Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Metformin Ameliorates Epithelial–Mesenchymal Transition of Renal Tubular Epithelial Cells in Diabetes by Increasing Vitamin D Receptor Expression
Received 20 September 2022
Accepted for publication 15 November 2022
Published 22 December 2022 Volume 2022:15 Pages 4001—4010
DOI https://doi.org/10.2147/DMSO.S389918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Wenjie Wen,1,2,* Bin Huang,1,2,* Shandong Ye1,2
1Department of Endocrinology and Laboratory for Diabetes, The First Affiliated Hospital of University of Science and Technology of China (USTC), Department of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China; 2Department of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shandong Ye, University of Science and Technology of China, No. 96 Jinzhai Road, Hefei, Anhui Province, 230026, People’s Republic of China, Email [email protected]
Background: Metformin is used as a first-line drug for the treatment of type 2 diabetes. Epithelial–mesenchymal transition (EMT) plays a significant role in the development of renal tubular damage in diabetic kidney disease. However, the underlying mechanisms of EMT in diabetic kidney disease are unclear and how to inhibit this process remains to be explored.
Methods: C57 mice were randomly divided into four groups, including the normal control group (NC group), the Type 2 diabetes group (T2DM group), the metformin group (MET group), and glibenclamide group (GLIB). Fasting blood glucose (FBG), glycated hemoglobin (HbA1c), urinary albumin, RBP, PCX, and creatinine were measured. Renal pathology was observed with HE staining. Molecular mechanism of VDR expression are regulated by metformin through wound healing assay, and Western blot analysis of VDR, Ecad, and SMA in HK2 cells.
Results: In animal experiments, compared with the NC group, the T2DM group showed decreased body weight, increased levels of FBG, HbA1c, UAlb/UCR, URBP/UCR, and UPCX/UCR, decreased levels of VDR protein and mRNA expression in renal tissues (P < 0.05), and significantly increased renal pathological damage in mice in the T2DM group. Compared with the T2DM group, mice in the GLIB and MET groups had higher body weight and lower FBG, HbA1c, UAlb/UCR, URBP/UCR, and UPCX/UCR (P < 0.05). In addition, renal pathological damage was significantly reduced in the MET group compared to the GLIB group. In HK2 cells, high glucose promoted the reduction of VDR and the development of EMT compared to the NC group. In addition, we found that Metformin can up-regulate VDR and inhibit EMT.
Conclusion: Our study shows that the renoprotective effect of metformin is independent of glycemic control and metformin is involved in the progression of EMT by regulating VDR expression.
Keywords: type 2 diabetes, diabetic kidney disease, metformin, VDR, EMT
Introduction
Metformin is currently the first-line drug of choice for the treatment of type 2 diabetes recommended by national guidelines.1 Recent studies have shown that in addition to lowering blood sugar, the drug may be beneficial to health.2 Metformin is known to inhibit mainly the mitochondrial respiratory complex and can improve autophagy and oxidative stress through the AMPK signaling pathway,2,3 but its key targets and molecular mechanisms remain unclear.
Diabetic kidney disease (DKD) is a common chronic complication of diabetes and one of the main causes of end-stage renal disease (ESRD) in diabetic patients.4–6 The main features of DKD are proteinuria, glomerular thylakoid hyperplasia, basement membrane thickening, and tubulointerstitial fibrosis.7,8 Although glomerular injury is the main pathological feature of DKD, a series of studies have shown that tubular injury is also critical in DKD and that tubulointerstitial fibrosis (TIF) is more associated with disease progression than glomerular injury.9,10 Epithelial–mesenchymal transition (EMT) is characterized by the loss of epithelial markers such as E-cadherin and the gain of mesenchymal markers such as α-smooth muscle actin (α-SMA).11 EMT occurs in tubular epithelial cells of diabetic patients as a basis for the progression of chronic kidney disease and leads to tubulointerstitial fibrosis (TIF).12 Experiments have confirmed that metformin inhibits DKD-related phenotypes such as EMT.13 However, the mechanism by which metformin protects EMT of DKD is still under further study.
