Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Metabolic Syndrome and Prediabetes Among Yemeni School-Aged Children
Authors Saeed W, AL-Habori M , Saif-Ali R, Al-Eryani E
Received 27 April 2020
Accepted for publication 28 June 2020
Published 20 July 2020 Volume 2020:13 Pages 2563—2572
DOI https://doi.org/10.2147/DMSO.S260131
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Walid Saeed, Molham AL-Habori, Riyadh Saif-Ali, Ekram Al-Eryani
Department of Biochemistry and Molecular Biology, Faculty of Medicine and Health Sciences, University of Sana`a, Sana`a, Yemen
Correspondence: Molham AL-Habori Email [email protected]
Purpose: In view of the high rate of obesity and physical inactivity as well as the rising incidence of Type 2 DM among children in the neighboring Gulf countries and Middle East region; the aim of this study was, therefore, to determine the prevalence of metabolic syndrome (MetS) and prediabetes in Yemeni school-aged children.
Patients and Methods: In this study, 1402 school children aged 12– 13 years old (grade 7) were recruited from public schools in the capital Sana’a during the period April–May 2013. Anthropometric measurements and BP were recorded and BMI was calculated. Fasting venous blood (5 mL) was collected for biochemical analysis including FBG, HbA1c, insulin and lipids profile. Insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were calculated.
Results: The prevalence of prediabetes (as defined by impaired fasting glucose) and MetS (as classified by the IDF 2007) were 0.86% and 0.5%, respectively. Our results also showed 5.21% and 20.26% of the children to have two or one factor(s) of the MetS criteria fulfilled, respectively, with low HDL-c (17%) being the most prevalent MetS component, followed by metabolic glucose (8%), raised TG (5.3%), DBP (1.4%), and high WC (0.5%). Moreover, the prevalence of overweight and obesity was 4.2% and 2.8%, respectively; and about 1.2% of children had abnormal high insulin levels. Children with impaired fasting glucose (IFG) had increased HOMA-IR (p = 0.016) and SBP (p = 0.042) and decreased HDL-c (p = 0.034) and HOMA-β (p < 0.001); whereas obese children had increased WC (p < 0.001) and TG (p = 0.049).
Conclusion: The main finding of this study is that Yemeni children are at potential risk of obesity, metabolic syndrome and prediabetes despite their low prevalences. These results highlight the need for early identification and close monitoring of children at risk of later Type 2 DM as an important primary care strategy that can effectively prevent or delay the onset of such condition.
Keywords: metabolic syndrome, prediabetes, obesity, insulin resistance, school-aged children
Introduction
Type 2 diabetes mellitus (Type 2 DM), previously considered an adult disease and non‐existent in the pediatric population in the late 20th century, has now become one of the fastest growing pediatric chronic diseases worldwide.1–4 The SEARCH study previously showed the incidence of Type 2 DM among children to rise in the last 2 decades.5,6 The highest rates of Type 2 DM in children and adolescents have been reported in studies from the USA,5,7,8 with the incidence rising from 9 to 12.5 cases per 100,000 between 2002 and 2012.9 This incidence in the USA is expected to substantially increase linearly and might almost quadruple between 2010 and 2050 as previously modeled.10 European countries report a lower incidence, with an Austrian study reporting 0.29 per 100,000 per year between 1999 and 2007.7 In Europe, the UK has the highest reported prevalence of Type 2 DM in children aged <17, rising from 0.53 per 100 000 in 2004–200511 to 0.72 per 100 000 in 2015–2016.12 Not surprisingly, data from the Arab world show similar figures for childhood Type 2 DM, whereby reports from Saudi Arabia showed a prevalence of 1 per 1000 Type 2 DM in children <18 years old,13 which was similar to the highest prevalence found in specific groups (American Indian and African American) in the United States.14
Risk factors for developing childhood Type 2 DM are similar to those for adulthood, and include obesity, family history and ethnicity; together with the rapid rise in urbanization, sedentary lifestyles, physical inactivity and high calorie diets.15 Despite early indications that the prevalence of obesity in children has plateaued in several countries;16 childhood and adolescent obesity remains the most prominent risk factor for Type 2 DM, with documented prevalence rates of 80% occurring at the time of diagnosis.17 Obesity prevalence in the UK and USA has increased dramatically over the last 20 years.18,19 In North Africa and the Middle East region, the prevalence of obesity was 8.4% in boys and 10.2% in girls aged <20 years old.20 Despite the increasing rates of childhood and adolescent’s obesity, pediatric metabolic syndrome (MetS), a cluster of cardiometabolic risk factors associated with an increased risk for Type 2 DM, remains controversial,21,22 and a consensus definition for MetS in children and adolescents has not yet been established. There are at least 40 definitions of MetS for this age group,23 and the prevalence of MetS varies depending on the definition used.24 In 2007, the International Diabetes Federation (IDF) established a new concept for defining MetS in children and adolescents, which considers increased waist circumference as the main component for defining MetS, as well as the presence of two or more of the clinical or laboratory criteria (low HDL-c and high blood pressure, triglycerides and high glucose). The prevalence of MetS worldwide range from 1.2% to 22.6% for youth25 and as high as 30–50% among overweight youth,26 depending on the definition of MetS used, the study design, the age group and study population. Previous studies debated that the environment, in particular the typical Western diet, plays a significant role in the development of MetS.27
