Back to Journals » Cancer Management and Research » Volume 13
Metabolic Syndrome-Related Hyperuricemia is Associated with a Poorer Prognosis in Patients with Colorectal Cancer: A Multicenter Retrospective Study
Authors Feng Q, Tang LJ, Luo DH, Wang Y, Wu N, Chen H, Chen MX, Jiang L , Jin R
Received 13 September 2021
Accepted for publication 12 November 2021
Published 24 November 2021 Volume 2021:13 Pages 8809—8819
DOI https://doi.org/10.2147/CMAR.S338783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Qian Feng,1 Liang-Jie Tang,2 Ding-Hai Luo,3 Ying Wang,1 Nan Wu,1 Hao Chen,1 Meng-Xia Chen,1 Lei Jiang,4 Rong Jin1,5
1Department of Gastroenterology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 2NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 3Department of Gastroenterology, Taizhou Hospital of Zhejiang Province, Taizhou, 317000, People’s Republic of China; 4Central Laboratory, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 5Department of Epidemiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Rong Jin
Department of Gastroenterology, Department of Epidemiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Email [email protected]
Lei Jiang
Central Laboratory, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Tel +86-577-55579127
Fax +86-577-55578999
Email [email protected]
Purpose: Hyperuricemia and metabolic syndrome (MetS) have been shown to correlate with prognosis in patients with malignant tumors. The present study evaluated the relationship between preoperative hyperuricemia and MetS in colorectal cancer (CRC) patients and analyzed the effect of this combination on prognosis within 5 years.
Patients and Methods: The study enrolled patients who had undergone radical CRC resection at three independent medical centers from January 2014 to December 2016. Patients were preoperatively categorized into four groups, those with hyperuricemia alone (H), those with MetS alone (MS), those with MetS-related hyperuricemia (MSH), and those with neither condition (control [C] group). The disease-free survival (DFS) and overall survival (OS) rates of these four groups were compared.
Results: The study population consisted of 1271 patients, with 114, 201, 101, and 855 patients categorized into the H, MS, MSH and C groups, respectively. Preoperative MetS was found to be significantly associated with hyperuricemia (P < 0.001). Multivariate Cox regression analysis showed that MetS-related hyperuricemia (hazard ratio [HR] = 2.728; P < 0.001) and MetS alone (HR = 1.631; P < 0.001) were independent predictors of death, whereas simple hyperuricemia was not (P > 0.1). Relative to the C group, the MSH group had the highest rate of tumor recurrence or metastasis (HR = 5.103, P < 0.001), followed by the MS (HR = 2.231, P < 0.001) group. In contrast, prognosis did not differ significantly in the H and C groups (P > 0.1). MetS was significantly associated with poor prognosis, with MetS-related hyperuricemia resulting in a significantly poorer prognosis. In contrast, hyperuricemia alone had no effect on the long-term prognosis of CRC patients.
Conclusion: This study highlights the prognostic importance of MetS-related hyperuricemia on the survival of patients with CRC.
Keywords: colorectal cancer, metabolic syndrome, hyperuricemia, prognosis
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths and the third most frequent malignancy worldwide. It is fourth in incidence among men and third among women.1 The International Agency for Research on Cancer estimated, that over 1,800,000 persons worldwide were newly diagnosed with CRC in 2018 and over 860,000 patients died of this disease.1,2 In China, CRC is the fifth most common malignant tumor, with the fourth highest cancer mortality rate; moreover, its incidence rate is still rising.3 Despite the availability of advanced diagnostic techniques and developments in surgical treatments and chemotherapy, the 5-year survival rate of patients with CRC remains low.4 The development of individualized treatment plans has increased the importance of timely assessment of disease progression and accurate evaluation of patient prognosis.5 The identification of prognostic risk factors can assist in evaluating the risks of postoperative recurrence and death, as well as identifying treatment targets.