Vitamin D receptor (VDR) is a transcription factor that belongs to a superfamily of nuclear receptors and ligand-activated transcription factors. It is widely distributed in various tissues.14,15 Studies have shown that VDR plays an important role in cardiovascular and neurodegenerative diseases, skeletal muscle cell remodeling, muscle regeneration, and inflammatory bowel disease.16,17 In addition, VDR has a protective effect on the kidneys.18 There is a relative lack of VDR in some kidney-related diseases such as glomerulonephritis, acute kidney injury, and DKD.19,20 In intervention research of kidney-related diseases, VDR is a very attractive drug target.21 At present, there are almost no reports on the relationship between metformin and VDR and its mechanism in diabetic kidney disease.
In this study, we found that metformin could reverse EMT by upregulating VDR, thereby inhibiting the development of diabetic kidney disease. Our findings provide a new rationale for the renal protective effect of metformin.
Materials and Methods
Model Building and Grouping
The feeding conditions of the mice were kept at 12 h light/dark alternating regular lighting, room temperature (20±1) °C, humidity (48±10) %, and free feeding. After the mice were adapted for 1 week, eight mice were randomly selected as the normal control (NC) group and fed a normal chow diet, and the remaining mice were fed with high-fat diets. Two months later, a small dose of STZ (50 mg/kg, dissolved in 0.1 mol/L citric acid buffer, pH=4.2, continuous intraperitoneal injection for 3 days) was intraperitoneally injected, and the NC group was injected with an equal amount of citrate buffer. After a week, the tail vein detected random blood glucose ≥16.7 mmol/L, which is a successful model for T2DM. The successfully modeled T2DM mice were randomly divided into the T2DM group, the metformin group (MET group), and the glibenclamide group (GLIB group), and eight animals in each group were continued to be fed a high-fat diet. These mice were randomly divided into three groups: the T2DM group, in which mice were administered sterile saline daily by gavage for 8 weeks; the MET group, in which mice were administered metformin (250 mg/kg/day) daily by gavage for 8 weeks; and the glibenclamide (GLIB, a clinically employed antidiabetic molecule) group, in which mice were administered GLIB (2.5 mg/kg/day) daily by gavage for 8 weeks. Before killing the mice, their urine and blood were collected and weighed. The animals were then euthanized.
Detection of Blood and Urine Index
After 8 weeks of intervention, all mice were fasted with water, and urine sample of each mouse were collected in a metabolic cage. ELISA method is used to detect blood hemoglobin A1c (HbA1c) (Cat No. MM-0159M1, MEIMIAN, China), glucose oxidase method is used to determine fasting blood glucose (FBG). ELISA method was used to detect Urine albumin (UAlb), Retinol-Binding Protein (RBP), and Podocalyxin (PCX). The picric acid method was used to determine urine creatinine (UCR), and UAlb/UCR, URBP/UCR, and UPCX/UCR ratio were calculated.
HE Staining
First, the kidney tissues in different groups were used to make frozen sections. Then, a hematoxylin–eosin (HE) staining kit was used for HE staining. Mouse kidney sections were observed at high magnification using a microscope (400 ×).
Immunohistochemistry
Immunohistochemistry was performed as previously described. In brief, after deparaffinizing the sections were treated using 0.3% H2O2 at room temperature for 10 min to quench endogenous peroxidase. Next, 1% BSA was used to incubate sections for 30 min at 37°C to block non-specific staining. Then, sections were incubated with primary antibody overnight at 4°C. After rinsing with PBS, sections were incubated with anti-Ecad 1:200 (Proteintech, China) or anti-SMA 1:200 (Proteintech, china) antibody at 37°C for 30 min and HRP-conjugated streptavidin at 37°C for 30 min, in turn. Finally, sections were incubated with 3.3′‐diaminobenzidine tetrahydrochloride (DAB) to show positive staining. Sections were observed under a high magnification (400×) with a microscope (Olympus, Japan).