Type 2 DM in children and adolescents is highly heterogeneous in terms of disease onset and progression. Since onset is progressive, diabetes is preceded by a range of glucose-related phenotypes characterized by a progressive decline in β-cell function defined as prediabetes.28 Prediabetes is a broad expression of altered glucose metabolism, including impaired fasting glucose (IFG), impaired glucose tolerance (IGT), elevated glycated haemoglobin (HbA1c), or combinations of these.29 Prediabetes is increasingly recognized as an important metabolic state; as well as predisposing individuals to future progression to Type 2 DM and many of the pathologies associated with diabetes.30 Prediabetes will progress to Type 2 DM in approximately 25% of subjects within 3–5 years, and as many as 70% of individuals with prediabetes will develop Type 2 DM within their lifetime.30 The rise in the prevalence of prediabetes in children and adolescents has paralleled the rise in childhood obesity observed over the past three decades.31 The prevalence of prediabetes has been reported in various countries across the world, including China (0.28%), Mexico (1.5%), Canada (2.6%), and UAE (5.4%).32–35
In the light of the high rate of obesity and physical inactivity among children in many countries as well as the rising incidence of Type 2 DM in children in the neighboring Gulf countries and Middle East region, the aim of this study was therefore to determine the prevalence of obesity, metabolic syndrome and prediabetes in Yemeni school children aged 12–13 years old.
Patients and Methods
Study Design, Subjects and Data Collection
In this study a cross sectional with simple clustering sampling was used for subject selection. The capital Sana’a was divided into 10 “educational areas” as classified by the Ministry of Education. All public schools in the Yemen are sex-segregated and therefore two male public primary schools were selected randomly in each area. Then, according to the population of each school and its ratio to the total population of elementary school students, a subsample was selected randomly from two classes from the 7th grade (aged 12–13 years old). The study protocol was approved by the institutional review board (IRB) of the Faculty of Medicine and Health Sciences, Sana’a University. In the first phase, 1700 male students were selected and a questionnaire (including family history of diabetes and hypertension) as well as an Informed Consent Form was sent to each student’s parents. The parents were requested to fill out the demographic and Consent Forms and return them to school if they agreed with their child’s participation in the study. Informed consent was obtained from all participants after explaining the purpose and nature of the study. Of the initial number recruited, 1402 (82.5%) students gave both blood samples and physical measurements.
All school visits were carried out in five weeks between April and May 2013, and data were collected through interviews using questionnaires, collection of anthropometric measurements and blood analysis. The enrolled students were asked to come to the school in the morning after 10 hour overnight fast. On the visit day, children were weighed without shoes or heavy clothing to the nearest 0.1 Kg, and their height was measured to the nearest 0.1 cm on a calibrated scale with attached stadiometer. A standard measuring tape was used to measure waist circumference (WC) at a point right above the iliac crest on the midaxillary line at minimal respiration and the results were rounded to the nearest 1.0 cm. Body mass index (BMI) was calculated as the ratio of weight to height squared (kg/m2) and BMI percentiles was classified according to percentile charts for age and sex from the Centers for Disease Control and Prevention (CDC).36 Blood pressure (BP) was measured in the seated position using a calibrated Omron M6 IntelliSense (Healthcare, Kyoto, Japan) automatic blood pressure monitors with at least a 10-min rest period before the measurement. Two measurements were taken for all subjects at 2 minutes intervals, and the average of BP readings was calculated and recorded.
Metabolic syndrome was classified according to the IDF 2007 criteria37 by abdominal obesity (≥90th percentile as assessed by WC), and the presence of two or more clinical features including raised triglyceride (TG), low HDL-cholesterol (HDL-c), high systolic blood pressure (SBP) and/or diastolic blood pressure (DBP), and raised fasting blood glucose (FBG). Prediabetes was defined as FBG 110–126 mg/dl, and diabetes as FBG ≥126 mg/dl according to the ADA guidelines.29 Children’s weights were classified as underweight: BMI < 5th% percentile, normal weight: BMI ≥ 5th to <85th% percentile, overweight: BMI ≥ 85th to < 95th% percentile, and obese: BMI ≥ 95th% percentile.36
Blood Collection and Biochemical Analysis
A fasting venous blood (5 mL) was collected from each individual after an overnight fast of more than 10 hours and divided into two vacuumed tubes; 4 mL into plain tubes for biochemical assay and 1 mL into a K2EDTA tube for HbA1c. The serum was separated within 30 min and stored at - 20ºC for biochemical analysis. Haemolysate was prepared immediately for HbA1c determination within 2 hours of blood collection. Serum TG, HDL-c, and FBG were measured by an automated analyser Cobas c311 (Roche Diagnostic, USA) using their respective kits (Roche Diagnostic, Germany). Glycated haemoglobin (HbA1c) was measured in an automated Cobas c311 chemistry (Roche Diagnostic, Germany). Insulin was measured by Electrochemiluminescence immunoassay (ECL) on Elecsys autoanalyzer (Roche Diagnostics, Germany). Insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were calculated using the Homeostasis Model Assessment (HOMA 2) Calculator v2.2 which is available from Oxford Centre for Diabetes, Endocrinology and Metabolism.