Metabolic syndrome (MetS), a major worldwide public health problem, has been associated with increased mortality rates in patients with common cancers and autoimmune diseases.6,7 MetS is actually a group of metabolic disorders, including obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low high-density lipoprotein (HDL) cholesterol concentrations.8 Due to urbanization, aging, and lifestyle changes, the incidence of MetS is rising significantly throughout the world.9 The strongest evidence in MetS and cancer association focused on insulin resistance and its effect on cancer cell proliferation was suggested to be with IGF-1 stimulation.10 Other studies proved that MetS and cancer had in common chronic inflammation and oxidative stress, which were constantly associated to metabolic alterations.11 MetS has been found to be associated with poor prognosis in patients with various types of cancer, including hepatocellular,12 prostate,13 breast,14 gastric,15 and colorectal cancers.16
Serum uric acid (SUA), the final product of nucleotide metabolism, is produced in the liver, muscles, and intestines.17 Abnormal purine metabolism or excretion can lead to an increase in SUA.18 The incidence of hyperuricemia in recent years has increased in developing countries.19,20 High SUA levels are frequently observed in patients with MetS, and increasing evidence indicated that high SUA levels could play a key role in the occurrence and development of MetS.21,22 Hyperuricemia has been associated with MetS, with insulin resistance playing a key role in this association. Hyperuricemia may be responsible, at least in part, for insulin resistance, leading to endothelial cell dysfunction and inhibiting the bioavailability of nitric oxide. Moreover, insulin resistance has been considered the key factor in the development of MetS. Furthermore, hyperinsulinemia resulting from insulin resistance could reduce renal excretion of SUA in the proximal tubules and lead to hyperuricemia.23 These findings suggest that, hyperuricemia and insulin resistance may have a bidirectional cause-and-effect relationship.
Hyperuricemia has also been found to increase cancer prevalence, and its pro-inflammatory properties have been postulated to play an important role in the pathogenesis of cancer,19 as well as to increase mortality rates of cancer patients.24 In view of the increasing incidence of MetS and hyperuricemia worldwide, this study evaluated the correlation between hyperuricemia and MetS in CRC patients treated at multiple clinical centers. The aim of this study was to assess the effects of these two conditions on the progression and prognosis of patients with CRC.
Patients and Methods
Study Population
This study enrolled patients who underwent radical resection of CRC at three independent hospitals in China from January 2014 to December 2016, with complete clinical and follow-up data. Patients were included if: (a) they had been pathologically diagnosed with CRC adenocarcinoma; (b) they had undergone radical resection of CRC; (c) they had undergone complete physical and relevant laboratory examinations within 1–2 weeks before surgery; and (d) detailed clinicopathological characteristics and follow-up data were available. Patients were excluded if they: (a) had been diagnosed with other types of carcinoma; (b) had received chemotherapy or radiotherapy before surgery; (c) had severe cardiovascular or cerebrovascular disease; or (d) had incomplete medical records or were lost to follow-up. Patients were preoperatively categorized into four groups, based on the diagnosis of MetS and/or hyperuricemia: patients with hyperuricemia alone (H group), patients with MetS alone (MS group), patients with both MetS and hyperuricemia (MSH group), and patients with neither MetS nor hyperuricemia (control [C] group). The study protocol was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (Clinical Research Review Issuing Number (2021) No: R045), which waived the requirement for written informed consent due to the retrospective nature of the study. The confidentiality of patient data was guaranteed, as required by the Ethics Committees, and the study was conducted in accordance with the Declaration of Helsinki.
Clinical Data Collection
Data collected for each enrolled patient included: (1) demographic characteristics, such as gender, age and body mass index (BMI); (2) preoperative blood parameters, including concentrations of plasma albumin (with hypoproteinemia defined as an albumin concentration < 35 g/L), creatinine, fasting blood glucose (FBG), triglycerides, HDL, triglycerides, SUA, carcinoembryonic antigen (CEA) and cancer antigen 199 (CA199); and (3) tumor characteristics, including tumor location, histological type, pathologic tumor– node–metastasis (pTNM) stage, as assessed according to the 8th American Joint Committee on Cancer (AJCC) criteria for CRC, tumor (T) stage, node (N) stage, and metastasis (M) stage.
