Back to Journals » Patient Preference and Adherence » Volume 14
Metformin Improves the Depression Symptoms of Women with Polycystic Ovary Syndrome in a Lifestyle Modification Program
Authors AlHussain F, AlRuthia Y , Al-Mandeel H, Bellahwal A, Alharbi F, Almogbel Y , Awwad O, Dala'een R, Alharbi FA
Received 31 December 2019
Accepted for publication 31 March 2020
Published 15 April 2020 Volume 2020:14 Pages 737—746
DOI https://doi.org/10.2147/PPA.S244273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Fatimah AlHussain,1 Yazed AlRuthia,2,3 Hazem Al-Mandeel,4 Arwa Bellahwal,2 Fadia Alharbi,2 Yasser Almogbel,5 Oriana Awwad,6 Roua Dala’een,6 Fawaz Abdullah Alharbi7
1Department of Pharmacoeconomics and Drug Pricing, Saudi Food and Drug Authority, Riyadh, Saudi Arabia; 2Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 3Pharmacoeconomics Research Unit, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 4Department of Obstetrics and Gynecology, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 5Department of Pharmacy Practice, College of Pharmacy, Qassim University, Buraydah, Saudi Arabia; 6Department of Biopharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, University of Jordan, Amman, Jordan; 7Drug Information and Poison Centre, Al Ansaar General Hospital, Medina, Saudi Arabia
Correspondence: Yazed AlRuthia
Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
Tel +996 114677483
Fax +966 114677480
Email [email protected]
Background: Metformin is commonly prescribed to manage polycystic ovary syndrome (PCOS), which is one of the most common endocrine disorders among women of childbearing age and is associated with high prevalence rates of depression and anxiety.
Objective: This study’s objective was to determine the impact of prescribed metformin on depression and anxiety levels of patients with PCOS.
Methods: This prospective, multi-center, cohort study examined the impact of prescribed metformin on the depression and anxiety of women with PCOS in four gynecology clinics in Saudi Arabia and Jordan. The women had recently been prescribed metformin along with lifestyle modifications, such as diet and exercise, and were compared to another group of women with PCOS who were prescribed lifestyle modifications only. Depression and anxiety were assessed at baseline and three months later using the Patient Health Questionnaire (PHQ-9) and the Generalized Anxiety Disorder (GAD-7) scale, respectively. Health-related quality of life was measured using the Short Form Health Survey (SF-36). Multiple logistic regression analyses were conducted to examine the impact of metformin on depression and anxiety.
Results: Eighty-six women participated in the study: 53 were prescribed metformin with lifestyle modifications, and 33 were prescribed lifestyle modifications only. The women on metformin had 70% lower odds of having major depression (PHQ-9≥ 10) (OR=0.302, P=0.045); however, no significant effect of metformin on anxiety (GAD-7≥ 10) was found.
Conclusion: Metformin may have a role in the management of depression symptoms among patients with PCOS; however, its potential antidepressant effect should be further examined in randomized double-blind placebo-controlled clinical trials.
Keywords: metformin, polycystic ovary syndrome, antidepressant activity
Introduction
Polycystic ovary syndrome (PCOS) is one of the most commonly encountered reproductive, endocrine, and hormonal disorders among women of reproductive age.1,2 For a woman to be diagnosed with PCOS, she must have irregular or absent ovulation, high levels of androgenic hormones, and/or enlarged ovaries with 12 or more follicles in each ovary based on the Rotterdam consensus criteria.3 In addition, PCOS includes a multitude of hormonal and metabolic abnormalities, such as hyperinsulinemia, impaired fasting plasma glucose, hypertension, hyperlipidemia, acne, and abdominal obesity.2,4,5 These disorders increase the patient’s risk for developing diabetes, metabolic syndrome, endometrial hyperplasia, and endometrial cancer.4,6 Depending on the diagnostic criteria used,7,8 PCOS affects 6% to 18% of women worldwide in their reproductive years.2,9 In the Middle East, the prevalence of PCOS is equally high and ranges from 5% based on the National Institutes of Health criteria, to 19% based on the Rotterdam consensus criteria.10–13
Several studies have reported a link between PCOS and reduced health-related quality of life (HRQoL).14–16 Among the factors contributing to the impairment of HRQoL is the frequent presence of poor self-perception and psychiatric disorders, such as anxiety and depression among women with PCOS.17–20 It has been suggested that the prominent symptoms of the syndrome, such as obesity, infertility, acne, and hirsutism cause mental stress and might be responsible for the higher prevalence of psychiatric illnesses in this group of patients.4,21 The higher likelihood of psychiatric disorders among women with PCOS might also be due to the hyperinsulinemia, which occurs because of insulin resistance and obesity.22,23