Statistical Analysis
The statistical analyses were performed on Social Package of Social Sciences (SPSS) version 11.5 (SPSS Inc, Chicago, IL, USA). Frequency was used to describe the parameters included in this study. ANOVA was used to detect the differences between means of metabolic syndrome factors (BMI, WC, systolic and diastolic blood pressure, TG, Cholesterol, and HDL-c) and diabetic parameters (Glucose, HbA1c, insulin, HOMA-IR and HOMA-β). The significant differences were indicated if p-value was <0.05.
Results
The frequency of prediabetes (as defined by impaired fasting glucose) and metabolic syndrome (as classified by the IDF 2007) was 0.86% and 0.5%, respectively. In addition, 73 children had two MetS factors fulfilled and a further 284 children having one factor. The frequency of the individual MetS factors (Figure 1) showed those with low HDL-cholesterol to be the most encountered (238 children). Other common factors include: metabolic glucose (112 children), raised TG (74), DBP (20), and high WC (7). Moreover, the distribution of the studied population according to BMI percentile; showed that out of the 1402 children: 36 were obese, 58 were overweight, 1009 were normal weight and 185 were underweight. Twelve of the children had impaired fasting glucose and 84 had metabolic glucose (100–110 mg/dl) with 1114 being normoglycaemic and 78 hypoglycaemic. In addition, 15 children were hyperinsulinaemic and 33 were insulin resistance with HOMA-IR > 3, and 226 with HOMA-IR between 2 and 3.
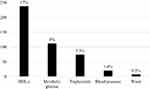 |
Figure 1 The frequency of the individual metabolic syndrome factors. |
Table 1 shows comparison of the metabolic syndrome factors and diabetic parameters according to BMI, glucose level and insulin level. Waist circumference and triglyceride were significantly (p < 0.001 and p = 0.049) higher with increasing BMI; whereas on increasing glucose level through to impaired fasting glucose; HOMA-IR and SBP were significantly (p = 0.016 and p = 0.042) increased; and both HOMA-β and HDL-c were significantly (p < 0.001, p = 0.034) decreased. However, on comparing those with abnormally high insulin levels (hyperinsulinaemic) with the normal insulin group showed HOMA-IR, HOMA-β, triglycerides and SBP to be significantly (p < 0.001, p < 0.001, p = 0.005, and p = 0.024, respectively) higher in the hyperinsulinaemic group; whereas HDL-c and BMI were significantly (p = 0.002, p = 0.049) lower.
 |
Table 1 Comparing of Metabolic Syndrome Factors and Diabetic Parameters According to BMI, Glucose and Insulin Levels |
Discussion
Unlike the rising incidence of Type 2 DM in children in the neighboring Gulf countries and Middle East region, this cross-sectional study of randomly selected 1402 Yemeni school children aged 12–13 years did not show the presence of Type 2 DM but clearly highlights the presence of prediabetes (0.86%) and metabolic syndrome (0.5%). The prevalence of Type 2 DM among randomly selected children and adolescents in Kuwait was estimated at 0.04% in children aged 6–18 years,38 and 0.12% in Saudi Arabia among children under 14 years old and 0.79% in younger adults 14–29 years.39 Higher prevalence was reported in overweight/obese children in Emirates (0.87%)34 and Turkey (2%).40 The higher prevalence of Type 2 DM in these Arabian Gulf countries is attributed to the rapid socioeconomic transition towards an affluent lifestyle leading to obesity and associated diseases, including diabetes, to an epidemic level.20
Moreover, the prevalence of prediabetes in our Yemeni children at 0.86% is not as high as its prevalence in neighboring countries; which could be attributed to the overall lower prevalence of obesity in our society. The prevalence of prediabetes was estimated in Emirati overweight/obese children and adolescents to be 5.4% based on FBG and/or OGTT,34 with more than half of their pre-diabetics having a first-degree relative with Type 2 DM. Abnormal glucose metabolism is also highly prevalent among Saudi children and adolescents, exceeding 10%.13 The prevalence of prediabetes defined by IFG and IGT among 10–19 year old adolescents in the US was 9.2% and 8.1%, respectively.41 The prevalence of prediabetes and diabetes in the obese pediatric population also varies substantially across different countries and ethnicities.28 Progression of IFG to Type 2 DM appears to be slower in obese children and adolescents than in adults;28,42 whereas the transition from IGT to Type 2 DM has been shown to be more rapid in children and adolescents than in adults.43