Quantitative Real-Time PCR
Kidney tissue RNA was extracted with TRizol and reverse transcribed into cDNA for qPCR. Then, Hieff® qPCR SYBR Green Master Mix (Cat No. 11201ES03; Yeasen, Shanghai, China) was used for real-time PCR detection in accordance with the protocol as follows: 95°C for 5 min, 95°C for 10 s, 60°C for 30 s, 40 cycles. The primer sequences are given as follows: VDR upstream primer: 5-GCTCAAACGCTGCGTGGACATT-3, the downstream primer: 5’-GGATGGCGATAATGTGCTGTTGC-3’; β-actin upstream primer: 5’-CATTGCTGACAGGATGCAGAAGG-3’, the downstream primer: 5’-TGCTGGAAGGTGGACAGTGAGG-3’. The results were analyzed using the 2−ΔΔCT method and shown as relative quantity (Gene/ACTB).
Cell Culture and Transfection
HK2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin. To mimic a high-glucose environment, HK2 cells were incubated in high-glucose concentration medium with 50 mM glucose (Sigma-Aldrich, USA). The siVDR (Generalbiol, China) was transfected with NanoTrans 40 (Bioogenetech, China) and negative siRNA (Generalbiol, China) was used as a control.
Wound Healing Assays
HK2 cells were seeded into 12-well plate and cultured until the cells were confluent. Once the cells reached 90% confluence, a plastic 10 µL pipette tip was used to create wounds. After washing 3 times in PBS, the cells were incubated for 24 h in DMEM without FBS. Photos were taken at 0 and 24 h after wound formation. Cell migration capacity is calculated in proportion to the wound.
Western Blot
Kidney cortical protein was extracted with RIPA lysate, and protein concentration was quantified by BCA method. Equal amount of protein was subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. The membranes were blocked by 5% skimmed milk for 1 h and then incubated with primary antibody at 4°C overnight, washed three times, then incubated with secondary antibody at room temperature for 1 h. The protein bands were detected by Gel Imaging System and analyzed with Image J.
Statistical Analysis
Statistical analyses were performed using GraphPad and SPSS 26.0. Data were expressed as the mean ± standard deviation (mean ±SD), and comparisons between two groups were performed using a Student’s t-test. One-way analysis of variance was used to compare the data between multiple groups. If the data were shown to be normally distributed, pairwise comparisons were conducted using the least significant difference test. Otherwise, pairwise comparisons were conducted using Dunnett’s T3 test. Statistical significance was set at P < 0.05.
Results
The Renal Protective Effect of Metformin in T2DM Mice is Independent of Glycemic Control
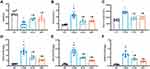
In our experiment, we used a clinically employed antidiabetic drug (GLIB) as a hypoglycemic control. As shown in Figure 1A, compared with the NC group, the body weight of each diabetes group decreased (p<0.05); compared with the T2DM group, the body weight of the MET group and the GLIB group increased (p<0.05); Compared with the NC group, FBG and HbA1c of the MET group and GLIB group were increased (p<0.05). Compared with the T2DM group, FBG and HbA1c of the MET group and GLIB group were decreased (p<0.05). There was no significant difference in FBG and HbA1c between the GLIB group and the MET group (Figure 1B and C). In addition, we measured urinary albumin (reflecting glomerular damage), RBP (reflecting proximal renal tubular dysfunction), and PCX (reflecting podocyte damage) excretion. We found that MET and GLIB significantly ameliorated the increased urine UAlb/UCR, UPCX/UCR, and URBP/UCR excretion levels in T2DM mice. Furthermore, compared to the GLIB group, the MET group significantly decreased the UAlb/UCR, UPCX/UCR, and URBP/UCR ratios, indicating that its renal protective effects were independent of glycemic control (Figure 1D–F). These results suggest that metformin treatment has renoprotective effects in T2DM mice independent of glycemic control.
Metformin Ameliorates Tubular EMT in T2DM Mice
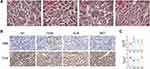
HE staining showed no obvious pathological changes in the NC group. The renal tubular cells in the T2DM group were distorted and arranged irregularly. Pathological damage was reduced in the MET group and was less severe than in the GLIB group (Figure 2A). These morphological changes may be hallmarks of EMT in renal tubular epithelial cells. Furthermore, metformin has been reported to protect renal tubular cells from EMT in various animal models of acute kidney disease (AKI).22 Therefore, we examined the expression of markers of EMT (SMA and Ecad) in diabetic kidney disease by IHC. IHC analysis showed that SMA expression increased in T2DM mice compared to NC group mice, while Ecad expression decreased. Their expression was significantly improved after metformin administration and was superior to the GLIB group (Figure 2B and C). Collectively, these findings suggest that metformin treatment may significantly improve renal tubular epithelial EMT in T2DM mice independent of glycemic control.