Definition of Metabolic Syndrome
MetS was defined according to the criteria proposed by the Chinese Diabetes Society (CDS, 2004) based on population characteristics.25 MetS was diagnosed in patients who met at least three of the following characteristics: (1) obesity/central obesity, defined as BMI ≥ 25 kg/m2; (2) hypertension, defined as blood pressure ≥ 140/90 mmHg or treatment with antihypertensive drugs; (3) impaired blood glucose regulation, defined as FBG ≥ 6.1 mmol/L, a diagnosis of diabetes or treatment with antidiabetic drugs; and (4) dyslipidemia, defined as a triglyceride level ≥ 1.7 mmol/L, or HDL<0.9 mmol/L in men or<1.0 mmol/L in women.
Definition of Hyperuricemia
Hyperuricemia was defined as an SUA concentration ≥ 420 μmol/L in men or ≥ 360 μmol/L in women.26
Survival Follow-Up
Patients were initially followed-up one month after surgery and every 3 to 6 months thereafter. Follow-up data were collected by telephone or from outpatient records. Overall survival (OS) was defined as the time in months from surgery to the date of death from any cause or the date of last follow-up. Disease-free survival (DFS) was defined as the time in months from surgery to the date of the first tumor metastasis or recurrence or the date of last follow-up. Patients were followed-up for at least 2 years, until March 31, 2021.
Statistical Analysis
Continuous data were reported as mean ± standard deviation (SD) or as median (interquartile range [IQR]); according to the data distribution, and compared in the four groups using the Kruskal‒Wallis test; whereas categorical data were reported as number (percent) and compared using the chi-squared test or Fisher’s exact test. Logistic regression models were constructed to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Survival curves were constructed using the Kaplan‒Meier method and compared by Log rank tests. Univariate and multivariate Cox regression analyses were performed to determine factors associated with OS or DFS rates. Tests for trend were performed using SUA concentration as a continuous variable. Variables with P-values < 0.1 in the univariate analysis and known prognostic factors were included in the multivariate regression analysis. Hazard ratios (HRs) and 95% CIs were calculated by the forward stepwise selection method. All statistical analyses were performed using SPSS version 26.0 software for Windows (IBM Corp., Armonk, NY, USA). All P-values were two-sided, with P-values <0.05 considered statistically significant.
Results
Patient Characteristics
The study population consisted of 1271 patients, with 114, 201, 101, and 855 patients categorized into the H, MS, MSH and C groups, respectively (Figure 1). Table 1 showed the detailed clinical characteristics of these groups of patients. Rates of hyperuricemia and MetS were higher in elderly than in younger patients, with CA199 concentrations comparable in the four groups. Age, BMI, creatinine concentration, and diagnoses of diabetes, hypertension, and dyslipidemia differed significantly in the four groups (all P<0.001).
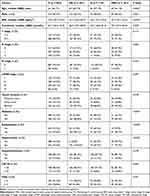 |
Table 1 Clinicopathological Characteristics of Patients |
 |
Figure 1 Patient flow chart for the study. |
Hyperuricemia and MetS
The relationship between hyperuricemia and MetS was assessed by logistic regression analysis. Based on the results of univariate analyses, diagnosis of MetS, diabetes, and hyperlipidemia, as well as age, BMI, and pTNM stage were included in the multivariate analysis. This analysis showed that a diagnosis of MetS (OR = 2.357; P = 0.003) was an independent predictor of hyperuricemia. In addition, age > 65 years, higher BMI, and a diagnosis of dyslipidemia were associated with an increased risk of hyperuricemia (all P < 0.05; Table 2).
