Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 9
Metabolic syndrome and associated factors among outpatients of Jimma University Teaching Hospital
Authors Abda E, Hamza L, Tesema F, Cheneke Gebisa WC
Received 2 October 2015
Accepted for publication 23 December 2015
Published 4 March 2016 Volume 2016:9 Pages 47—53
DOI https://doi.org/10.2147/DMSO.S97561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Edris Abda,1 Leja Hamza,2 Fasil Tessema,3 Waqtola Cheneke4
1Department of Medicine, College of Medicine and Health Sciences, Madda Walabu University, Bale Robe, 2Department of Internal Medicine, 3Department of Epidemiology, 4Department of Medical Laboratory Sciences and Pathology, College of Health Sciences, Jimma University, Jimma, Ethiopia
Background: Developing countries are now experiencing the epidemiologic transition, whereby the burden of chronic diseases, like metabolic syndrome, is increasing. However, no study had previously been conducted to show the status of metabolic syndrome among outpatients of Jimma University Teaching Hospital. Therefore, this study was designed to determine the prevalence of metabolic syndrome and associated factors among adult (≥20 years) patients.
Methods: A cross-sectional hospital-based study was conducted in July 2014 among adult (≥20 years) patients attending Jimma University Teaching Hospital, outpatient department. All patients attending the outpatient department and were willing to participate in the study were included. Anthropometric and biochemical measurements were undertaken for all the study subjects to know the status of metabolic syndrome. Metabolic syndrome was identified using the National Cholesterol Education Program’s Adult Treatment Panel III criteria.
Results: A total of 225 participants were included in the study, of whom 106 (47.1%) were males and 119 (52.9%) were females. A total of 59 (26%) adults were found to have metabolic syndrome, which was seen more than twice as much in females, 42 (35%), as compared with males, 17 (16%), (P<0.01). The most frequent metabolic syndrome parameters were hypertension (45%), hyperglycemia (39%), decreased high-density lipoprotein (HDL) (31%), central obesity (26%), and elevated triglycerides (18%). Elevated blood pressure is more common in females (44.5%) than in males (34.9%). Decreased HDL-cholesterol was observed among 37% of females versus 24% males (P<0.001) and 6% of males versus 45% females had central obesity (P<0.001). Hypertension and body mass index were significantly lower among males (35% and 14%) than females (45% and 41%) (P<0.01 and P<0.001), respectively.
Conclusion: It is demonstrated that metabolic syndrome is prevalent in adult outpatients in Jimma and increases as age increases; it is more common among females than males. Among the five diagnostic criteria for metabolic syndrome, hypertension, hyperglycemia, and low HDL-cholesterol were the most prevalent. As metabolic syndrome is rising at an alarming rate, we recommend that relevant prevention, diagnostics, and therapy in adult outpatients are undertaken.
Keywords: metabolic syndrome, Jimma, outpatients, high-density lipoprotein, obesity
Background
Metabolic syndrome, which comprises visceral obesity, dyslipidemia, hyperglycemia, and hypertension, has become one of the major public-health challenges worldwide. It is a condition that increases the risk of cardiovascular disease (CVD) by twofold and the risk of developing type 2 diabetes mellitus by threefold. The principal pathophysiology is assumed to be linked to abdominal obesity and insulin resistance.1
The eventual significance of metabolic syndrome is that it helps to classify persons at high risk of both type 2 diabetes mellitus (DM) and CVD. Numerous skilled groups have therefore tried to create diagnostic criteria. The first attempt was by a World Health Organization (WHO) diabetes group in 1999, which suggested a definition that could be amended as more information became available. The criteria contained insulin resistance or its substitutes, impaired glucose tolerance, or diabetes as essential components. Additionally, at least two of the following conditions were considered: raised blood pressure (BP), hypertriglyceridemia, and/or low high-density lipoprotein (HDL)-cholesterol, obesity (as measured by waist circumference or body mass index (BMI)), and microalbuminuria.2
The European Group for the Study of Insulin Resistance then modified the WHO criteria by rejecting people with diabetes, wanting hyperinsulinemia to be present, and waist circumference should be the measure of obesity (with different cutoffs for male and female).