Similarly, the prevalence of MetS (0.5%) is by far less than those reported in other countries. The criteria for MetS used in our study are based on the proposed IDF criteria in 2007, which had been used worldwide for comparison of data from different countries.44 The prevalence of MetS, according to the IDF criteria, among adolescents aged 10–16 years old attending public schools in Dubai was 3.7%.45 Similarly, the prevalence of MetS was 3% among Qatari school children,46 2.3% among Turkish school children aged 10–19 years,47 and 4.7% in Canadian Tsimshian Nation youth aged 6–18 years.48 Higher prevalence of 6.3% was reported in another Turkish study in 7–15 years old children49 and 16.5% among school-aged children in Pakistan based on various definitions.50 However, studies that have assessed the prevalence of MetS using different diagnostic criteria have consistently reported different results sometimes differing by more than two-fold.45
Several reports indicate that the prevalence of childhood MetS has substantially increased during childhood and adolescence due to the increasing rate of childhood obesity on a global scale.49,51 The prevalence of MetS among obese children was reported to be more serious than that among normal weight children.52 Metabolic syndrome was more common in Iranian obese children with a prevalence of 0.9% in normal-weighed students, 11.3% in the overweight and 36.2% in the obese group,53 which is higher than those reported in Chinese,54 Egyptian,55 and Turkish children49 but less than another study from Turkey.40 Moreover, the prevalence of MetS in overweight/obese French children aged 10–16 years old was 8.9%,56 and that of Mexican children aged 6–12 years was 6.7%.57 Earlier studies have demonstrated an association between obesity and the clustering of metabolic abnormalities in early life and their persistence during adulthood.58,59 Children with MetS are at increased risk of developing Type 2 DM and cardiovascular disease (CVD) in later life; however, if the occurrence of MetS in children and adolescents is identified early, risk stratification of future cardiovascular events can be performed.60
Moreover, our study also showed that in addition to those already classified as MetS, 5.21% and 20.26% of the children have two or one factor(s) of the MetS criteria fulfilled, respectively. Low HDL-c (17%) was the most prevalent MetS component, followed by metabolic glucose (8%), raised TG (5.3%), DBP (1.4%), and high WC (0.5%). Our results are in agreement with several studies reporting HDL as the predominant risk factor among adolescents, followed by high BP, in both Saudi Arabia and the UAE;45,61,62 with ~87% of Saudi children and adolescents aged 10–18 years old had low HDL levels.61 Moreover, a recent UAE study suggested that if MetS did not account for HDL levels, their prevalence would drop from 3.7% to 0.8%.45 Studies investigating the genetic susceptibility to having low HDL have identified several predisposing variants among Arabs.63 On the other hand, an Iranian study reported that 46.5% of children had at least one of the MetS components with the most prevalent MetS component was hypertension followed by abdominal obesity, hypertriglyceridemia and low HDL-C;53 while a Turkish study showed that the variables of greatest frequency in pre-pubertal children were hypertension, hypertriglyceridemia, low HDL-C and hyperinsulinemia, with a prevalence of 32.9%, 29.4%, 23.1%, and 14.1%, respectively.40 All this together indicate that a high percentage of youths with high probability of future worsening in cardiometabolic risks.
Our results also showed that out of the 1402 children studied, 4.1% and 2.4% were overweight and obese, which is lower than the rates reported in a number of neighboring countries. In the children and adolescents of Saudi Arabia, the prevalence of overweight, obesity, and severe obesity was reported to be 23.1%, 9.3%, and 2%, respectively;64 whereas in Qatar the prevalence of obesity and overweight among 12–17 years old boys was 23.1% and 23%.65 On the other hand, childhood obesity in the UAE is as high as 40–50% among school children.45,66 The recent increase in obesity prevalence among children and adolescents in Gulf countries and Middle East region and most developing countries, especially those with higher socio-economic status,67 could be associated with the sedentary lifestyle, lack of physical activity, urbanization, increased income, family dietary patterns and family history.
