acta Bio-medica de L’Ateneo parmense. 1993;64(5–6):139–145.
81. Aranibar N, Vassallo JD, Rathmacher J, et al. Identification of 1- and 3-methylhistidine as biomarkers of skeletal muscle toxicity by nuclear magnetic resonance-based metabolic profiling. Anal Biochem. 2011;410(1):84–91. doi:10.1016/j.ab.2010.11.023
82. Wade AM, Tucker HN. Antioxidant characteristics of L-histidine. J Nutr Biochem. 1998;9(6):308–315. doi:10.1016/S0955-2863(98)00022-9
83. Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosc. 2015;38(3):145–157. doi:10.1016/j.tins.2014.12.006
84. Lee CH, Giuliani F. The Role of Inflammation in Depression and Fatigue. Front Immunol. 2019;10:1696. doi:10.3389/fimmu.2019.01696
85. Engeland CG, Nielsen DV, Kavaliers M, Ossenkopp KP. Locomotor activity changes following lipopolysaccharide treatment in mice: a multivariate assessment of behavioral tolerance. Physiol Behav. 2001;72(4):481–491. doi:10.1016/S0031-9384(00)00436-4
86. Renard CE, Dailly E, David DJ, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17(4):449–455. doi:10.1046/j.1472-8206.2003.00160.x
87. Moreau J. Validation of an animal model of anhedonia, a major symptom of depression. L’encephale. 1997;23(4):280–289.
88. Scheggi S, De Montis MG, Gambarana C. Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol. 2018;21(11):1049–1065. doi:10.1093/ijnp/pyy083
89. Machado DG, Cunha MP, Neis VB, et al. Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol Biochem Behav. 2012;103(2):220–229. doi:10.1016/j.pbb.2012.08.024
90. Awan S, Hashmi A, Taj R, et al. Genetic association of butyrylcholinesterase with major depressive disorder. Biochem Genetics. 2022;60(2):720–737. doi:10.1007/s10528-021-10125-z
91. Hodes GE, Pfau ML, Leboeuf M, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci. 2014;111(45):16136–16141. doi:10.1073/pnas.1415191111
92. Lotrich FE. Inflammatory cytokine-associated depression. Brain Res. 2015;1617:113–125. doi:10.1016/j.brainres.2014.06.032
93. Tang M-M, Lin W-J, Pan Y-Q, Guan X-T, Li Y-C. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behavior. 2016;161:166–173. doi:10.1016/j.physbeh.2016.04.034
94. Frühauf-Perez PK, Temp FR, Pillat MM, et al. Spermine protects from LPS-induced memory deficit via BDNF and TrkB activation. Neurobiol Learn Mem. 2018;149:135–143. doi:10.1016/j.nlm.2018.02.012
95. Jin Y, Sun LH, Yang W, Cui RJ, Xu SB. The role of BDNF in the neuroimmune axis regulation of mood disorders. Front Neurol. 2019;10:515. doi:10.3389/fneur.2019.00515
96. Kitagishi Y, Kobayashi M, Kikuta K, Matsuda S. Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress Res Treat. 2012;2012:752563. doi:10.1155/2012/752563
97. Deng Z, Yuan C, Yang J, et al. Behavioral defects induced by chronic social defeat stress are protected by Momordica charantia polysaccharides via attenuation of JNK3/PI3K/AKT neuroinflammatory pathway. Ann Transl Med. 2019;7(1):6. doi:10.21037/atm.2018.12.08
98. Fang K, Li H-R, Chen -X-X, et al. Quercetin alleviates LPS-induced depression-like behavior in rats via regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front Pharmacol. 2020;10:1544. doi:10.3389/fphar.2019.01544
99. Silva Dos Santos J, Gonçalves CJP, de Oliveira Carvalho P, Ortega MM. The pharmacological action of kaempferol in central nervous system diseases: a review. Front Pharmacol. 2020;11:565700. doi:10.3389/fphar.2020.565700
100. Saleem H, Zengin G. New insights into the phytochemical composition, enzyme inhibition and antioxidant properties of desert cotton (Aerva javanica (Bum.f) Shult. -Amaranthaceae). Nat Prod Res. 2021;35(4):664–668. doi:10.1080/14786419.2019.1587427
101. Gao W, Wang W, Peng Y, Deng Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab Brain Dis. 2019;34(2):485–494. doi:10.1007/s11011-019-0389-5
102. de la Garza AL, Garza-Cuellar MA, Silva-Hernandez IA, et al. Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients. 2019;11(3):572. doi:10.3390/nu11030572
103. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36(2):764–785. doi:10.1016/j.neubiorev.2011.12.005
104. Szwajgier D, Borowiec K, Pustelniak K. The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients. 2017;9(5):477. doi:10.3390/nu9050477
105. Mazlan NA, Mediani A, Abas F, et al. Antioxidant, antityrosinase, anticholinesterase, and nitric oxide inhibition activities of three Malaysian macaranga species. Sci World J. 2013;2013:1–8. doi:10.1155/2013/312741
106. Joca SR, Guimarães FS. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacol. 2006;185(3):298–305. doi:10.1007/s00213-006-0326-2
107. Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198–209. doi:10.1080/10253890701754068
108. Wu F, Li H, Zhao L, et al. Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2013;148(3):861–868. doi:10.1016/j.jep.2013.05.026
109. Lin YE, Lin SH, Chen WC, et al. Antidepressant-like effects of water extract of Gastrodia elata Blume in rats exposed to unpredictable chronic mild stress via modulation of monoamine regulatory pathways. J Ethnopharmacol. 2016;187:57–65. doi:10.1016/j.jep.2016.04.032
110. Smagin GN, Swiergiel AH, Dunn AJ. Peripheral administration of interleukin-1 increases extracellular concentrations of norepinephrine in rat hypothalamus: comparison with plasma corticosterone. Psychoneuroendocrinology. 1996;21(1):83–93. doi:10.1016/0306-4530(95)00019-4
111. Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23(8):1148–1155. doi:10.1016/j.bbi.2009.07.007
112. Peng C-H, Chiou S-H, Chen S-J, et al. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18(2):128–140. doi:10.1016/j.euroneuro.2007.05.002
113. LN Costa, Carneiro BA, Alves GS, et al. Metabolomics of major depressive disorder: a systematic review of clinical studies. Cureus. 2022;14(3):e23009. doi:10.7759/cureus.23009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.