Another approach originated from the US National Cholesterol Education Program: Adult Treatment Panel III in 2001, giving attention to CVD risk. Particular concern was to assist clinical diagnosis of high-risk individuals. This approach was less glucocentric than the definition from WHO and the European Group for the Study of Insulin Resistance, needing the presence of any three of the following five components: central obesity (waist circumference >102 cm [males], >88 cm [females]), raised BP (≥130 mmHg systolic or ≥85 mmHg diastolic or specific medication), raised triglycerides (TG ≥150 mg/dL or specific medication), low HDL-cholesterol (<40 and <50 mg/dL, respectively for men and women, or specific medication), and fasting hyperglycemia (fasting blood sugar [FBS] ≥100 mg/dL or specific medication or previously diagnosed type 2 DM).3,4
Of the different approaches that produce the best prediction of subsequent diabetes and CVD is the central theme. Thus, Adult Treatment Panel III was superior to WHO in the San Antonio Study, but WHO gave better prediction of CVD in Finnish men.5,6 Another problem with the WHO and the Adult Treatment Panel definitions has been their applicability to different ethnic groups, especially as relates to obesity cutoffs.7
On a global scale, obesity prevalence remains worse both in developed and developing countries. This epidemic is now followed by a worldwide epidemic of metabolic syndrome. Younger children and adults are being challenged with the hazards of dietary carelessness and physical inactivity. Clearly, consistent methods for defining metabolic syndrome are needed not only to account for geographical and ethnic difference, but also for proper data analysis in epidemiological studies.8
The highest documented prevalence of metabolic syndrome worldwide is in Native Americans, with nearly 60% of women aged 45–49 years and 45% of men aged 45–49 years meeting the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP: ATP III) criteria.9 In the US, metabolic syndrome is less common in African–American men and more common in Mexican–American women. Based on data from the National Health and Nutrition Examination Survey 1999–2000, the age-adjusted prevalence of the metabolic syndrome in the US adults who did not have diabetes was 28% for men and 30% for women.10,11
In France, a cohort of 30–60 year-old adults has shown a <10% prevalence of metabolic syndrome for each sex, although 17.5% are affected in the age range 60–64 years. Greater industrialization worldwide is associated with rising rates of obesity, which is anticipated to increase prevalence of the metabolic syndrome dramatically, especially as the age of population grows. The prevalence of metabolic syndrome in African population ranges from 0% to about 50% or even higher depending on the population setting.12
In sub-Saharan Africa, the prevalence of metabolic syndrome is rising in an alarming rate and approaching that of the developed nation. It could be because of risk factors such as consumption of calorie-dense foods, sedentary lifestyle, tobacco use, and use of anti-HIV drugs in those areas. Only one study has systematically assessed the prevalence of metabolic syndrome among occupied adults in Addis Ababa and determined the overall prevalence of metabolic syndrome to be 14.5% and 17.9%, according to ATP III and International Diabetes Federation definitions, respectively.13–15
In recent years, quick economic growth and aging population as well as pronounced sedentary lifestyle in sub-Saharan countries like Ethiopia has increased CVD and the diabetes epidemic.16,17
Especially in our study area there was almost no study done on metabolic syndrome. So this study would clearly show the prevalence and related factors of metabolic syndrome in outpatients of Jimma University Teaching Hospital (JUTH). This would help the physicians to provide the best possible care for outpatients and would also help policy makers to have evidence for their action towards metabolic syndrome management among outpatients.
Materials and methods
This hospital-based cross-sectional study was conducted from July 1 to 30, 2014 in JUTH, which is located in the Southwest of Ethiopia in Oromia region, 352 km away from the capital city, Addis Ababa.
The study included all adult patients (≥20 years) attending outpatients of internal medicine after overnight fasting and willing to participate in the study. Similar to the NCEP: ATP III studies, we included only adults (≥20 years) excluding those under the age of 20. We also excluded pregnant mothers because of unreliable waist circumference measurement and BMI.
We used the single population proportion formula [n=z21–α/2 p(1–p)/d2], where n stands for minimu sample size, p stands for proportion, z stands for level of confidence, and d stands for margin of error, to calculate the minimum sample size considering the following parameters: the prevalence of metabolic syndrome conducted in Addis Ababa among bank workers (14.5%), standardized normal distribution at 95% confidence interval, and margin of error tolerated (5%) and inclusion of 10% for the nonresponse rate.7 Hence, the minimum sample size (participants) for the study was 225. For recruiting the study participants, we randomly selected one of the outpatient departments (OPDs) and included all adult patients consecutively coming to the OPD and those willing to participate in the study till the minimum sample size was fulfilled.
For this study, we considered independent study variables such as: age, sex, religion, educational status, smoking status, alcohol consumption, waist circumference, hypertension or those on medication, known DM or those on medication, taking a lipid-lowering drug, impaired/diabetic range using FBS, serum HDL, and serum TG.