Women with PCOS are treated based on their presenting signs/symptoms and their preferences. Lifestyle modifications, such as restricted caloric intake and regular exercise, are considered the first line of therapy for obese and overweight women with PCOS.24,25 Hormonal contraception is used to manage hirsutism and menstrual irregularities if pregnancy is not desired.24 In addition to hormonal contraception, metformin is used to manage insulin resistance.25 Metformin is also used to manage infertility and anovulation among women who desire to become pregnant either alone or in combination with clomiphene.25,26 The value of metformin in the management of PCOS stems from its insulin-sensitizing activity, improving insulin resistance, which is common among PCOS patients;27–29 lowering the insulin level is associated with a reduction in free testosterone and androstenedione.30 In addition, metformin reduces hepatic production of glucose,31 thereby decreasing the release of free fatty acids from the adipose tissue, and lowering the level of fasting free fatty acids.32 Metformin alone does not seem to reduce the body mass index (BMI) or weight of patients with PCOS;33 however, when it is combined with lifestyle interventions, such as a hypocaloric diet and exercise, it contributes to a weight-reducing effect.34
The results obtained in a small clinical trial that was conducted at a single medical center in China with a group of 54 patients with type 2 diabetes mellitus raised the possibility that metformin may have an antidepressant effect.35 The results and conclusion were empirically supported by animal studies, and the downregulation of expression of the c-Jun gene in the hippocampus was proposed to mediate the antidepressant action of metformin.36,37 Metformin was also found to improve the psychosocial aspects of HRQoL among patients with PCOS after six months of treatment.38 However, not all of the results of these studies are in agreement. In a randomized controlled trial, the HRQoL scores of patients with PCOS receiving clomiphene plus metformin were significantly lower than the scores for those receiving clomiphene plus a placebo; this difference was attributable primarily to the higher incidence of gastrointestinal side effects among the patients on metformin.39 Another randomized controlled trial that examined the efficacy of two insulin sensitizers (metformin and pioglitazone) in depressed and obese patients with PCOS, indicated that pioglitazone resulted in a significantly better improvement in depression after six weeks of follow-up than metformin, although some improvement with metformin was also noted.33
Given the inconclusive results regarding the impact of metformin on depression in patients with PCOS, and evidence that ethnicity influences metabolic and hormonal aberrations associated with PCOS,40–42 the objective of the present study was to examine whether metformin has any antidepressant or anxiolytic effects among Middle Eastern women with PCOS. The impact of metformin on their HRQoL was also explored.
Methods
This prospective, multi-center, cohort study was conducted in four gynecology clinics in Riyadh, Buraydah, Medinah (Saudi Arabia), and Amman (Jordan). Patient recruitment started in January 2016 and ended in July 2018. Participants enrolled in the study were newly diagnosed with PCOS using the Rotterdam consensus criteria.3,43 All of the patients signed a written informed consent form in Arabic, which explained the purpose of the study and duration of enrollment. The study was approved by the Institutional Review Boards of King Saud University College of Medicine, University of Jordan, Al Ansaar General Hospital, and Qassim University. This study adhered to the ethical principles of the Helsinki Declaration.44
The inclusion criteria were: the absence of diabetes, lack of a previous diagnosis of any mental disorder, including depression or anxiety, and the absence of any thyroid gland disorder, including hypothyroidism. Patients taking metformin (for PCOS or any other indication), who were treated with antidepressants, anti-anxiety agent, thyroxine, or any other medication for PCOS, such as clomiphene, letrozole, oral contraceptive, tamoxifen, or a herbal supplement, were excluded from the study. The recruited women were divided into two groups. The first group included patients who were prescribed metformin 850 mg twice a day plus lifestyle modifications, such as a calorie-restricted diet and regular exercise (eg, brisk walking for ≥ 30 minutes at least five days a week). The other group included women who were treated with lifestyle modifications only. Patients who were prescribed metformin were seen by physicians who usually started their patients with PCOS on metformin along with lifestyle modifications compared with the other group’s physicians who believed that PCOS should be managed first by lifestyle modifications only.