 |
Table 2 Univariate and Multivariate Analyses the Risk of Hyperuricemia |
Correlation of the Presence of MetS and Hyperuricemia with DFS and OS
Kaplan‒Meier analysis showed that DFS differed significantly in the four groups of CRC patients (P < 0.001), with median DFS being shorter in the MSH group than in the other groups (Figure 2A). Multivariate Cox regression analysis showed that the T stage, an absence of chemotherapy, and the presence of MetS-related hyperuricemia were independent predictors of DFS rate (all P < 0.05) (Table 3). Compared with the C group, the MSH group had the highest risk of disease recurrence (HR = 5.103, P < 0.001). In contrast, the prognoses of patients in the H and the C groups did not differ significantly (P > 0.1).
 |
Table 3 Univariate and Multivariate Analyses of Factors in Relation to Disease-Free Survival and Overall Survival |
Kaplan-Meier analysis also showed that OS differed significantly in the four groups of CRC patients (P < 0.001; Figure 2B). The OS rate was lowest in the MSH group, followed by the MS group. Multivariate Cox regression analysis results showed that the presence of MetS-related hyperuricemia, T stage, N stage, and CEA concentrations were independent predictors of OS rate (all P < 0.05) (Table 3). Compared with the C group, the MSH group had the highest overall mortality rate among the four groups (HR = 2.728, P < 0.001), followed by the MS group (HR = 1.631, P < 0.001). In contrast, the prognoses for the H and C groups showed no significant differences (P > 0.1).
Correlation of the Presence of MetS and SUA Levels with DFS and OS in Men and Women
We also assessed how the presence of MetS and different SUA levels affected the risk of recurrence in men and women with CRC (Table 4). Although the number of CRC patients in each group decreased due to the stratified analyses, we still observed a series of significant elevations in recurrence risk. Notably, we found that SUA concentrations were positively associated with recurrence rate in male CRC patients with MetS (P < 0.001). Specifically, the risk of recurrence among male CRC patients with MetS was higher in those with SUA ≥ 460 μmol/L than in those with SUA < 460 μmol/L (OR = 3.94; 95% CI, 1.146 to 13.544). Similarly, the risk of recurrence among the four groups was highest when SUA levels were ≥ 500 μmol/L, (OR = 6.005; 95% CI, 2.354 to 15.361). However, elevated SUA levels were not significantly associated with the risk of recurrence among female CRC patients with MetS. SUA concentrations, however, did not affect recurrence rates in either men or women CRC patients without MetS.
 |
Table 4 MetS, Different Serum Uric Acid Levels and Risk of Colorectal Cancer Recurrence and Death in Men and Women |
Meanwhile, we analyzed the effects of MetS and different SUA levels on the mortality risk in men and women with CRC (Table 4). SUA concentrations were associated with mortality in male CRC patients with MetS (P < 0.001), with the risk of death in male CRC patients with MetS being higher in those with SUA ≥ 460 μmol/L than in those with < 460 μmol/L (OR = 4.431; 95% CI, 1.019 to 19.259). Similarly, among the four groups, the risk of mortality was highest when SUA was ≥ 500 μmol/L (OR = 6.421; 95% CI, 2.031 to 20.304). In summary, mortality increased as SUA levels elevated in male CRC patients with MetS. However, elevated SUA levels were not significantly associated with the risk of mortality among male CRC patients without MetS or among female CRC patients with or without MetS.
Discussion
Accurate prognostic evaluation of CRC patients is clinically important. The screening of high-risk populations facilitates better classification and management. To our knowledge, the present study is the first to evaluate the correlation between hyperuricemia and MetS in CRC patients and explore their effects on tumor recurrence and survival. This multicenter retrospective cohort study found that the presence of MetS was related to hyperuricemia and confirmed that MetS-related hyperuricemia would lead to a worse clinical prognosis than either MetS or hyperuricemia alone.