Participants were interviewed by a trained health officer and a nurse using the modified WHO STEPwise approach to Surveillance of noncommunicable diseases-structured questionnaire. The survey questionnaire was first written in English, translated into Amharic, and then translated back into English. For those who could neither understand English nor Amharic, the data collectors translated for them.
BP was measured manually with participants sitting, after resting for at least 5 minutes. Three BP measurements were taken with at least 3-minute intervals between consecutive measurements. The mean systolic and diastolic BP from the second and third recording was analyzed.
Anthropometric measurements and blood sample collections were undertaken by a trained staff health officer. The waist circumference was taken at the borderline between the lower boundary of the last palpable rib and the top of the iliac crest. BMI was calculated by dividing weight in kilograms by height squared in meters (kg/m2) with regular monitoring and adjustment of the beam-balance. Weight categories were formed using the National Heart, Lung, and Blood Institute’s classification system.
Blood specimen, 5 mL, was collected after an 8-hour overnight fasting from every participant to determine participants’ FBS and lipid profiles in the university’s clinical chemistry laboratory using VegaSys automated chemistry analyzer (from AMS Via E. Barsanti 17/a, 00012 Guidonia – Rome, Italy). TG concentrations were measured by standard enzymatic assays using glycerol phosphate oxidase method.18 HDL cholesterol was determined after sample pretreatment with a precipitating reagent and centrifugation.19 Participants’ FBS was determined using glucose oxidase method.20
Normal and pathological quality control materials were run every day to detect any analytical errors and validate the laboratory values. Standard operating procedures for all the quality assurance phases were utilized.
The collected data were fed into SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. In multivariate logistic regression test, P<0.05 was taken as cutoff value for significant association among the dependent and independent variables.
Ethical consideration
Ethical clearance was obtained from Jimma University College of Health Sciences Institutional Review Board, and an official letter of permission was obtained from the hospital. Patients’ confidentiality was kept throughout the study and those who were diagnosed to have hypertension, DM, etc were called on their private number for treatment and follow-up. There were no risky procedures performed on the participants. Oral informed consent was obtained before inclusion into the study.
Results
Sociodemographic characteristics
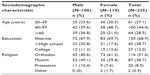
A total of 225 participants were included in the study, of whom 106 (47.1%) were males and 119 (52.9%) were females. The mean age (± standard deviation) of the participants was 48.6 (±15.1) years and 63% were in the age range of 40–69 years. The majority of the participants, 155 (69%) were illiterate and only 27 (12%) attended their education up to college level. From a religious point of view, Orthodox Christians and Muslims constituted 51.6% and 38.7%, respectively (Table 1).
  | Table 1 Sociodemographic characteristics of adult patients in JUTH internal medicine outpatient department, July 2014 |
Clinical and biochemical patient characteristics
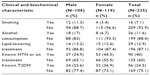
A total of 16 (7.1%) participants were smokers, of whom about four times as many were male. History of alcohol consumption, from patients self-report, was found among 26 (11.6%), of whom more than two-thirds were males. About 12.9% of the respondents were on lipid-lowering medications, which were similar percentages in both sexes. It was found that 90 (40%) of the participants had hypertension and 56 (24.5%) had diabetes and both conditions were more common among females than males (hypertension (female =44.5%, male =34.9%); DM (female =37%, male =23%)) (Table 2). The mean (± standard deviation) BMI and waist circumference were 22.7±4.4 kg/m2 and 83.1±12.3 cm, respectively. The mean systolic and diastolic BP values were 124.8±17.3 and 80.3±11.1 mmHg, respectively. The mean fasting blood glucose, HDL-cholesterol, and TG were 104.6±42.6, 72.1±38.7, and 64.8±44.9 mg/dL, respectively.
Association of predictors with metabolic syndrome
Among 225 study participants, a total of 59 (26.2%) adult patients had metabolic syndrome. It was more than twice as likely to occur in females as in males (odds ratio [OR] =2.86; 95% confidence interval [CI] 1.51–5.42; P<0.01). The risk of its development increased in those >40 years of age (40-59 year-olds are 2.84 times more at risk, and >60 year-olds are 3.24 times more at risk of developing metabolic syndrome compared to those under the age of 40 years).
The risk of metabolic syndrome was increased among cigarette smokers, though statistically not significant (P=0.64). Similarly, alcohol drinkers were found to be slightly more at risk of metabolic syndrome than their counterparts (OR =1.04; 95% CI 0.41–2.62; P=0.93).