Obese children are more likely to become overweight in adulthood than healthy-weight children resulting in a health risk later in life. Youth who are overweight/obese have ~5-fold increased risk of excess adiposity in adulthood with a high risk for obesity-related comorbidities.68 Obese children and adolescents are at a higher risk for glucose intolerance, insulin resistance, Type 2 DM and CVD. It is now well established that higher BMI values in children, even at levels far below current overweight classifications, are associated with increased risks of Type 2 DM in adulthood.69 Moreover, overweight during puberty, from 13 years of age to early adulthood is associated with a higher risk of Type 2 DM than is development of overweight by early adulthood.70 Moreover, it has long been viewed that CVD has its origins in childhood and epidemiological evidence has revealed that many children exhibit at least one CVD risk factor71 such as high cholesterol or high blood pressure, which places them at a high risk for developing diabetes later in life.4,17,45,72,73
Our results showed waist circumference and triglycerides to be increased with increasing BMI, which is in agreement with others observing strong associations between BMI with WC and TG.74,75 Moreover, a strong relationship between both BMI and waist circumference centile with fasting insulin was reported in children of both sexes suggesting that even in the healthy weight range, fasting insulin increases with increasing adiposity.74 A recent study also demonstrated that elevated TG level and WC influences the risk of insulin resistance in healthy male adolescents with parental history of Type 2 DM.76 Furthermore, TG and WC were recently reported to be independently associated with insulin resistance in adults, with 75% of insulin resistance being attributed to the TG level even when TG was in the normal range.77
Our results also showed that on increasing glucose level through to impaired fasting glucose; HOMA-IR and SBP were significantly increased; whereas HOMA-β and HDL-c were significantly decreased. In addition, our results showed 15 children (1.2%) to be hyperinsulinaemic with the overall general characteristics of insulin resistance/pre-diabetes including: low HDL-c, high TG, and raised HOMA-IR and HOMA-β;78 thus reflecting what is known as β-cell compensation to accommodate for the rising insulin resistance and decreased insulin sensitivity.79 Moreover, 2.56% of the children were insulin resistance with HOMA-IR > 3, and an additional 17.55% with HOMA-IR between 2 and 3. Insulin resistance has been shown to precede the development of pancreatic β-cell dysfunction in people at risk for developing diabetes thus suggesting that insulin resistance is the primary abnormality in Type 2 DM.80,81 Although different studies have made an attempt to identify normal values of insulin resistance for children and adolescents, reliable reference ranges of HOMA-IR are not available yet.82,83 An earlier study conducted on 2573 Caucasian children and adolescents demonstrated that, even though age, gender and body adiposity are responsible for insulin resistance physiological changes, values > 1.68 in normal-weight subjects define a “non-physiological state” and may place the patient at an increased risk for CVD. However, if subjects are overweight and obese, the cut-off rises to 3.42.84 Variations in the underlying prevalence of obesity over time may contribute to discrepancies in insulin resistance and to the increasing incidence of Type 2 DM. Factors that contribute to compromised insulin secretion are not well known and may include epigenetic dysregulation, which is yet to be elucidated.85
The strength of our study is that it is the first national study to apply the IDF consensus definition of MetS to Yemeni school-aged children which will allow for more accurate comparisons with future studies on MetS in children and adolescents. On the other hand, the limitations of our study are: First, generalization should be restricted to Yemeni children aged 12–13 years old. Second, our sample population was only male children. Third, the enrolled children were from public schools, which mainly consist of students of low socio-economic status and therefore, inclusion of private schools may have produced different results. Fourth, our sample population was collected only from one region of Yemen. Finally, international WC cut-off points were used since no WC cut-off values were available for our population.
Conclusion
The main finding of this study is that Yemeni school-aged children between 12 and 13 years old who attended public schools in Sana’a are at potential risk of obesity, metabolic syndrome and prediabetes despite their low prevalence. Our findings highlight the importance of early screening interventions for metabolic abnormalities in children, given the negative health outcomes associated with overweight/obesity and the risk that children with MetS could develop chronic diseases, such as Type 2 DM, later in life. Such early primary care strategy may allow a targeted approach by lifestyle measures to prevent or delay the onset of these conditions in later life. Our study also showed that low HDL-c levels were the predominant MetS factor, which needs further investigations to explore the possible genetic susceptibility leading to the low HDL levels.
Abbreviations
BMI, Body mass index; BP, Blood pressure; CDC, Centre for Disease Control and Prevention; CVD, Cardiovascular disease; DBP, Diastolic blood pressure; ECL, Electrochemiluminescence immunoassay; ELISA, Enzyme-linked immunoassay; FBG, Fasting blood glucose; HbA1c, Glycated hemoglobin; HDL-c, High-density lipoprotein cholesterol; HOMA, Homeostasis model assessment; HOMA-IR, Insulin resistance; HOMA-β, β-cell function; IFG, Impaired fasting glucose; IGT, Impaired glucose tolerance; LDL-c, Low-density lipoprotein cholesterol; MetS, Metabolic syndrome; SD, Standard deviation; SPSS, Statistically Package for Social Sciences; SBP, Systolic blood pressure; TG, Triglycerides; WC, Waist circumference.
Data Sharing Statement
The data set generated and/or analyzed during this study are included in this submitted manuscript and is available from the corresponding author on reasonable request.
Ethical Approval
The study was approved by the Institutional Ethical Committee, Faculty of Medicine and Health Sciences, Sana`a University. The study was in compliance with the Declaration of Helsinki for clinical research. All children and their parents both provided written informed consent before participating in the study.
Consent to Publish
All authors approved the submitted manuscript.
Acknowledgments
The authors thank all the study participants.
Disclosure
All authors have no conflicts of interest to declare.
