Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Metastatic Hepatic Epithelioid Hemangioendothelioma Treated with Olaratumab: A Falling Star Rising?
Authors Kyriazoglou A, Koutsoukos K, Zagouri F, Liontos M , Dimitriadis E, Tiniakos D, Dimopoulos MA
Received 25 June 2019
Accepted for publication 12 November 2019
Published 27 February 2020 Volume 2020:16 Pages 141—146
DOI https://doi.org/10.2147/TCRM.S220804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Anastasios Kyriazoglou, 1 Konstantinos Koutsoukos, 1 Flora Zagouri, 1 Michalis Liontos, 1 Efthimios Dimitriadis, 2 Dina Tiniakos, 3, 4 Meletios Athanasios Dimopoulos 1
1Department of Clinical Therapeutics, General Hospital Alexandra, Athens, Greece; 2Department of Genetics, Agios Savvas Hospital, Athens, Greece; 3Department of Pathology Aretaion Hospital, National & Kapodistrian University of Athens, Athens, Greece; 4Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK
Correspondence: Anastasios Kyriazoglou
Vasilisis Sofias 80, Athens 11528, Greece
Tel +302132162545
Fax +302132162511
Email [email protected]
Abstract: Epithelioid hemangioendothelioma (EHE) is a rare vascular malignant tumor with indolent course. Liver transplantation for local disease is the treatment of choice. In the metastatic setting there is no consensus regarding the appropriate systemic treatment. We present two cases of metastatic hepatic epithelioid hemangioendothelioma (hEHE) treated with the combination of Doxorubicin and Olaratumab. Both patients showed Stable Disease (SD) as a response, after the completion of six cycles of this combination therapy.
Keywords: Olaratumab and Doxorubicin, metastatic hepatic epithelioid hemangioendothelioma, 1st line treatment
Introduction
Epithelioid hemangioendothelioma (EHE) is a rare vascular sarcoma of intermediate malignant potential with an indolent course.1 Hepatic epithelioid hemangioendothelioma (hEHE) presents usually with multifocal lesions and unpredictable progression. Recurrence and metastases to several distant sites such as bones, lungs and soft tissues can occur at any time.1
Recent development in the diagnosis of these tumors is the identification of two specific fusion genes; WWTR1(TAZ)-CAMTA1 and YAP1-TFE3, which are pathognomonic for EHE.2–4 Further, they shed light on the biological mechanism involved in the development of these tumors. Both TAZ and YAP are co-transcription factors, being the principal effectors of Hippo signaling pathway. TAZ and YAP via TEAD transcription factor alter the expression of their downstream targets. Interestingly, Hippo pathway gains a pivotal role in the tumorigenesis of hEHE.5,6
Treatment of hEHE is still surgical. For localized disease; hepatic transplantation is the treatment of choice.7,8 However, when metastatic disease exists; systemic treatment should be considered.9,10 Regarding the selection of the most appropriate systemic treatment there is no consensus. European Society of Medical Oncology (ESMO) and National Comprehensive Cancer network (NCCN) guidelines do not recommend any specific regimens for Stage IV EHE and clinicians treat those patients like any other patient with a soft tissue sarcoma.10–12 Anthracycline-based chemotherapy is the standard of choice for 1st line treatment. Recently, a Phase II randomized trial showed that the addition of Olaratumab (a anti PDGFRα monoclonal antibody) to standard Doxorubicin resulted in a 11.8 month survival benefit as compared to Doxorubicin monotherapy in patients with advanced soft tissue sarcoma of various histology.13 This combination regimen was incorporated in both ESMO and NCCN guidelines, despite original skepticism. However, according to a recent press release by the Olaratumab manufacturer, the primary endpoint of overall survival (OS) benefit with the combination of Olaratumab plus Doxorubicin was not met for patients with advanced or metastatic soft tissue sarcoma in the Phase III ANNOUNCE clinical trial.14
Based on the initial indication of the drug, we present herein two cases of hEHE treated with the combination of Doxorubicin and Olaratumab in the 1st line setting.
Both patients have provided written informed consent to have the case details and the accompanying images. The ethics committee of Alexandra General Hospital approved the study and provided approval to publish the case details
Patients and Methods
Patient 1
A 33-year-old male presented with the diagnosis of metastatic hEHE. In a routine blood test, alkaline phosphatase and γ-glutamyl transferase were found over the highest normal level as an incidental finding. Subsequent imaging with Ultrasound of the abdomen revealed multiple hepatic lesions. Colonoscopy and gastroscopy were normal. A CT scan of the chest and the abdomen was performed revealing a lytic lesion of the 5th right rib and confirming the multiple hepatic lesions. Brain MRI showed a lytic lesion of the clivus bone. Imaging was completed with a PET CT which confirmed the lesions described from previous tests. Biopsy of the hepatic lesions favored the diagnosis hEHE.