Information on patients’ age, BMI, educational level, other comorbidities, any menstrual cycle irregularities, and marital status were collected from the patients and from their medical records at baseline. All of the women were screened for depression, anxiety, and HRQoL at baseline and three months later, as the antidepressant effects of some medications can take up to three months before they are observed.45
The level of depression was evaluated using the Arabic version of the 9-item Patient Health Questionnaire (PHQ-9), which consists of nine questions and is scored from 0 to 27, with higher scores indicating more severe depression.46 The women’s anxiety was assessed using the Arabic version of the Generalized Anxiety Disorder-7 (GAD-7) scale; this questionnaire yields scores from 0 to 21, with higher scores indicating a more severe level of anxiety.47 Patients’ HRQoL was determined using the Arabic version of the 36-item Short Form Health Survey (SF-36), which consists of eight scales (physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health); each scale is scored from 0 to 100 with the higher scores indicating better HRQoL.48 The women were also screened for health literacy using the Arabic version of the single-item literacy screener (SILS). The SILS consists of a single question that assesses the patient’s need for someone to help him/her read and understand medical instructions and information contained in prescription medication leaflets, labels, and other educational medical materials. There are five possible responses (1-never, 2-rarely, 3-sometimes, 4-often, and 5-always), with a score of 3 or higher suggesting limited health literacy.49,50
Statistical Analysis
The minimum sample size, needed for a two-tailed t-test based on α=0.05, β=0.2, power=0.8, and a medium effect size (Cohen’s d=0.33) for the difference in the mean scores of the PHQ-9 and GAD-7 at baseline and three months follow-up, was estimated to be 75 patients. To ensure patient confidentiality, all data were coded, and no patient identifiers were recorded. Student’s t-test, the chi-square test, and Fisher’s exact test were performed as appropriate to compare the baseline data between the metformin group and the lifestyle modifications only group. Paired t-tests were used to compare the scores of both groups on the PHQ-9, GAD-7, and the eight scales of SF-36 at baseline and three months later.
Multiple logistic regression analyses were conducted to examine whether metformin had any antidepressant and/or anxiolytic effects. The dependent variables in all of the regression models were the PHQ-9 scores dichotomized into two categories (PHQ-9≥10 and PHQ-9<10) and the GAD-7 scores dichotomized into two categories (GAD-7 ≥10 and GAD-7<10) to examine the impact of metformin use on depression and anxiety, respectively. A PHQ-9 score ≥10 indicates major depression, and was found to have 88% sensitivity and specificity for major depression.51 On the other hand, a GAD-7 score of ≥10 indicates generalized anxiety disorder and was found to have 89% sensitivity and 82% specificity for generalized anxiety disorder.52 Eight logistic regression models were conducted to examine the impact of metformin use on depression and anxiety, controlling for the baseline PHQ-9 and GAD-7 scores, respectively. In addition to the baseline PHQ-9 and GAD-7 scores, Models II to VII controlled for age, other comorbidities, education, BMI, marital status, and health literacy, separately. In Model VIII, all of the aforementioned variables (age, other comorbidities, education, BMI, marital status, and health literacy) plus the baseline PHQ-9 and GAD-7 scores were controlled for. Statistical significance was defined as P<0.05. All analyses were conducted using the SAS statistical software (version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
During the period from March 2016 to June 2018, 128 women with a confirmed diagnosis of PCOS in the four participating clinics were approached. Of those, 102 women met the inclusion criteria. However, five women did not consent to participate, and 11 were lost to follow-up, resulting in a total enrollment of 86 women of which 53 were prescribed metformin and lifestyle modifications and 33 were managed using lifestyle modifications only. Women in both groups were advised to follow a lifestyle modification program, which included a hypocaloric diet and regular exercise.
The demographic and clinical characteristics of the recruited women are listed in Table 1. The mean age of both groups was not significantly different: 27.64 versus 28.90 years among the metformin and lifestyle modifications only groups, respectively. The overall mean BMI of both groups (28.58) was indicative of overweight. Almost all of the women completed at least high school, and more than one-third graduated from college. Accordingly, health literacy was very high (96.51%). There was no statistically significant difference between the two groups with regard to educational level or health literacy (P≥0.05). Approximately half of the women were single and the other half were married. Irregular menstrual cycles were experienced by 56.6% of the women in the metformin group compared to 66.6% of their counterparts in the lifestyle modifications only group; however, this differences was not significant (P=0.353). Comorbidities were not frequent; single cases of hypertension, seizure, and asthma were present in both groups, and one case of dyslipidemia was encountered in the metformin group (Table 1).