Those adults with previously known history of hypertension had more than fivefold increased risk of metabolic syndrome whether on treatment or not (OR =5.44; 95% CI 2.85–10.39; P<0.01) than their counterparts. Similarly, known diabetic patients were shown to have about seven times higher likelihood of developing metabolic syndrome (OR =7.01; 95% CI 3.59–13.71; P<0.01) than their counterparts. Dyslipidemic respondents who were not on lipid-lowering agents had more than fourfold likelihood of developing metabolic syndrome (OR =4.16; 95% CI 2.92–5.92; P<0.01).
Elevation in systolic BP, diastolic BP, and BMI significantly increased the risk of metabolic syndrome by more than eight-, six-, and fivefold (OR =8.12, 95% CI 3.99–16.56, P<0.01; OR =6.77, 95% CI 3.53–12.98, P<0.01; OR =5.5; 95% CI 2.90–10.54, P<0.01), respectively. All male patients (n=6) with waist circumference >102 cm had metabolic syndrome, while only about 12% risk increment was seen in female respondents (OR =0.12; 95% CI 0.05–0.28; P<0.01). Low HDL-cholesterol (<40 mg/dL for female and <50 mg/dL for male) increased prevalence of metabolic syndrome by about threefold (OR =2.92; 95% CI 0.93–9.20; P=0.07).
FBS of ≥100 mg/dL had increased risk of metabolic syndrome by more than fourfold and was comparable in both sexes (OR =4.20; 95% CI 2.24–7.86; P<0.01). Similarly, serum TG level of 150 mg/dL and above was shown to raise the risk of metabolic syndrome by more than four times (OR =4.34; 95% CI 1.32–14.24; P=0.02) (Table 3).
Multivariate analysis of predictors of metabolic syndrome
Alcohol drinking, age ≥40 years, and history of hypertension or on antihypertensives increased the risk of metabolic syndrome by about twofold, but was statistically not significant after adjustment. Metabolic syndrome risk was more than five times more likely in females than male respondents after adjustment (adjusted odds ratio [AOR] =5.49; 95% CI 1.85–16.24; P<0.01).
Dyslipidemic patients on lipid-lowering treatment had about six times less likelihood of developing metabolic syndrome than those untreated (AOR =0.06; 95% CI 0.01–0.22; P<0.01). DM or high FBS, TG level ≥150 mg/dL, and BMI >25 kg/m2 were found to be independent predictors of metabolic syndrome risk (P=0.01, P<0.01, P=0.04, respectively). Known diabetics had more than four times higher likelihood of developing metabolic syndrome (AOR =4.23; 95% CI 1.39–12.91). The risk of developing metabolic syndrome was about eight times higher in those with FBS of 100 mg/dL or above (AOR =7.95; 95% CI 2.62–24.14; P<0.01).
Both systolic and diastolic BPs ≥130 and/or ≥85 mmHg were found to increase metabolic syndrome risk by more than eight- and threefold (OR =8.05; 95% CI 2.08–31.07; P<0.01) (AOR =3.40; 95% CI 0.95–12.16; P=0.06), respectively. Finally, BMI ≥25 kg/m2 was associated with more than twice the risk of developing metabolic syndrome (AOR =2.81; 95% CI 1.07–7.38) (Table 4).
Discussion
This study was conducted to assess the burden of metabolic syndrome and associated factors. In this study, it was found that the prevalence of metabolic syndrome was about double (26.2%) than that of adult bank workers in Addis Ababa (14.5%).7 It is clear that the study populations in Addis Ababa and ours are different. The study population in Addis Ababa are apparently health bank workers whose job style is mainly of the sedentary lifestyle. People with sedentary lifestyles might be at risk of developing metabolic syndrome. However, these current study populations are patients visiting medical OPD for any illness. The prevalence of metabolic syndrome in this current study is about two times more than that in Addis Ababa. The rate of occurrence varies depending on components of the metabolic syndrome; hypertension and hyperglycemia are predominant in this study, which is also similar to the study in Addis Ababa. Hypertension (40%), hyperglycemia (39%), and low HDL-cholesterol (31%) were the most frequently occurring risk factors for metabolic syndrome. Central obesity was 26% and 18% of adults had hypertriglyceridemia or were on lipid-lowering treatment. This finding is not amazing as it is understood that hypertension and central obesity play a crucial role in the development of the metabolic syndrome and look to come first before the appearance of the other metabolic syndrome components.4
There were variations in the prevalence of each of the distinct risk factors by sex. Several studies have identified the sex differences in prevalence of metabolic syndrome. For instance, a study in France, MONICA showed a significantly elevated body weight, waist circumference, and low HDL-cholesterol in women than in men, which is similar to our study.21 The results of female sex dominance seen in our study were similar to the findings in Russia, Korea, China, and Ethiopia. It was worrisome that the increase in prevalence of the metabolic syndrome was higher in women (16%–35%) than in men (10%–16%).7,11–13
All males with waist circumference >102 cm had metabolic syndrome as compared with 45% of females. Moreover, there were variations among different age groups for both sexes in the prevalence of abdominal obesity, hypertriglyceridemia, hypertension, and hyperglycemia in this current study.