Metformin Intervention Upregulates the Expression of VDR in DKD
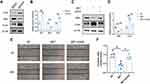
A recent trial showed a reduction in VDR in rats with diabetic kidney disease.23 To further verify the results, we detected the level of VDR mRNA and protein in each group of kidney tissues. Compared with the NC group, the VDR mRNA level of each diabetes group decreased, and it was further found that the VDR mRNA level of the MET group was higher than that of the T2DM group and the GLIB group (p<0.05) (Figure 3A). Similarly, WB results showed (Figure 3B and C) that compared with the NC group, the relative expression of VDR protein in each diabetes group decreased, and the expression level of VDR protein in the MET group was higher than that in the T2DM group and the GLIB group (p<0.05).
Metformin Ameliorates VDR Expression and EMT in the HK-2 Cell Model of Hyperglycemia Injury
Subsequently, we investigated the effects of VDR expression and EMT in HK2 cells cultured in vitro under high-glucose and metformin intervention. HK2 cells were treated with normal glucose (NG), high-glucose (HG), and high-glucose plus metformin (HG+MET). By analyzing the WB results, we found that metformin rescued the reduction of VDR under a high-glucose environment (Figure 4A and B). At the same time, we further found that metformin could rescue the changes of Ecad and SMA under a high-glucose environment. Similarly, in HK2 cells, metformin intervention also rescued the increase in cell motility and invasion induced by high-glucose intervention (Figure 4C and D). Collectively, these findings suggest that metformin improves VDR expression and EMT in HK-2 cells model of high-glucose injury.
Metformin Inhibits EMT by Upregulating VDR in HK2 Cells
It has been reported that VDR activation alleviates intestinal fibrosis by inhibiting fibroblast activation and epithelial mitochondria-mediated EMT.24 To confirm whether VDR expression could affect EMT in DKD, we intervened cells with the VDR agonist calcitriol. Compared with the DMSO group, calcitriol intervention reduced SMA and increased Ecad expression in HK2 cells (p<0.05) (Figure 5A and B). After that, we further found that VDR knockdown is sufficient to rescue MET intervention on Ecad and SMA expression in HK2 cells (Figure 5C and D). Furthermore, compared to the NC group, the MET group was found to repress cell motility and invasion. However, these downregulations were inhibited after transfection with siVDR (Figure 5E and F). In conclusion, these findings suggest that metformin improves EMT in HK-2 cells by modulating VDR.
Discussion
Metformin is used as first-line therapy for type 2 diabetes (T2D). Based on its various pharmacologic actions, the renoprotective effects of metformin have been extensively studied.13 Although several signaling pathways by which metformin regulates EMT have been previously reported, the detailed molecular mechanisms of diabetes-induced renal tubular EMT are not fully understood. Here, we used HK2 cells cultured in HG as an in vitro model of DKD and demonstrated that VDR expression has an important role in HG-induced EMT. In particular, our findings suggest that VDR knockdown rescues MET intervention on EMT in HK2 cells. Based on our findings, we believe that VDR may serve as a potential molecular target for blocking tubular EMT and fibrosis in DKD.
Diabetes is one of the leading causes of end-stage renal disease (ESRD), and approximately one-third of people with type 2 diabetes will eventually develop DKD.25 Reducing the progression of kidney damage and managing associated complications are goals of DKD treatment.26 Metformin is widely used in the treatment of diabetes due to its various pharmacological effects.27 In addition, many studies have shown that metformin also has a protective effect on the kidneys and can delay the occurrence and progression of DKD.28 Our study showed that urinary albumin (reflecting glomerular injury), RBP (reflecting proximal tubular function injury), and PCX (reflecting podocyte injury) excretion were significantly increased in type 2 diabetic mice compared with normal controls. HE staining showed the existence of significant renal injury in mice in the T2DM group under hyperglycemic condition. These symptoms are similar to those previously reported for kidney disease.29 After MET and GLIB intervention, the above urinary indexes and renal histopathological changes were significantly improved. In addition, we further found similar glycemic control in the MET and GLIB groups, but renal improvement was more significant in the MET group. It is suggested that the renal protection of diabetic mice by metformin treatment is partially independent of glycemic control.