 |
Table 1 Baseline Characteristics of the Study’s Cohort |
The baseline as well as the 3-month follow-up scores of the PHQ-9, GAD-7, and eight scales of the SF-36 for both the metformin and lifestyle modifications only groups are shown in Table 2. The mean PHQ-9 score significantly decreased by 2.75 points following 3 months of metformin therapy, indicating improvement in the symptoms of depression (P=0.002). However, the small difference found in the mean PHQ-9 score among women in the lifestyle modifications only group (−0.702) did not reach statistical significance (P=0.378) (Table 2). This improvement in symptoms of depression among the women receiving metformin was reflected in the decreased number of women exhibiting major depression, defined as a PHQ-9 score ≥10. At baseline, 46.51% of the women had a PHQ-9 score ≥10, with no significant difference between the metformin and lifestyle modifications only groups (47.17% vs 45.45%; P=0.876). After three months of follow-up, the percentage of women with a PHQ-9 score ≥10 had decreased to 26.42% among those in the metformin group but remained unchanged (45.45%) among those in the lifestyle modifications only group; however, this difference was not statistically significant (P=0.069).
 |
Table 2 Baseline and Follow-Up Scores for Patient-Reported Outcomes |
The mean GAD-7 scores did not change significantly from baseline to three months later among the women in the metformin and lifestyle modifications only groups (Table 2). Similarly, no significant difference was found in the percentage of women with a GAD-7 score ≥10, indicating the presence of generalized anxiety disorder at baseline. However, the difference was significant after three months of follow-up, shifting downward from 30.19% to 26.42% among the women in the metformin group, and upward from 42.42% to 48.48% among the women in the lifestyle modifications only group (P=0.036).
Scores on the eight scales of the SF-36 at baseline and three months later are presented in Table 2. Both groups’ scores on role limitations due to emotional problems improved after three months of follow-up. Furthermore, the women who received metformin felt more energetic/less fatigued, and experienced improved emotional well-being. The women who were treated only with lifestyle modifications reported fewer role limitations due to physical health and better general health. Self-reported physical functioning, social functioning, and level of pain were not affected by either type of treatment.
Women on metformin had 3.3 times lower odds of having major depression (PHQ-9 score ≥10) compared to those in the lifestyle modifications only group, controlling for the PHQ-9 score at baseline, age, educational level, health literacy, marital status, BMI, and the number of comorbidities (OR=0.302; 95% confidence interval (CI), 0.093–0.976; P=0.045). This significant relationship remained significant in all of the regression models, except the model that controlled for marital status and the baseline PHQ-9 score (Table 3). On the other hand, the odds of having a GAD-7 score ≥10, which indicates generalized anxiety disorder, was 2.91 times lower among women in the metformin group than their counterparts in the lifestyle modifications only group, but the difference was not statistically significant in most of the regression models that controlled for the GAD-7 score at baseline as well as age, educational level, health literacy, marital status, BMI, and the number of comorbidities (OR=0.344; 95% CI, 0.117–1.011; P=0.052) (Table 4).
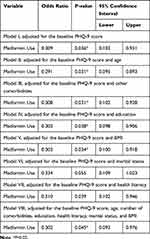 |
Table 3 Logistic Regression Analyses for the Association Between Treatment with Metformin and Scores on the Patient Health Questionnaire 9-Items (PHQ-9) |
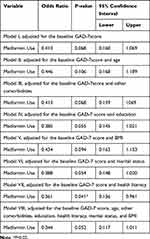 |
Table 4 Multiple Logistic Regression Analyses for the Association Between Treatment with Metformin and Scores on the Generalized Anxiety Disorder 7-Item (GAD-7) Scale |
Discussion
The findings of this study suggest that metformin might have mental-health benefits for women affected by PCOS. This conclusion was drawn from the results of two types of analyses. First, the mean score on the PHQ-9 scale, which was used to screen patients for depression, decreased by 2.75 points at the 3-month follow-up in the patients treated with metformin and lifestyle modifications, while no significant change was observed in the women treated with lifestyle modifications only. Furthermore, the women who received metformin had lower odds of having major depression, as indicated by a PHQ-9 score ≥10, even after controlling for the baseline PHQ-9 score, age, educational level, health literacy, BMI, and the number of comorbidities. However, metformin combined with lifestyle modifications failed to show a significant reduction in the mean GAD-7 score, even after controlling for the baseline GAD-7 score, age, educational level, health literacy, BMI, and the number of comorbidities. With regard to the patients’ HRQoL, the mean score on the SF-36 scale measuring role limitations due to emotional problems improved among the women in both treatment groups after three months of follow-up. However, the mean scores on the energy/fatigue and emotional well-being scales showed significant improvement after three months of follow-up only among the patients in the metformin with lifestyle modifications group. Of note, the scores on the perception of general health and role limitations due to physical health did not improve among the women treated with metformin; better scores at follow-up were achieved by the women in the lifestyle modifications only group.