References
1. Rhodes E, Prosser L, Hoerger T, Lieu T, Ludwig D, Laffel L. Estimated morbidity and mortality in adolescents and young adults diagnosed with type 2 diabetes mellitus. Diabet Med. 2012;29(4):453–463. doi:10.1111/j.1464-5491.2011.03542.x
2. Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37(2):
3. Saydah S, Imperatore G, Geiss L. Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among US adolescents: results from the continuous NHANES, 1999–2010. Am J Epidemiol. 2014;179(3):
4. Dean HJ, Sellers EA. Children have type 2 diabetes too: an historical perspective. Biochem Cell Biol. 2015;93(5):
5. Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi:10.1001/jama.2014.3201
6. Lee JH, Kim YM, Kwak MJ, et al. Incidence trends and associated factors of diabetes mellitus in Korean children and adolescents: a retrospective cohort study in Busan and Gyeongnam. Ann Pediatr Endocrinol Metab. 2015;20:206–212. doi:10.6065/apem.2015.20.4.206
7. Fazeli Farsani S, Van Der Aa MP, Van Der Vorst MM, Knibbe CA, De Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471–1488. doi:10.1007/s00125-013-2915-z
8. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336–3344. doi:10.2337/dc14-0574
9. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376:1419–1429. doi:10.1056/NEJMoa1610187
10. Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515–2520. doi:10.2337/dc12-0669
11. Haines L, Wan KC, Lynn R, Barrett TG, Shield JPH. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30:1097–1101. doi:10.2337/dc06-1813
12. Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35(6):737–744. doi:10.1111/dme.13609
13. Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population based study (SAUDI-DM). J Epidemiol Community Health. 2015;69:1045–1051. doi:10.1136/jech-2015-205710
14. Zeitler P, Fu J, Tandon N, et al. ISPAD clinical practice consensus guidelines 2014: type 2 diabetes in the child and adolescent. Pediatr Diabetes. 2014;15(Suppl 20):26–46. doi:10.1111/pedi.12179
15. Wilmot EG, Davies MJ, Yates T, Benhalima K, Lawrence IG, Khunti K. Type 2 diabetes in younger adults: the emerging UK epidemic. Postgrad Med J. 2010;86:711–718. doi:10.1136/pgmj.2010.100917
16. Olds T, Maher C, Zumin S, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6(5–6):342–360. doi:10.3109/17477166.2011.605895
17. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):
18. van Jaarsveld CH, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Arch Dis Child. 2015;100:214–219. doi:10.1136/archdischild-2014-307151
19. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–1725. doi:10.1001/jama.2018.3060
20. Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12:1–13. doi:10.1111/j.1467-789X.2010.00750.x
21. Higgins V, Adeli K. Pediatric metabolic syndrome: pathophysiology and laboratory assessment. EJIFCC. 2017;28(1):25–42.
22. Felix A, John RM. Pediatric metabolic syndrome. Nurse Pract. 2019;44(7):18–25. doi:10.1097/01.NPR.0000559841.45754.73
23. Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr. 2008;152(2):160–164. doi:10.1016/j.jpeds.2007.07.056
24. Vanlancker T, Schaubroeck E, Vyncke K, et al. Comparison of definitions for the metabolic syndrome in adolescents: the HELENA study. Eur J Pediatr. 2017;176(2):241–252. doi:10.1007/s00431-016-2831-6
25. Tailor AM, Peeters PH, Norat T, Vineis P, Romaquera D. An update on the prevalence of the metabolic syndrome in children and adolescents. Int J Pediatr Obes. 2010;5(3):202–213. doi:10.3109/17477160903281079
26. Marcovecchio ML, Chiarelli F. Metabolic syndrome in youth: chimera or useful concept? Curr Diab Rep. 2013;13(1):56–62. doi:10.1007/s11892-012-0331-2
27. Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281(1):123–140. doi:10.1111/nyas.12030
28. Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, Caprio S. Prediabetes in youths: mechanisms and biomarkers. Lancet Child Adolesc Health. 2017;1:240–248. doi:10.1016/S2352-4642(17)30044-5
29. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81–90. doi:10.2337/dc14-S081
30. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi:10.1016/S0140-6736(12)60283-9
31. Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi:10.1038/nm0106-75
32. Zhu H, Zhang X, Li MZ, Xie J, Yang XL. Prevalence of type 2 diabetes and pre‐diabetes among overweight or obese children in Tianjin, China. Diabet Med. 2013;30(12):
33. Zvarova K, Zvarova Z, Callas P, Malone‐Rising D. New estimates of pre‐diabetes and type 2 diabetes prevalence in Mexican Quintana Roo. Int J Diabetes Dev Ctries. 2013;33(1):
34. Al Amiri E, Abdullatif M, Abdulle A, et al. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health. 2015;15:1298–1306. doi:10.1186/s12889-015-2649-6
35. Spurr S, Bally J, Allan D, Bullin C, McNair E. Prediabetes: an emerging public health concern in adolescents. Endocrinol Diabetes Metab. 2019;2:e00060. doi:10.1002/edm2.60
36. Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2000;2002(246):1–190.
37. Zimmet P, Alberti KGMM, Kaufman F, et al. The metabolic syndrome in children and adolescents: an IDF consensus repot. Pediatr Diabetes. 2007;8(5):299–306. doi:10.1111/j.1399-5448.2007.00271.x
38. Moussa MA, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Prevalence of type 2 diabetes mellitus among Kuwaiti children and adolescents. Med Princ Pract. 2008;17:270–275. doi:10.1159/000129604
39. El-Hazmi MA, Warsy AS, Al-Swailem AR, Al-Swailem AM, Sulaimani R. Diabetes mellitus as a health problem in Saudi Arabia and its complications. East Mediterr Health J. 1998;4:58–67.