This study found that CRC patients with MetS had higher recurrence and mortality rates than other groups of CRC patients. MetS and its related complications are serious health problems, with the global prevalence of MetS exceeding 23.7%.27 Emerging evidence has demonstrated that MetS is an important factor for the development and malignant progression of various cancers.26 Patients with MetS are at higher risks of increased 30-day postoperative mortality, postoperative complications, and recurrence of colorectal adenoma.28 Biological links between MetS and cancer risk involve many factors and signaling pathways, such as the deregulation of cytokine production, a chronic inflammatory state, the insulin-like growth factor pathway, and concentrations of hormones and proinflammatory cytokines.29 Therefore, the relationship between preoperative MetS and CRC was close.
Several recently surveys have reported close correlations between MetS and SUA concentrations in the general population,21,22 a finding consistent with our results showing that hyperuricemia was independently correlated with MetS, as well as with MetS complications, including a high BMI and dyslipidemia, but was not independently correlated with diabetes. In some studies, hyperuricemia was shown to be associated with each of the individual components of MetS: obesity, hypertension, high triglyceride levels, and low HDL levels,30–32 as well as with elevated fibrinogen levels. Insulin resistance and central obesity are regarded as the critical components of MetS, with both leading to glucose intolerance and dysglycemia.33 Historically, elevated SUA levels in MetS had been attributed to hyperinsulinemia because insulin reduced the renal excretion of SUA.34 Hyperuricemia often preceded the development of hyperinsulinemia,35 obesity,36 and diabetes.37 However, the relationship between hyperuricemia and hyperglycemia was unclear. Although most studies suggested that hyperglycemia and hyperuricemia were related to the pathophysiological mechanism of MetS, others reported that hyperuricemia was negatively correlated with fibrinogen levels in adult residents of Taiwan38 and that there was an inverse association between hyperuricemia and diabetes in Asian men.39
We found that patients with both hyperuricemia and MetS had several risk factors for shorter DFS, including an absence of postoperative chemotherapy and a higher T stage, which could explain the survival curve. These patients with both MetS and hyperuricemia also had several risk factors for shorter OS, including higher T and N stages and higher CEA concentrations. The vicious cycle linking MetS and hyperuricemia makes it easier to understand the impact of hyperuricemia on the prognosis of CRC patients with MetS. However, the present study found no obvious correlation between hyperuricemia alone and the poor prognosis of CRC patients (P > 0.1). Although the association between SUA levels and cancer has not yet been clarified, with few relevant studies to date higher SUA levels were thought to protect against the development of cancer.40,41 This hypothesis was based on findings showing that lipid peroxidation was inhibited and free oxygen radicals were cleared through xanthine oxidoreductase when SUA concentrations were high.24 More recent studies, however, found that high concentrations of the main monosodium form of SUA at physiological pH significantly increased cancer mortality rates in both genders.19,42 Other studies have confirmed that obesity, MetS and the comorbidity associated with SUA levels were important prognostic factors, especially in patients with breast cancer, with these factors resulting in reduced survival rates and increased mortality rates.43 Cox regression analysis of the effects of hyperuricemia, alone or combined with MetS, on the prognosis of CRC patients showed that prognosis was poorer in patients with MetS-related hyperuricemia than in patients with hyperuricemia alone.
The prevalence of MetS has been reported higher in men than in women with hyperuricemia.23,44 To explore the relationship of hyperuricemia and MetS with prognosis in patients with CRC, SUA was stratified in both men and women. These findings showed high SUA (≥ 460 μmol/L) in male CRC patients with MetS was associated with poorer prognosis. In contrast, elevated SUA levels did not significantly affect prognosis in female CRC patients with MetS, although the latter may have been caused by the small sample size in our study.
The present study had several limitations. First, the pathological slides were interpreted by experienced pathologists separately at each center rather than by a centralized pathology review. However, the criteria for MetS and hyperuricemia employed by the three centers were uniform. Second, the specific mechanisms of interactions among MetS, hyperuricemia, and CRC have not been determined, indicating a need for further studies. Third, the number of women patients with MetS-related hyperuricemia was small, limiting validation of the results in women. Fourth, although this was a multicenter study, it included only Chinese patients limiting the applicability of our results, especially because the diagnostic criteria for MetS and hyperuricemia criteria in China differ from those in Western populations. Therefore, the findings of this study require verification in other ethnic groups.