Several studies have documented a beneficial impact of metformin on diabetes-related depression clinically,33,35,53 and experimentally,36,54 although in some cases, the antidepressant effect was not reported.27,55 Metformin has also demonstrated an anti-anxiety effect among patients with diabetes56 as well as in animal models.57 Despite these encouraging results, few studies have investigated the antidepressant or anxiolytic effects of metformin among patients with PCOS.33,38,39,58,59 These studies have reported conflicting results confirming33,38,58 and refuting39,59 the positive effect of metformin in the management of depression in this patient population. The present work suggests that metformin with lifestyle modifications has a positive impact on depression, but not on anxiety among women with PCOS. Interestingly, it is noteworthy that the latter finding is in agreement with the only study conducted thus far on the effect of metformin on the anxiety of women with PCOS. In this study, stress associated with public speaking was used as the measure of anxiety.59
The reasons for the discrepancies in the results of the published studies are difficult to explain. However, in addition to differences in study design and the small number of women examined, the ethnicity and genetics of the participants might have played a significant role as confounding factors.60–62 Genome-wide association studies have highlighted the need to include a large sample size and patients representing multiple ethnic groups in the context of diabetes treatment.63 Similarly, differences in the metabolic manifestations associated with PCOS are present among patients from various geographical regions.41 Middle Eastern women are more likely to be diagnosed with PCOS at a younger age and have a higher rate of insulin resistance compared to their Caucasian counterparts with PCOS.64 Hirsutism is more frequent in patients with PCOS from the Middle East than in those of European ancestry.65 African American and Hispanic women have significantly higher BMIs and waist circumferences compared with their Northern European and Asian counterparts.66 Thus, it is very likely that the discrepancies among the previous studies addressing the therapeutic impact of metformin on PCOS-related depression are also related to population genetics. This possibility highlights the necessity to obtain data pertaining to different ethnicities. The present study addresses this imperative by providing relevant data for a cohort of Middle Eastern women, which have previously been unavailable.
To understand fully, the effect of metformin on depression in PCOS and the basis for ethnic differences, its mechanism of action must be explained. Although it is most often assumed that metformin counteracts depression in women with diabetes and PCOS by alleviating the multiple sequelae of the underlying disease, alternative mechanisms have been recently proposed. Experimentally, metformin has been shown to act as an antidepressant by regulating DNA hydroxymethylation via the AMPK/Tet2 pathway, resulting in upregulation of a neuroprotective peptide, brain-derived neurotrophic factor (BDNF).67 Metformin can also activate the formation of BDNF by activating CREB and Akt/GSK3 signaling pathways.68 In addition, research into the insulin resistance-independent modes of action of metformin on the brain may provide additional insights into the clinical application of this drug. The need for novel information is critical because at present there is a perceivable paucity of data on pharmacological treatment options for the management of depression and anxiety among patients with PCOS.18
Limitations
In comparison with previously published research, the current investigation has the advantage of being conducted in four clinical centers and being designed as a prospective study, providing additional strength to the conclusions reached.69 Moreover, the effects of metformin on multiple variables – depression, anxiety, and HRQoL – were analyzed. However, some limitations have to be acknowledged. The sample size, although it satisfied the pre-established level of statistical power, was relatively small. One of the reasons for not being able to recruit more participants was the difficulty of recruiting women with PCOS, given the cultural barriers in the Middle East. It should be recognized, however, that the sample size was comparable to those employed in similar studies.58,59 Despite the effort to recruit the same number of patients for both study groups, the number of patients in the metformin with lifestyle modifications group was significantly higher than the number in the lifestyle modifications only group. However, the study was an observational one, and the number of patients in each arm of treatment could not be controlled, as no randomization was performed and the investigators did not have any influence over the number of patients in either treatment group. In addition, depression and anxiety were assessed using the PHQ-9 and GAD-7 scales, which are widely used in assessments of levels of depression and anxiety with high levels of specificity and sensitivity,51,52 however, they are used in the assessment and screening for depression and anxiety, respectively, and not for diagnostic purposes. Moreover, the impact of metformin on body weight over the three months of follow-up was not assessed; this is something that could have a role in explaining the difference between the PHQ-9 scores at baseline and follow-up. Finally, adherence to medication and the required lifestyle modifications were not addressed in this study. Therefore, a causal effect of the use of metformin combined with lifestyle modifications on the improvement of symptoms of depression among patients with PCOS cannot be established.