For both sexes, the prevalence of each of these risk factors increased with subsequent age group. Males of 40 years and over were more likely to have more risk factors than the younger age groups, which were seen in different sub-Saharan countries.10–12
Conclusion
Based on the NCEP: ATP III guidelines, more than a quarter of adults attending medical OPD in JUTH were characterized as having metabolic syndrome. The metabolic syndrome increased with age and was higher in females. Abdominal obesity, hypertension, and hyperglycemia were the most prevalent conditions among the five diagnostic criteria for metabolic syndrome.
This finding shows the need for prevention, diagnosis, and management of metabolic syndrome and its related factors among adults in Ethiopia. Individuals having or at risk of metabolic syndrome should be advised to be compliant to treatment for hypertension, DM, and dyslipidemia.
Acknowledgments
The authors would like to thank Jimma University for the financial support. Their appreciation also goes to the study participants for their willingness and the data collectors. The authors would also like to thank Desta Hiko, Ahmed Zeinudin, Wondimagegn Addisu, and Tsigereda Limenih for their unreserved assistance.
Author contributions
EA designed the study, developed protocol, supervised data collection and data entry, conducted the data analysis, and wrote the manuscript. LH, FT, and WC participated in the formulation of the study design, participated in protocol development, reviewed the data analysis, and contributed to revising the manuscript. All authors have read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Robert HE. The metabolic syndrome. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:1992–1997. | |
Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity and body mass index: United States 2003–2006. Natl Health Stat Report. 2009 May 5;(13):1–7. | |
Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82(5):509–524. | |
Lao XQ, Zhang YH, Wong MC, et al. The prevalence of metabolic syndrome and cardiovascular risk factors in adults in southern China. BMC Public Health. 2012;12:64. | |
Okafor CI. The metabolic syndrome in Africa: current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. | |
Motala AA, Mbanya JC, Ramaiya KL. Metabolic syndrome in sub-Saharan Africa. Ethn Dis. 2009;19(Suppl 2):S2-8–S2-10. | |
Tran A, Gelaye B, Girma B, et al. Prevalence of metabolic syndrome among working adults in Ethiopia. Int J Hypertens. 2011;2011:193719. | |
Alegría E, Cordero A, Laclaustra M, et al. Prevalence of metabolic syndrome in the Spanish working population: MESYAS registry. Rev Esp Cardiol. 2005;58(7):797–806. | |
Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. | |
Mutimura E, Crowther NJ, Stewart A, Cade WT. The human immunodeficiency virus and the cardiometabolic syndrome in the developing world: an African perspective. J Cardiometab Syndr. 2008;3(2):106–110. | |
World Health Organization. Preventing chronic diseases: a vital investment; WHO global report. World Health Organization, Geneva; 2005. Available from: www.who.int/entity/chp/chronic_disease_report/contents/foreword.pdf. Accessed January 30, 2016. | |
Kelliny C, William J, Riesen W, Paccaud F, Bovelt P. Metabolic syndrome according to different definitions in a rapidly developing country of the African region. Cardiovasc Diabetol. 2008;7(27):683–689. | |
Albrink MJ, Meigs JW. The relationship between serum triglycerides and skin fold thickness in obese subjects. Ann N Y Acad Sci. 1965;131(1):673–683. | |
Avogaro P, Battaglia G, Pujatti G, et al. Metabolic syndrome and cardiac complication. In: Manual of Cardiovascular Medicine. Philadelphia, PA: Lippincott Williams and Wilkins; 2007;V8:631–647. | |
Reaven GM. Banting lecture 1988, role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. | |
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. | |
Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366(9491):1059–1062. | |
Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–2080. | |
Maddison A, Motwani R, Speaight AB. Serum high density lipoprotein cholesterol determination: a simple modification. Clin Chim Acta. 1979;92(2):307–310. | |
Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24. | |
Balkau B, Charles MA. Comment on the provisional report from the WHO Consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999;16(5):442–443. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.