Conclusion
In conclusion, the present study found that preoperative MetS was independently associated with hyperuricemia in patients with CRC. MetS-related hyperuricemia was associated with increased risks of tumor recurrence and mortality. Although MetS alone affected the prognosis of CRC patients, hyperuricemia alone did not. These findings indicate that CRC patients with MetS-related hyperuricemia require more prognostic risk assessments and clinical interventions than patients without this disorder.
Abbreviations
BMI, body mass index; CA199, cancer antigen 199; CEA, carcinoembryonic antigen; CI, confidence interval; CRC, colorectal cancer; DFS, disease-free survival; FBG, fasting blood glucose; HDL, high-density lipoprotein; HR, hazard ratio; OR, odds ratio; OS, overall survival; pTNM, pathology tumor– node–metastasis stage; MetS, Metabolic syndrome; SUA, serum uric acid.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY19H160025), Medicine and Health Technology Program of Zhejiang Province (2020KY635), and Wenzhou Science & technological Project (No. Y20170180).
Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
4. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117.
5. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi:10.1200/JCO.2005.05.0245
6. Pan B, Zhang Q, Zhou H, Ma ZF. Prevalence of components of metabolic syndrome among adults with the presence of autoimmune thyroid condition in an iodine-sufficient region. Biol Trace Elem Res. 2021;199(8):2837–2843. doi:10.1007/s12011-020-02413-3
7. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. doi:10.2337/dc12-0336
8. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645.
9. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi:10.1111/obr.12229
10. Uzunlulu M, Telci Caklili O, Oguz A. Association between metabolic syndrome and cancer. Ann Nutr Metab. 2016;68(3):173–179. doi:10.1159/000443743
11. Battelli MG, Bortolotti M, Polito L, Bolognesi A. Metabolic syndrome and cancer risk: the role of xanthine oxidoreductase. Redox Biol. 2019;21:101070. doi:10.1016/j.redox.2018.101070
12. Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100(1):113–121. doi:10.1002/bjs.8963
13. Shiota M, Yokomizo A, Takeuchi A, et al. The feature of metabolic syndrome is a risk factor for biochemical recurrence after radical prostatectomy. J Surg Oncol. 2014;110(4):476–481. doi:10.1002/jso.23677
14. Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147(1):159–165. doi:10.1007/s10549-014-3076-6
15. Hu D, Peng F, Lin X, et al. Preoperative metabolic syndrome is predictive of significant gastric cancer mortality after gastrectomy: the Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;15:73–80. doi:10.1016/j.ebiom.2016.12.004
16. Han F, Wu G, Zhang S, Zhang J, Zhao Y, Xu J. The association of metabolic syndrome and its components with the incidence and survival of colorectal cancer: a systematic review and meta-analysis. Int J Biol Sci. 2021;17(2):487–497. doi:10.7150/ijbs.52452
17. Huang J, Ma ZF, Zhang Y, et al. Geographical distribution of hyperuricemia in mainland China: a comprehensive systematic review and meta-analysis. Glob Health Res Policy. 2020;5(1):52. doi:10.1186/s41256-020-00178-9
18. Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology. 2005;20(2):125–133. doi:10.1152/physiol.00039.2004
19. Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1(1):16. doi:10.1186/2001-1326-1-16
20. Huang J, Ma ZF, Tian Y, Lee YY. Epidemiology and prevalence of gout in Mainland China: an updated systematic review and meta-analysis. SN Compr Clin Med. 2020;2(9):1593–1606. doi:10.1007/s42399-020-00416-8
21. Caliceti C, Calabria D, Roda A, Cicero AFG. Fructose intake, serum uric acid, and cardiometabolic disorders: a critical review. Nutrients. 2017;9(4):395. doi:10.3390/nu9040395
22. Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res. 2017;8(5):537–548. doi:10.1016/j.jare.2016.11.004
23. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–216. doi:10.1097/BOR.0b013e32835d951e
24. Tanriverdi O, Cokmert S, Oktay E, et al. Prognostic significance of the baseline serum uric acid level in non-small cell lung cancer patients treated with first-line chemotherapy: a study of the Turkish descriptive oncological researches group. Med Oncol. 2014;31(10):217. doi:10.1007/s12032-014-0217-z
25. Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China health and nutrition survey in 2009. Prev Med. 2013;57(6):867–871. doi:10.1016/j.ypmed.2013.09.023
26. Li Y, You A, Tomlinson B, et al. Insulin resistance surrogates predict hypertension plus hyperuricemia. J Diabetes Investig. 2021;12(11):2046-2053.