Conclusion
Despite the shortcomings of this study, the collected data suggest that metformin treatment combined with lifestyle modifications improves the mental well-being of patients with PCOS. The current findings support the notion that all women with PCOS should undergo psychological screening and receive appropriate interventions whenever required. Finally, future studies should verify the findings of this study using more robust study designs, such as randomized double-blind placebo-controlled clinical trials.
Data Sharing Statement
The data will be available from the corresponding author upon reasonable request.
Ethical Approval
The study was approved by the Institutional Review Boards of King Saud University College of Medicine, University of Jordan, Al Ansaar General Hospital, and Qassim University.
Acknowledgments
The authors would like to acknowledge the financial support for this study from the Researchers Supporting Project (No. RSP-2019/16), King Saud University, Riyadh, Saudi Arabia, and the Deanship for Scientific Research, University of Jordan, Amman, Jordan. The funders had no role in the study’s design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no financial or non-financial conflicts of interest.
References
1. Baldani DP, Skrgatic L, Ougouag R. Polycystic ovary syndrome: important underrecognised cardiometabolic risk factor in reproductive-age women. Int J Endocrinol. 2015;2015:786362. doi:10.1155/2015/786362
2. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet (London, England). 2007;370(9588):685–697. doi:10.1016/S0140-6736(07)61345-2
3. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. doi:10.1016/j.fertnstert.2018.05.004
4. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi:10.2147/CLEP.S37559
5. Pfieffer ML. Polycystic ovary syndrome: diagnosis and management. Clin Med Res. 2019;44:30–35.
6. Ali AT. Polycystic ovary syndrome and metabolic syndrome. Ceska Gynekol. 2015;80(4):279–289.
7. Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781–785. doi:10.1210/jc.2005-2153
8. Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab. 2006;91(3):786–789. doi:10.1210/jc.2005-2501
9. Skiba MA, Islam RM, Bell RJ, Davis SR. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(6):694–709. doi:10.1093/humupd/dmy022
10. Guraya SS. Prevalence and ultrasound features of polycystic ovaries in young unmarried Saudi females. J Microsc Ultrastruct. 2013;1:30–34. doi:10.1016/j.jmau.2013.06.002
11. Al Bassam NM, Ali S, Rahman SR. Polycystic ovarian syndrome (PCOS), awareness among female students, qassim university, Qassim Region, Saudi Arabia. Int J Res Granthaalayah. 2018;6:395–406.
12. Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62(3):238–242.
13. Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351–96358. doi:10.18632/oncotarget.19180
14. Coffey S, Bano G, Mason HD. Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the short form-36 (SF-36). Gynecol Endocrinol. 2006;22(2):80–86. doi:10.1080/09513590600604541
15. Barnard L, Ferriday D, Guenther N, et al. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. 2007;22(8):2279–2286. doi:10.1093/humrep/dem108
16. Li Y, Li Y, Yu Ng EH, et al. Polycystic ovary syndrome is associated with negatively variable impacts on domains of health-related quality of life: evidence from a meta-analysis. Fertil Steril. 2011;96(2):452–458. doi:10.1016/j.fertnstert.2011.05.072
17. Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26(6):1399–1407. doi:10.1093/humrep/der071
18. Cooney LG, Dokras A. Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Curr Psychiatry Rep. 2017;19(11):83. doi:10.1007/s11920-017-0834-2
19. Chaudhari AP, Mazumdar K, Mehta PD. Anxiety, depression, and quality of life in women with polycystic ovarian syndrome. Indian J Psychol Med. 2018;40(3):239–246. doi:10.4103/IJPSYM.IJPSYM_561_17
20. Farkas J, Rigo A, Demetrovics Z. Psychological aspects of the polycystic ovary syndrome. Gynecol Endocrinol. 2014;30(2):95–99. doi:10.3109/09513590.2013.852530
21. Hung J-H, Hu L-Y, Tsai S-J, et al. Risk of psychiatric disorders following polycystic ovary syndrome: a nationwide population-based cohort study. PLoS One. 2014;9(5):e97041. doi:10.1371/journal.pone.0097041
22. Watson K, Nasca C, Aasly L, McEwen B, Rasgon N. Insulin resistance, an unmasked culprit in depressive disorders: promises for interventions. Neuropharmacol. 2018;136:327–334. doi:10.1016/j.neuropharm.2017.11.038