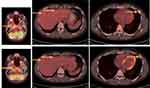
The patient requested consultation from Cleveland Clinic, Cleveland, OH, USA, where a diagnosis of YAP1/TFE3 fused EHE was made based on negative CAMTA1 and diffuse strongly positive nuclearTFE3 immunostain in tumor cells. The patient was treated with the combination of Doxorubicin (75mg/m2)-Olaratumab (15mg/kg) for six cycles and continued with Olaratumab (15mg/kg) maintenance until the removal of the product from the market. The patient had no adverse effects from the treatment. Restaging with CT scans after the completion of the six cycles of chemotherapy revealed SD. In addition, a PET CT was performed and revealed decreased absorption of 18-FDG of the known lesions, indicative of Partial Response (PR) (Figure 1).
Patient 2
A 62-year-old male, receiving chronic treatment for chronic obstructive pulmonary disease, presented with imaging that showed multiple hepatic lesions. He has been diagnosed with testicular seminoma 20 years ago and had received several lines of treatment for advanced disease including 4 cycles of Bleomycin, Etoposide, Cisplatin (BEP), 4 cycles of Vepeside, Ifosfamide, Cisplatin (VIP), laparotomy, autologous transplantation (June of 1998) and 7 cycles of Carboplatin-Etoposide until May 2001. Since then, the patient remained in full remission on annual or biannual follow up. CT scans of abdomen and chest on September of 2018 revealed multiple hepatic and lung lesions, further confirmed by imaging with FDG PET/CT. CT guided needle biopsy of one of the liver tumors favored the diagnosis of hEHE. Molecular testing could not be performed due to limited remaining diagnostic tissue material.
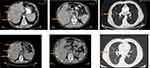
The patient was treated with the combination of Doxorubicin (75mg/m2)-Olaratumab (15mg/kg) for 6 cycles. The patient tolerated the treatment well, without any severe adverse effects. Restaging with CT scans after the completion of the 6 cycles of chemotherapy revealed SD, with some liver tumors showing a decrease in diameter (Figure 2). The patient tolerated therapy very well, with minor toxicities, mainly low grade neutropenia, anemia and nausea. Granulocyte-colony stimulation factor (GCSF) was administered for prophylaxis in every cycle. Following the negative outcome of the ANNOUNCE trial, it was decided to permanently discontinue Olaratumab, since the patient did not experience any clinically meaningful response and reimbursement re-approval by Greek Public Insurance was also needed.
Results
Histopathology
In patient 1, histologic examination of the hepatic tumor biopsy specimen showed medium-sized epithelioid tumor cells with Ki67 Labeling index (LI) 8–10%, positive for the endothelial markers CD34, CD31 and FVIII, and also positive for EGFR, CD10 and Vimentin while HepPar1, AFP, CEA, HHV-8 and K8/18 were negative, in keeping with hEHE.
In patient 2, biopsy of one of the hepatic tumors showed epithelioid neoplastic cells with an intravascular pattern of growth and Ki67:<1%. Tumour cells were positive for CD31 and CD34, while keratins, OCT3/4, PLAP, TTF1 and napsin A were negative in keeping with hEHE and excluding metastatic seminoma.
Genetics
Real time PCR for the detection of WWTR1(TAZ)/CAMTA1 or YAP1/TFE3 was not informative for patient 1, while for patient 2 there was no tissue material available for testing.
Discussion
HEHE is a rare vascular mesenchymal malignancy with indolent course. Surgery is the treatment of choice when this can lead to R0 excision. However, recurrence or metastasis can occur at any time.