27. Stocks T, Lukanova A, Bjørge T, et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer. 2011;117(11):2398–2407. doi:10.1002/cncr.25772
28. Kim MC, Jung SW, Kim CS, Chung TH, Yoo CI, Park NH. Metabolic syndrome is associated with increased risk of recurrent colorectal adenomas in Korean men. Int J Obes. 2012;36(7):1007–1011. doi:10.1038/ijo.2011.177
29. Mendonça FM, de Sousa FR, Barbosa AL, et al. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64(2):182–189. doi:10.1016/j.metabol.2014.10.008
30. Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc. 2006;69(11):512–516. doi:10.1016/S1726-4901(09)70320-X
31. Xu A, Tso AW, Cheung BM, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115(12):1537–1543. doi:10.1161/CIRCULATIONAHA.106.647503
32. Zhang Q, Zhang C, Song X, et al. A longitudinal cohort based association study between uric acid level and metabolic syndrome in Chinese Han urban male population. BMC Public Health. 2012;12:419. doi:10.1186/1471-2458-12-419
33. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
34. Quiñones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268(1 Pt 1):E1–E5.
35. England LJ, Levine RJ, Qian C, et al. Glucose tolerance and risk of gestational diabetes mellitus in nulliparous women who smoke during pregnancy. Am J Epidemiol. 2004;160(12):1205–1213. doi:10.1093/aje/kwh340
36. Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480. doi:10.1161/01.HYP.0000091371.53502.D3
37. Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31(2):361–362. doi:10.2337/dc07-1276
38. Liu PW, Chang TY, Chen JD. Serum uric acid and metabolic syndrome in Taiwanese adults. Metabolism. 2010;59(6):802–807. doi:10.1016/j.metabol.2009.09.027
39. den Engelsen C, Gorter KJ, Salomé PL, Rutten GE. Development of metabolic syndrome components in adults with a healthy obese phenotype: a 3-year follow-up. Obesity. 2013;21(5):1025–1030. doi:10.1002/oby.20049
40. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi:10.1073/pnas.78.11.6858
41. Strasak AM, Rapp K, Hilbe W, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. 2007;18(11):1893–1897. doi:10.1093/annonc/mdm338
42. Xia X, He F, Wu X, Peng F, Huang F, Yu X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis. 2014;64(2):257–264. doi:10.1053/j.ajkd.2013.08.027
43. Menotti A, Lanti M, Nedeljkovic S, Nissinen A, Kafatos A, Kromhout D. The relationship of age, blood pressure, serum cholesterol and smoking habits with the risk of typical and atypical coronary heart disease death in the European cohorts of the Seven Countries Study. Int J Cardiol. 2006;106(2):157–163. doi:10.1016/j.ijcard.2004.12.092
44. Uaratanawong S, Suraamornkul S, Angkeaw S, Uaratanawong R. Prevalence of hyperuricemia in Bangkok population. Clin Rheumatol. 2011;30(7):887–893. doi:10.1007/s10067-011-1699-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