The vitamin D receptor (VDR) is a nuclear steroid hormone receptor that binds 1.25 dihydroxyvitamin D3 (1,25(OH) 2D3) with high affinity and regulates calcium metabolism as well as cell proliferation and differentiation.30,31 DKD has a very close relationship with VDR.32 In the diabetic state, VDR regulates JAK/STAT signaling to protect mesenchymal cells from high-glucose damage.33 Da et al showed that calcitriol activates VDR-suppressed p38MAPK signaling and reduces apoptosis in renal tubular epithelial cells in diabetic nephropathy.34 In addition, activation of VDR in DKD has good effects in reducing proteinuria, alleviating podocyte damage, renal tubulopathy, interstitial fibrosis, and anti-inflammatory effects.18 The results of this study showed that compared with the NC group, the expression of VDR mRNA and protein in the kidney tissue of the T2DM group decreased. After metformin intervention treatment, it increased significantly, and was higher than that in the glibenclamide group, suggesting that MET intervention treatment could upregulate the expression of VDR in the kidneys of diabetic mice, which was partially independent of glycemic control. Similarly, in vitro experiments showed that high glucose treatment of HK2 cells decreased VDR expression, which was significantly increased after metformin intervention. Although we show that metformin decreases VDR expression, the mechanism behind this regulation is unclear, and this needs to be further explored.
EMT is an important biological process during which epithelial cells can transdifferentiate into mesenchymal cells and contribute to pathological fibrosis, chronic inflammation, and wound healing.35 EMT is also one of the important causes of tubulointerstitial fibrosis in DKD, so EMT of renal tubular cells is considered to be a key process in the development of tubulointerstitial fibrosis in DKD.36 By analyzing the expression of SMA, Ecad, and wound-healing assays, we found that EMT occurred in renal tubular epithelial cells in a high-glucose environment and recovered after metformin intervention. In addition, we found that the increase of VDR was accompanied by the down-regulation of EMT by calcitriol intervention. Similarly, studies have reported VDR as an important regulator of EMT in RCC cells.37 Therefore, we further hypothesize that MET can improve EMT through VDR in DKD. Ultimately, we demonstrate that knockdown of VDR can alter the therapeutic effect of MET on EMT.
Due to some limitations of our study, there is still a need for further research on this topic. Firstly, due to relevant conditions, clinical DKD kidney specimens have not been collected for examination. Second, we did not explore the mechanism of VDR activation by metformin in detail. Therefore, further studies are needed in the future to improve the more detailed understanding of metformin by increasing VDR expression.
Conclusion
In conclusion, the present study showed that inhibition of EMT process and upregulation of VDR were found in renal tubular epithelial cells when treated with metformin. Further, overexpression of VDR inhibited the EMT process. Mechanistic analysis showed that metformin inhibits EMT by upregulating VDR in diabetic kidney disease. In summary, our findings provide a new theoretical basis for the treatment of diabetic kidney disease with metformin.
Ethical Approval
All animal experimental procedures were performed in accordance with the guidelines of the Regulation for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 1988, revised in March 2017) and approved by the ethics committee of the First Affiliated Hospital of University of Science and Technology of China. This study was also conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals (China).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the local scientific and technological development project guided by the Central Government of China (no. 2017070802D147).
Disclosure
The authors declare that they have no competing interests.