Surgical treatment of hEHE includes liver resection or liver transplantation. Konstantinidis et al, in their study of 131 patients with angiosarcoma or hEHE compared liver resection and transplantation, showing similar median overall survival (mOS).7 Especially for hEHE patients both approaches had similar OS. The same conclusion was reported from Mayo clinic, Rochester, MN, USA, in a retrospective study of 30 patients with hEHE. However, it must be highlighted that metastasis is not a contraindication for liver transplantation. Smaller studies included hEHE patients who underwent liver transplantation without extrahepatic disease, with very good outcomes.8,15–17
Systemic treatment for metastatic or progressed hEHE is still not well established. There is no consensus on the therapeutic algorithm which clinicians should follow.9 Chemotherapy with several regimens such as Doxorubicin with or without ifosfamide, paclitaxel, epirubicin and dacarbazine, 5FU and mitomycin have been reported in small series.18–22 Tyrosine kinase inhibitors such as pazopanib, sunitinib, apatinib and sorafenib have been studied showing PRor stabilization of the disease.23–28 Targeting angiogenesis with bevacizumab, interferon alpha 2b, thalidomide and lenalidomide revealed contradictory results.29–33 Case reports using metronomic cyclophosphamide and sirolimus have also been published.34,35 The largest cohort of patients reported includes 32 patients from the Royal Marsden Hospital, London, UK, and depicts the heterogeneous therapeutic approach of patients with these rare tumors ranging from observation to systemic treatment including cytotoxic regimens and targeted therapies.10
Recently, two specific fusion genes have been identified for EHE. WWTR1/CAMTA1 and YAP1/TFE3 are pathognomonic for the diagnosis.2,3,36 WWTR1 (TAZ) and YAP1 are two co-transcription factors, which represent the main molecular effectors of Hippo pathway.37 Both fusion genes consist of the N-terminus of WWTR1 and YAP1, containing the WW (tryptophan-tryptophan) domain and TEAD binding domain respectively, fused with the C-terminus of CAMTA1 and TFE3 transcription factors.5 Targeting YAP and TAZ interaction with TEAD or inhibiting Hippo pathway is a potent systemic treatment for EHE. Clinical trials testing MEK inhibitor trametinib and anti-microtubular agent eribulin are still running.
The addition of Olaratumab to Doxorubicin offered a benefit of 11.8 months in OS; in a phase Ib-II clinical trial comparing the doublet to Doxorubicin monotherapy.13 This advancement on the treatment of patients with soft tissue sarcomas was highlighted by the inclusion of Doxorubicin and Olaratumab combination in the recent versions of both NCCN and ESMO guidelines.11,12 However, ESMO members have raised important criticism regarding the unknown mechanism of action of Olaratumab and the small benefit of PFS in the study.12 Unfortunately, Olaratumab did not meet the primary endpoints of OS both to overall population and the subgroup of leiomyosarcomas.
We have treated two patients with metastatic hEHE with the combination of Doxorubicin and Olaratumab on 1st line setting. Both patients had multifocal liver disease and metastases making the option of liver transplantation or hepatectomy impossible. Under the perspective of a metastatic soft tissue sarcoma without any targeted therapies approved, Olaratumab and Doxorubicin were chosen. The phase Ib-II clinical trial did not include any patients with EHE in the arm of Olaratumab.13 We are the first to report real world data with the administration of this combination to hEHE patients, with SD as best response for both of them.
Real world data reporting the use of Olaratumab are scarce. We have recently published the poor outcome of Olaratumab administration to two patients with phyllodes tumor of the breast.38 Herein, we report the potentially beneficial addition of Olaratumab to the 1st line treatment of metastatic hEHE.
The molecular mechanism by which PDGFR inhibition works in EHE is unknown. Though, it is intriguing to hypothesize that WWTR1/CAMTA1 and YAP1/TFE3 fusions can be associated to PDGFR inhibition. Interestingly, PDGFR has been shown to crosstalk with Hippo pathway. Smoot et al have described PDGFR regulation of YAP localization and expression via SFK kinase phosphorylation in cholangiocarcinoma cell lines, xenografts and mice.6 Pharmacologic inhibition of PDGFR signaling with crenolanib had a profound effect on Hippo pathway’s downstream targets expression, such as CTGF and Cyr61.6 All these data demonstrate a hint for a possible interaction of PDGFR inhibition through Olaratumab and –– the principal for the development of EHE deregulation – Hippo pathway through the formation of WWTR1/CAMTA1 and YAP1/TFE3 fusion genes.
It is reasonable to support that the response of our patients was due to the action of Doxorubicin alone. However, in the few cases reported and treated with Doxorubicin; best response ranged from PR to SD or PD.10,18,39,40 A possible explanation for the response of the two cases of metastatic hEHE described herein; may be the biologic behavior of this tumor type, which progresses slowly. Therapeutic targeting of angiogenesis appears important; supporting a potential benefit of Olaratumab addition to the therapeutic approach of this rare tumor.