23. Sartorius N. Depression and diabetes. Dialogues Clin Neurosci. 2018;20(1):47–52.
24. Mathur R, Levin O, Azziz R. Use of ethinylestradiol/drospirenone combination in patients with the polycystic ovary syndrome. Ther Clin Risk Manag. 2008;4:487–492. doi:10.2147/TCRM.S6864
25. Wang R, Kim BV, Van Wely M, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. bmj. 2017;356:j138.
26. Abu Hashim H, Foda O, Ghayaty E. Combined metformin-clomiphene in clomiphene-resistant polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2015;94(9):921–930. doi:10.1111/aogs.12673
27. Palomba S, Falbo A, Zullo F, Orio JF. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30(1):1–50.
28. Fux Otta C, Wior M, Iraci GS, et al. Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double-blind, and placebo control trial. Gynecol Endocrinol. 2010;26(3):173–178. doi:10.3109/09513590903215581
29. Macut D, Bjekic-Macut J, Rahelic D, Doknic M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163–170. doi:10.1016/j.diabres.2017.06.011
30. Kurzthaler D, Hadziomerovic-Pekic D, Wildt L, Seeber BE. Metformin induces a prompt decrease in LH-stimulated testosterone response in women with PCOS independent of its insulin-sensitizing effects. Reprod Biol Endocrinol. 2014;12(1):98. doi:10.1186/1477-7827-12-98
31. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi:10.1007/s00125-017-4342-z
32. Castro Cabezas M, van Wijk JP, Elte JW, Klop B. Effects of metformin on the regulation of free fatty acids in insulin resistance: a double-blind, placebo-controlled study. J Nutr Metab. 2012;2012:394623. doi:10.1155/2012/394623
33. Kashani L, Omidvar T, Farazmand B, et al. Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology. 2013;38(6):767–776. doi:10.1016/j.psyneuen.2012.08.010
34. Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107(4):E55. doi:10.1542/peds.107.4.e55
35. Guo M, Mi J, Jiang QM, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41(9):650–656.
36. Shivavedi N, Kumar M, Tej G, Nayak PK. Metformin and ascorbic acid combination therapy ameliorates type 2 diabetes mellitus and comorbid depression in rats. Brain Res. 2017;1674:1–9. doi:10.1016/j.brainres.2017.08.019
37. Khedr SA, Elmelgy AA, El-Kharashi OA, et al. Metformin potentiates cognitive and antidepressant effects of fluoxetine in rats exposed to chronic restraint stress and high fat diet: potential involvement of hippocampal c-jun repression. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(4):407–422. doi:10.1007/s00210-018-1466-8
38. Hahn S, Benson S, Elsenbruch S, et al. Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod. 2006;21(7):1925–1934. doi:10.1093/humrep/del069
39. Moll E, van Wely M, Lambalk CB, Bossuyt PM, van der Veen F. Health-related quality of life in women with newly diagnosed polycystic ovary syndrome randomized between clomifene citrate plus metformin or clomifene citrate plus placebo. Hum Reprod. 2012;27(11):3273–3278. doi:10.1093/humrep/des310
40. Sirota I, Stein DE, Vega M, Keltz MD. Insulin Resistance and β-cell Function Calculated by Homeostasis Model Assessment in Lean, Overweight, and Obese Women with Polycystic Ovary Syndrome. J Reprod Med .2016;61:3–10.
41. Louwers Y, Lao O, Fauser BC, Kayser M, Laven JS. The impact of self-reported ethnicity versus genetic ancestry on phenotypic characteristics of polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2014;99(10):E2107–E2116.
42. Huang Z, Yong EL. Ethnic differences: is there an Asian phenotype for polycystic ovarian syndrome? Best Pract Res Clin Obstet Gynaecol. 2016;37:46–55. doi:10.1016/j.bpobgyn.2016.04.001
43. Wang R, Mol BWJ. The Rotterdam criteria for polycystic ovary syndrome: evidence-based criteria? Hum Reprod. 2017;32(2):261–264. doi:10.1093/humrep/dew287
44. Carlson RV, Boyd KM, Webb DJ. The revision of the declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi:10.1111/j.1365-2125.2004.02103.x
45. Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10(4):419–430.