References
1. Tulipano G. Integrated or independent actions of metformin in target tissues underlying its current use and new possible applications in the endocrine and metabolic disorder area. Int J Mol Sci. 2021;22(23):13068. doi:10.3390/ijms222313068
2. Bost F, Rena G, Viollet B. Editorial: metformin: beyond diabetes. Front Endocrinol. 2019;10:851. doi:10.3389/fendo.2019.00851
3. Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective effects of metformin in diabetic nephropathy. J Cell Physiol. 2017;232(4):731–742. doi:10.1002/jcp.25598
4. Narongkiatikhun P, Chattipakorn SC, Chattipakorn N. Mitochondrial dynamics and diabetic kidney disease: missing pieces for the puzzle of therapeutic approaches. J Cell Mol Med. 2021;26:249–273. doi:10.1111/jcmm.17116
5. Zhang X, Zhou Y, Ma R. Potential effects and application prospect of angiotensin receptor-neprilysin inhibitor in diabetic kidney disease. J Diabetes Complications. 2021;36:108056. doi:10.1016/j.jdiacomp.2021.108056
6. Kong L, Andrikopoulos S, MacIsaac R, et al. The role of the adaptive immune system in diabetic kidney disease (Dkd). J Diabetes Investig. 2021;13:213–226. doi:10.1111/jdi.13725
7. Ahmad AA, Draves SO, Rosca M. Mitochondria in diabetic kidney disease. Cells. 2021;10(11):2945. doi:10.3390/cells10112945
8. Kleinaki Z, Kapnisi S, Theodorelou-Charitou SA, Nikas IP, Paschou SA. Type 2 diabetes mellitus management in patients with chronic kidney disease: an update. Hormones. 2020;19(4):467–476. doi:10.1007/s42000-020-00212-y
9. Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–906. doi:10.1056/NEJMc1602469
10. Liu H, Yan R, Liang L, et al. The role of Cdx2 in renal tubular lesions during diabetic kidney disease. Aging. 2021;13(5):6782–6803. doi:10.18632/aging.202537
11. Xu Y, Huang J, Xin W, et al. Lipid accumulation is ahead of epithelial-to-mesenchymal transition and therapeutic intervention by acetyl-coa carboxylase 2 silence in diabetic nephropathy. Metabolism. 2014;63(5):716–726. doi:10.1016/j.metabol.2014.02.010
12. Li Y, Xue M, Hu F, et al. Klotho prevents epithelial-mesenchymal transition through Egr-1 downregulation in diabetic kidney disease. BMJ Open Diabetes Res Care. 2021;9(1):e002038. doi:10.1136/bmjdrc-2020-002038
13. Kawanami D, Takashi Y, Tanabe M. Significance of metformin use in diabetic kidney disease. Int J Mol Sci. 2020;21(12):4239. doi:10.3390/ijms21124239
14. Janjusevic M, Gagno G, Fluca AL, et al. The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci. 2021;289:120193. doi:10.1016/j.lfs.2021.120193
15. Voutsadakis IA. Vitamin D receptor (Vdr) and Metabolizing Enzymes Cyp27b1 and Cyp24a1 in breast cancer. Mol Biol Rep. 2020;47(12):9821–9830. doi:10.1007/s11033-020-05780-1
16. Battistini C, Ballan R, Herkenhoff ME, Saad SMI, Sun J. Vitamin D modulates intestinal microbiota in inflammatory bowel diseases. Int J Mol Sci. 2020;22(1):362. doi:10.3390/ijms22010362
17. Crescioli C. Vitamin D restores skeletal muscle cell remodeling and myogenic program: potential impact on human health. Int J Mol Sci. 2021;22(4):1760. doi:10.3390/ijms22041760
18. Lei M, Liu Z, Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. Biomed Res Int. 2020;2020:4137268. doi:10.1155/2020/4137268
19. Gembillo G, Cernaro V, Salvo A, et al. Role of vitamin D status in diabetic patients with renal disease. Medicina. 2019;55(6). doi:10.3390/medicina55060273
20. Gembillo G, Siligato R, Amatruda M, Conti G, Santoro D. Vitamin D and Glomerulonephritis. Medicina. 2021;57(2). doi:10.3390/medicina57020186