Under the perspective of the recent failure of Olaratumab to add any treatment benefit, a more critical view to the design of sarcoma clinical trials is highlighted. Histology-based reports, even with small number of cases, due to the rarity of these neoplasms, are urgently needed. Real world data with Olaratumab administration and subanalysis of the ANNOUNCE phase III Clinical trial are highly awaited, in order to assess the effectiveness of this new drug.
Acknowledgments
We would like to thank Dr. Evangelia Skoura MD, MSc, PhD for her assistance with the assessment of patients’ imaging.
Disclosure
Dr Michalis Liontos reports personal fees from Janssen, personal fees from Astellas, non-financial support from Sanofi, non-financial support from Roche, personal fees from MSD, non-financial support from Ipsen, non-financial support from BMS, outside the submitted work. Professor Meletios Athanasios Dimopoulos reports personal fees from Amgen, personal fees from Takeda, personal fees from Janssen, personal fees from BMS, personal fees from Celgene, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ravi V, Patel S. Vascular sarcomas. Curr Oncol Rep. 2013;15(4):347–355. doi:10.1007/s11912-013-0328-2
2. Lee SJ, Yang WI, Chung WS, et al. Epithelioid hemangioendotheliomas with TFE3 gene translocations are compossible with CAMTA1 gene rearrangements. Oncotarget. 2016;7(7):7480–7488. doi:10.18632/oncotarget.7060
3. Errani C, Zhang L, Sung YS, et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50(8):644–653. doi:10.1002/gcc.20886
4. Tanas MR, Sboner A, Oliveira AM, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3(98):98ra82. doi:10.1126/scitranslmed.3002409
5. Lamar JM, Motilal Nehru V, Weinberg G. Epithelioid hemangioendothelioma as a model of YAP/TAZ-driven cancer: insights from a rare fusion sarcoma. Cancers (Basel). 2018;10:7. doi:10.3390/cancers10070229
6. Smoot RL, Werneburg NW, Sugihara T, et al. Platelet-derived growth factor regulates YAP transcriptional activity via Src family kinase dependent tyrosine phosphorylation. J Cell Biochem. 2018;119(1):824–836. doi:10.1002/jcb.26246
7. Konstantinidis IT, Nota C, Jutric Z, et al. Primary liver sarcomas in the modern era: resection or transplantation? J Surg Oncol. 2018;117(5):886–891. doi:10.1002/jso.24979
8. Agrawal N, Parajuli S, Zhao P, et al. Liver transplantation in the management of hepatic epithelioid hemangioendothelioma: a single-center experience and review of the literature. Transplant Proc. 2011;43(7):2647–2650. doi:10.1016/j.transproceed.2011.06.035
9. Thomas RM, Aloia TA, Truty MJ, et al. Treatment sequencing strategy for hepatic epithelioid haemangioendothelioma. HPB (Oxford). 2014;16(7):677–685. doi:10.1111/hpb.12202
10. Yousaf N, Maruzzo M, Judson I, et al. Systemic treatment options for epithelioid haemangioendothelioma: the Royal Marsden Hospital experience. Anticancer Res. 2015;35(1):473–480.
11. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563. doi:10.6004/jnccn.2018.0025
12. Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv51–iv67. doi:10.1093/annonc/mdy096
13. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised Phase 2 trial. Lancet. 2016;388(10043):488–497. doi:10.1016/S0140-6736(16)30587-6
14. Tap WD, Wagner AJ, Papai Z, et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS). J Clin Oncol. 2019;37(suppl; abstr LBA3). doi:10.1200/JCO.2019.37.18_suppl.LBA3
15. Remiszewski P, Szczerba E, Kalinowski P, et al. Epithelioid hemangioendothelioma of the liver as a rare indication for liver transplantation. World J Gastroenterol. 2014;20(32):11333–11339. doi:10.3748/wjg.v20.i32.11333
16. Lai Q, Feys E, Karam V, et al. Hepatic epithelioid hemangioendothelioma and adult liver transplantation: proposal for a prognostic score based on the analysis of the ELTR-ELITA registry. Transplantation. 2017;101(3):555–564. doi:10.1097/TP.0000000000001603
17. Jung DH, Hwang S, Hong SM, et al. Clinicopathological features and prognosis of hepatic epithelioid hemangioendothelioma after liver resection and transplantation. Ann Transplant. 2016;21:784–790. doi:10.12659/AOT.901172
18. Ye B, Li W, Feng J, et al. Treatment of pulmonary epithelioid hemangioendothelioma with combination chemotherapy: report of three cases and review of the literature. Oncol Lett. 2013;5(5):1491–1496. doi:10.3892/ol.2013.1217
19. Kelly H, O’Neil BH. Response of epithelioid haemangioendothelioma to liposomal doxorubicin. Lancet Oncol. 2005;6(10):813–815. doi:10.1016/S1470-2045(05)70392-2
20. Treska V, Daum O, Svajdler M, et al. Hepatic epithelioid hemangioendothelioma - a rare tumor and diagnostic dilemma. In Vivo. 2017;31(4):763–767. doi:10.21873/invivo.11128
21. Gurung S, Fu H, Zhang WW, et al. Hepatic epithelioid hemangioendothelioma metastasized to the peritoneum, omentum and mesentery: a case report. Int J Clin Exp Pathol. 2015;8(5):5883–5889.