46. AlHadi AN, AlAteeq DA, Al-Sharif E, et al. An arabic translation, reliability, and validation of patient health questionnaire in a Saudi sample. Ann Gen Psychiatry. 2017;16(1):32. doi:10.1186/s12991-017-0155-1
47. Sawaya H, Atoui M, Hamadeh A, Zeinoun P, Nahas Z. Adaptation and initial validation of the patient health questionnaire - 9 (PHQ-9) and the generalized anxiety disorder - 7 questionnaire (GAD-7) in an Arabic speaking Lebanese psychiatric outpatient sample. Psychiatry Res. 2016;239:245–252. doi:10.1016/j.psychres.2016.03.030
48. Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the RAND-36 health survey and its equivalence to the US-English version. Med Care. 1998;36(3):428–432. doi:10.1097/00005650-199803000-00018
49. Al-Jumaili AA, Al-Rekabi MD, Sorofman B. Evaluation of instruments to assess health literacy in Arabic language among Iraqis. Res Social Adm Pharm. 2015;11(6):803–813. doi:10.1016/j.sapharm.2015.02.002
50. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7(1):21. doi:10.1186/1471-2296-7-21
51. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
52. Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. doi:10.7326/0003-4819-146-5-200703060-00004
53. Wang CP, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J Diabetes Complications. 2017;31(4):679–686. doi:10.1016/j.jdiacomp.2017.01.013
54. Aswar U, Chepurwar S, Shintre S, Aswar M. Telmisartan attenuates diabetes induced depression in rats. Pharmacol Rep PR. 2017;69(2):358–364. doi:10.1016/j.pharep.2016.12.004
55. Moulton CD, Hopkins CWP, Ismail K, Stahl D. Repositioning of diabetes treatments for depressive symptoms: A systematic review and meta-analysis of clinical trials. Psychoneuroendocrinology. 2018;94:91–103. doi:10.1016/j.psyneuen.2018.05.010
56. Tu HP, Lin C-H, Hsieh H-M, et al. Prevalence of anxiety disorder in patients with type 2 diabetes: a nationwide population-based study in Taiwan 2000–2010. Psychiatr Q. 2017;88(1):75–91. doi:10.1007/s11126-016-9436-0
57. Reddy BR, Maitra S, Jhelum P, et al. Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high-fructose-fed prediabetic rats. J Biosci. 2016;41(3):407–417. doi:10.1007/s12038-016-9627-8
58. Erensoy H, Niafar M, Ghafarzadeh S, Aghamohammadzadeh N, Nader ND. A pilot trial of metformin for insulin resistance and mood disturbances in adolescent and adult women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):72–75. doi:10.1080/09513590.2018.1498476
59. Benson S, Arck PC, Tan S, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2009;34(5):727–735. doi:10.1016/j.psyneuen.2008.12.001
60. Kawamura S, Maesawa C, Nakamura K, et al. Predisposition for borderline personality disorder with comorbid major depression is associated with that for polycystic ovary syndrome in female Japanese population. Neuropsychiatr Dis Treat. 2011;7:655–662. doi:10.2147/NDT.S25504
61. Cesta CE, Kuja-Halkola R, Lehto K, Iliadou AN, Landen M. Polycystic ovary syndrome, personality, and depression: A twin study. Psychoneuroendocrinology. 2017;85:63–68. doi:10.1016/j.psyneuen.2017.08.007
62. Day F, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. doi:10.1371/journal.pgen.1007813
63. Srinivasan S, Yee SW, Giacomini KM. Pharmacogenetics of antidiabetic drugs. Adv Pharmacol. 2018;83:361–389. doi:10.1016/bs.apha.2018.04.005
64. Song DK, Hong YS, Sung YA, Lee H. Insulin resistance according to beta-cell function in women with polycystic ovary syndrome and normal glucose tolerance. PLoS One. 2017;12(5):e0178120. doi:10.1371/journal.pone.0178120
65. Glintborg D, Mumm H, Hougaard D, Ravn P, Andersen M. Ethnic differences in Rotterdam criteria and metabolic risk factors in a multiethnic group of women with PCOS studied in Denmark. Clin Endocrinol (Oxf). 2010;73(6):732–738. doi:10.1111/j.1365-2265.2010.03873.x
66. Welt CK, Arason G, Gudmundsson JA, et al. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab. 2006;91(11):4361–4368. doi:10.1210/jc.2006-1191
67. Wang Y, Liu B, Yang Y, et al. Metformin exerts antidepressant effects by regulated DNA hydroxymethylation. Epigenomics. 2019;11(6):655–667. doi:10.2217/epi-2018-0187
68. Keshavarzi S, Kermanshahi S, Karami L, et al. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicol. 2019;72:74–84. doi:10.1016/j.neuro.2019.02.004
69. Vandenbroucke JP. Observational research, randomised trials, and two views of medical science. PLoS Med. 2008;5(3):e67. doi:10.1371/journal.pmed.0050067
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