40. Atabek ME, Eklioglu BS, Akyurek N. Reevaluation of the prevalence of metabolic syndrome in an urban area of Turkey. J Clin Res Pediatr Endocrinol. 2013;5:50–54. doi:10.4274/Jcrpe.778
41. Vijayakumar P, Nelson RG, Hanson RL, Knowler WC, Sinha M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care. 2017;40:16–21. doi:10.2337/dc16-1358
42. Hagman E, Danielsson P, Brandt L, Ekbom A, Marcus C. Association between impaired fasting glycaemia in pediatric obesity and type 2 diabetes in young adulthood. Nutr Diabetes. 2016;6:e227. doi:10.1038/nutd.2016.34
43. Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28:902–909. doi:10.2337/diacare.28.4.902
44. Schwandt P, Kelishadi R, Ribeiro RQ, Haas GM, Poursafa P. A three-country study on the components of the metabolic syndrome in youths: the big study. Int J Pediatr Obes. 2010;5:334–341. doi:10.3109/17477160903497043
45. Haroun D, Mechli R, Sahuri R, et al. Metabolic syndrome among adolescents in Dubai, United Arab Emirates, is attributable to the high prevalence of low HDL levels: a cross-sectional study. BMC Public Health. 2018;18:
46. Cizmecioglu FM, Etiler N, Hamzaoglu O, Hatun S. Prevalence of metabolic syndrome in schoolchildren and adolescents in Turkey: a population based study. J Pediatr Endocrinol Metab. 2009;22:703–714. doi:10.1515/JPEM.2009.22.8.703
47. Zorzi A, Wahi G, Macnab AJ, Panagiotopoulos C. Prevalence of impaired glucose tolerance and the components of metabolic syndrome in Canadian Tsimshian Nation youth. Can J Rural Med. 2009;14:61–67.
48. Rizk N, Amin M, Yousef M. A pilot study on metabolic syndrome and its associated features among Qatari school children. Int J Gen Med. 2011;4:521–525. doi:10.2147/IJGM.S21103
49. Battaloglu IB. Metabolic syndrome in school children in Mardin, South-Eastern of Turkey. Eurasian J Med. 2014;46(3):156–163. doi:10.5152/eajm.2014.39
50. Iqbal AZ, Basharat S, Basharat A, Basharat S. Prevalence of the metabolic syndrome and its component abnormalities among school age Pakistani children. J Ayub Med Coll Abbottabad. 2014;26(2):194–199.
51. Taheri F, Namakin K, Zardast M, Chakand T, Kazemi T, Bijari B. Cardiovascular risk factors: a study on the prevalence of MS among 11–18 years old school children in East of Iran, 2012. Nutr Food Sci Res. 2015;2(1):27–34.
52. Xu H, Li Y, Liu A, et al. Prevalence of the metabolic syndrome among children from six cities of China. BMC Public Health. 2012;12:13–20. doi:10.1186/1471-2458-12-13
53. Zardast M, Namakin K, Chahkandi T, Taheri F, Kazemi T, Bijari B. Prevalence of metabolic syndrome in elementary school children in East of Iran. J Cardiovasc Thorac Res. 2015;7(4):158–163. doi:10.15171/jcvtr.2015.34
54. Wang Q, Yin J, Xu L, et al. Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health. 2013;13:
55. Zaki ME, Mohamed SK, Bahgat KA, Kholoussi SM. Metabolic syndrome components in obese Egyptian children. Ann Saudi Med. 2012;32(6):603–610. doi:10.5144/0256-4947.2012.603
56. Druet C, Ong K, Levy Marchal C. Metabolic syndrome in children: comparison of the International Diabetes Federation 2007 consensus with an adapted National Cholesterol Education Program definition in 300 overweight and obese French children. Horm Res Paediatr. 2010;73:181–186. doi:10.1159/000284359
57. Elizondo-Montemayor L, Serrano-González M, Ugalde-Casas PA, Cuello-García C, Borbolla-Escoboza JR. Metabolic syndrome risk factors among a sample of overweight and obese Mexican children. J Clin Hypertens. 2010;12:380–387. doi:10.1111/j.1751-7176.2010.00263.x
58. Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 2003;107:1448–1453. doi:10.1161/01.cir.0000060923.07573.f2
59. Li Y, Yang X, Zhai F, et al. Prevalence of the metabolic syndrome in Chinese adolescents. Br J Nutr. 2008;99:565–570. doi:10.1017/S0007114507797064
60. Rosini N, Moura SA, Rosini RD, Machado MJ, Silva EL. Metabolic syndrome and importance of associated variables in children and adolescents in Guabiruba - SC, Brazil. Arq Bras Cardiol. 2015;105(1):37–44. doi:10.5935/abc.20150040
61. Al-Daghri NM. Extremely high prevalence of metabolic syndrome manifestations among Arab youth: a call for early intervention. Eur J Clin Investig. 2010;40(12):1063–1066. doi:10.1111/j.1365-2362.2010.02341.x
62. Mehairi AE, Khouri AA, Naqbi MM, et al. Metabolic syndrome among Emirati adolescents: a school based study. PLoS One. 2013;8(2):e56159. doi:10.1371/journal.pone.0056159