22. Lee YJ, Chung MJ, Jeong KC, et al. Pleural epithelioid hemangioendothelioma. Yonsei Med J. 2008;49(6):1036–1040. doi:10.3349/ymj.2008.49.6.1036
23. Tolkach Y, Petrov S, Lerut E, et al. Epithelioid hemangioendothelioma of the kidney treated with sunitinib. Onkologie. 2012;35(6):376–378. doi:10.1159/000338944
24. Saada E, Saint Paul MC, Gugenheim J, et al. Metastatic hepatic epithelioid hemangio-endothelioma: long-term response to sunitinib malate. Oncol Res Treat. 2014;37(3):124–126. doi:10.1159/000360208
25. Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer. 2013;119(14):2639–2644. doi:10.1002/cncr.28109
26. Kollar A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56(1):88–92. doi:10.1080/0284186X.2016.1234068
27. Semenisty V, Naroditsky I, Keidar Z, et al. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer. 2015;15:402. doi:10.1186/s12885-015-1395-6
28. Zheng Z, Wang H, Jiang H, et al. Apatinib for the treatment of pulmonary epithelioid hemangioendothelioma: a case report and literature review. Medicine (Baltimore). 2017;96(45):e8507. doi:10.1097/MD.0000000000008507
29. Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24(1):257–263. doi:10.1093/annonc/mds237
30. Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22(4):351–355. doi:10.1097/CCO.0b013e32833aaad4
31. Pallotti MC, Nannini M, Agostinelli C, et al. Long-term durable response to lenalidomide in a patient with hepatic epithelioid hemangioendothelioma. World J Gastroenterol. 2014;20(22):7049–7054. doi:10.3748/wjg.v20.i22.7049
32. Demir L, Can A, Oztop R, et al. Malignant epithelioid hemangioendothelioma progressing after chemotherapy and Interferon treatment: a case presentation and a brief review of the literature. J Cancer Res Ther. 2013;9(1):125–127. doi:10.4103/0973-1482.110386
33. Soape MP, Verma R, Payne JD, et al. Treatment of hepatic epithelioid hemangioendothelioma: finding uses for thalidomide in a new era of medicine. Case Rep Gastrointest Med. 2015;2015:326795. doi:10.1155/2015/326795
34. Lakkis Z, Kim S, Delabrousse E, et al. Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol. 2013;58(6):1254–1257. doi:10.1016/j.jhep.2013.01.043
35. Stacchiotti S, Provenzano S, Dagrada G, et al. Sirolimus in advanced epithelioid hemangioendothelioma: a retrospective case-series analysis from the Italian rare cancer network database. Ann Surg Oncol. 2016;23(9):2735–2744. doi:10.1245/s10434-016-5331-z
36. Flucke U, Vogels RJ, Somerhausen ND, et al. Epithelioid hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi:10.1186/1746-1596-9-131
37. Varelas X. The hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. doi:10.1242/dev.102376
38. Kyriazoglou A, Zagouri F, Dimopoulos MA. Olaratumab administered in two cases of phyllodes tumor of the breast: end of the beginning? ESMO Open. 2019;4:e000479. doi:10.1136/esmoopen-2018-000479
39. Afrit M, Nasri M, Labidi S, et al. Aggressive primary hepatic epithelioid hemangioendothelioma: a case report and literature review. Cancer Biol Med. 2017;14(2):187–190. doi:10.20892/j.issn.2095-3941.2016.0105
40. Idilman R, Dokmeci A, Beyler AR, et al. Successful medical treatment of an epithelioid hemangioendothelioma of liver. Oncology. 1997;54(2):171–175. doi:10.1159/000227683
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.