21. Yang S, Li A, Wang J, et al. Vitamin D receptor: a novel therapeutic target for kidney diseases. Curr Med Chem. 2018;25(27):3256–3271. doi:10.2174/0929867325666180214122352
22. Guan M, Li W, Xu L, et al. Metformin improves epithelial-to-mesenchymal transition induced by tgf-beta1 in renal tubular Epithelial Nrk-52e Cells Via Inhibiting Egr-1. J Diabetes Res. 2018;2018:1031367. doi:10.1155/2018/1031367
23. Song Z, Xiao C, Jia X, et al. Vitamin D/Vdr protects against diabetic kidney disease by restoring podocytes autophagy. Diabetes Metab Syndr Obes. 2021;14:1681–1693. doi:10.2147/DMSO.S303018
24. Yu M, Wu H, Wang J, et al. Vitamin D receptor inhibits emt via regulation of the epithelial mitochondrial function in intestinal fibrosis. J Biol Chem. 2021;296:100531. doi:10.1016/j.jbc.2021.100531
25. Ahmad N, Veerapalli H, Lankala CR, et al. Endothelin receptor antagonists as a potential treatment of diabetic nephropathy: a systematic review. Cureus. 2021;13(11):e19325. doi:10.7759/cureus.19325
26. Zhang X, Shi Z, Liu Q, Quan H, Cheng X. Effects of coenzyme Q10 intervention on diabetic kidney disease: a systematic review and meta-analysis. Medicine. 2019;98(24):e15850. Doi:10.1097/MD.0000000000015850
27. Salvatore T, Pafundi PC, Morgillo F, et al. Metformin: an old drug against old age and associated morbidities. Diabetes Res Clin Pract. 2020;160:108025. doi:10.1016/j.diabres.2020.108025
28. Beladi Mousavi SS, Nasri H, Rafieian-Kopaei M, Tamadon MR.Metformin improves diabetic kidney disease. J Nephropharmacol. 2012;1(1):1–2.
29. Wei L, Li Y, Yu Y, et al. Obesity-related glomerulopathy: from mechanism to therapeutic target. Diabetes Metab Syndr Obes. 2021;14:4371–4380. doi:10.2147/DMSO.S334199
30. Becker AL, Carpenter EL, Slominski AT, Indra AK. The role of the vitamin D receptor in the pathogenesis, prognosis, and treatment of cutaneous melanoma. Front Oncol. 2021;11:743667. doi:10.3389/fonc.2021.743667
31. Campbell MJ, Trump DL. Vitamin D receptor signaling and cancer. Endocrinol Metab Clin North Am. 2017;46(4):1009–1038. doi:10.1016/j.ecl.2017.07.007
32. Libby AE, Jones B, Lopez-Santiago I, Rowland E, Levi M. Nuclear receptors in the kidney during health and disease. Mol Aspects Med. 2021;78:100935. doi:10.1016/j.mam.2020.100935
33. Yang Y, Lei Y, Liang Y, et al. Vitamin D protects glomerular mesangial cells from high glucose-induced injury by repressing jak/stat signaling. Int Urol Nephrol. 2021;53(6):1247–1254. doi:10.1007/s11255-020-02728-z
34. Guo Y, Xie X, Zhao Y, Zhou M, Yang Y, Zhang X. Calcitriol attenuates renal tubular epithelial cells apoptosis via inhibiting P38MAPK signaling in diabetic nephropathy. Acta Diabetol. 2020;57(11):1327–1335. doi:10.1007/s00592-020-01554-0
35. Dong HH, Xiang S, Chen XP, et al. The epithelial-mesenchymal transition promotes transdifferentiation of subcutaneously implanted hepatic oval cells into mesenchymal tumor tissue. Stem Cells Dev. 2009;18(9):1293–1298. doi:10.1089/scd.2008.0321
36. Han J, Pang X, Shi X, Zhang Y, Peng Z, Xing Y. Ginkgo biloba extract Egb761 ameliorates the extracellular matrix accumulation and mesenchymal transformation of renal tubules in diabetic kidney disease by inhibiting endoplasmic reticulum stress. Biomed Res Int. 2021;2021:6657206. doi:10.1155/2021/6657206
37. Xu S, Zhang ZH, Fu L, et al. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing smad2/3-, Stat3- and beta-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. 2020;111(1):59–71. doi:10.1111/cas.14237
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.