63. Wakil SM, Ram R, Muiya NP, et al. A common variant association study reveals novel susceptibility loci for low HDL-cholesterol levels in ethnic Arabs. Clin Genet. 2016;90(6):518–525. doi:10.1111/cge.12761
64. El Mouzan MI, Foster PJ, Al Herbish AS, et al. Prevalence of overweight and obesity in Saudi children and adolescents. Ann Saudi Med. 2010;30(3):203–208. doi:10.4103/0256-4947.62833
65. Al-Thani M, Al-Thani A, Al-Chetachi W, Akram H. Obesity and related factors among children and adolescents in Qatar. Int J Basic Sci Med. 2017;2(4):161–165. doi:10.15171/ijbsm.2017.30
66. Al Junaibi A, Abdulle A, Sabri S, Hag-Ali M, Nagelkerke N. The prevalence and potential determinants of obesity among school children and adolescents in Abu Dhabi, United Arab Emirates. Int J Obes. 2013;37(1):68–74. doi:10.1038/ijo.2012.131
67. Musaiger AO. Overweight and obesity in eastern Mediterranean region: prevalence and possible causes. J Obes. 2011;2011:e407237. doi:10.1155/2011/407237
68. Juonala M, Magnussen C, Berenson G, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi:10.1056/NEJMoa1010112
69. Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sorensen TIA, Baker JL. Childhood body mass index and development of type 2 diabetes throughout adult life: a large-scale Danish cohort study. Obesity (Silver Spring). 2017;25:965–971. doi:10.1002/oby.21820
70. Bjerregaard LG, Jensen BW, Angquist L, Osler M, Sorensen TI, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N Engl J Med. 2018;378:1302–1312. doi:10.1056/NEJMoa1713231
71. Thomas NE, Rowe DA, Murtagh EM, Stephens JW, Williams R. Associations between metabolic syndrome components and markers of inflammation in Welsh school children. Eur J Pediatrics. 2018;177:409–417. doi:10.1007/s00431-017-3065-y
72. Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: national Health and Nutrition Examination Survey (2001–2006). Arch Pediatr Adolesc Med. 2009;163(4):371–377. doi:10.1001/archpediatrics.2009.3
73. Panagiotopoulos C, Hadjiyannakis S, Henderson M. Type 2 diabetes in children and adolescents. Can J Diabetes. 2018;42(Suppl 1):
74. Bell L, Hung J, Knuiman M, et al. Body mass index and waist circumference: relationship to cardiometabolic risk factors in children - Busselton Health Study 2005–2007. J Paediatr Child Health. 2013;49:955–962. doi:10.1111/jpc.12298
75. Sardinha LB, Santos DA, Silva AM, Grøntved A, Andersen LB, Ekelund U. A comparison between BMI, waist circumference, and waist-to-height ratio for identifying cardio-metabolic risk in children and adolescents. PLoS One. 2016;11(2):e0149351. doi:10.1371/journal.pone.0149351
76. Makbul Aman A, Rasyid H, Bakri S, Patellongi IJ. The association between parents history of Type 2 diabetes with metabolic syndrome component and insulin resistance in non-diabetic young adult male. Acta Med Indones. 2018;50(4):309–313.
77. Bi C, Wang L, Sun C, et al. Association between normal triglyceride and insulin resistance in US adults without other risk factors: A cross-sectional study from the US National Health and Nutrition Examination Survey, 2007–2014. BMJ Open. 2019;9:e028652. doi:10.1136/bmjopen-2018-028652
78. Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. doi:10.1016/j.cca.2006.07.005
79. Fonseca VA. The metabolic syndrome, hyperlipidemia, and insulin resistance. Clin Cornerstone. 2005;7:61–72. doi:10.1016/S1098-3597(05)80069-9
80. Sathiyapriya V, Bobby Z, Agrawal A, Selvaraj N. Protein glycation, insulin sensitivity and pancreatic beta-cell function in high-risk, non-diabetic, first-degree relatives of patients with type 2 diabetes. Indian J Physiol Pharmacol. 2009;53(2):163–168.
81. Usgaokari AP, Dhume CY, Amonkar SD. Comparative assessment of insulin sensitivity and pancreatic beta cell function in diabetics and non-diabetics. Int J Pharma Bio Sci. 2012;3(1):405–413.
82. Tobisch B, Blatniczky L, Barkai L. Cardiometabolic risk factors and insulin resistance in obese children and adolescents: relation to puberty. Pediatr Obes. 2015;10:37–44. doi:10.1111/j.2047-6310.2013.00202.x
83. Yin J, Li M, Xu L, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5:71. doi:10.1186/1758-5996-5-71
84. Shashaj B, Luciano R, Contoli B, et al. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2015;53:251–260. doi:10.1007/s00592-015-0782-4
85. Dayeh T, Ling C. Does epigenetic dysregulation of pancreatic islets contribute to impaired insulin secretion and type 2 diabetes? Biochem Cell Biol. 2015;93:511–521. doi:10.1139/bcb-2015-0057
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
