Back to Journals » Cancer Management and Research » Volume 11
Meta-analysis of the prognostic value of lncRNA DANCR for cancer patients in China
Authors Fan Y, He Y, Zhou X, Liu Y, Wang F
Received 27 November 2018
Accepted for publication 30 January 2019
Published 5 March 2019 Volume 2019:11 Pages 2027—2037
DOI https://doi.org/10.2147/CMAR.S196071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yanghua Fan,1,* Yu He,2,* Xi Zhou,2,* Yong Liu,2 Fu Wang3
1Department of Central Laboratory, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China; 2Department of Orthopedics, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China; 3Department of Orthopedic Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Ji’nan, Shandong, China
*These authors contributed equally to this work
Background: Abnormal expression of long non-coding RNA anti-differentiation noncoding RNA (lncRNA DANCR) can frequently be detected in cancer. Because of this, it is of vital necessity to perform a meta-analysis to clarify the value of lncRNA DANCR as a prognostic marker in malignant tumors.
Methods: Related studies were retrieved from electronic databases including Web of Science, PubMed, and OVID, from inception to November 21, 2018. The HRs and corresponding 95% CIs were also calculated to explore the relationship of lncRNA DANCR expression with patient survival. Moreover, ORs were computed to assess the association of lncRNA DANCR expression with the pathological parameters.
Results : A total of 14 studies involving 1,117 patients were included in this meta-analysis. The pooled HR suggested that high lncRNA DANCR expression was correlated with poor overall survival (OS; HR =1.85, 95% CI: 1.56–2.18) and disease-free survival (DFS; HR =2.49, 95% CI: 1.75–3.56) in cancer patients. Besides, High lncRNA DANCR expression was related to poor histological grade (PHG; OR =2.01, 95% CI: 1.08–3.75), high tumor stage (HTS; OR =3.52, 95% CI: 1.67–7.43), lymph node metastasis (LNM; OR =3.47, 95% CI: 1.42–8.49), and distant metastasis (DM; OR =4.76, 95% CI: 2.39–9.51). However, no evidence of obvious asymmetry was found for DFS (Pr>|z|=0.308), PHG (Pr>|z|=0.707), LNM (Pr>|z|=0.174), and DM (Pr>|z|=0.734) using Begg’s funnel plot.
Conclusion: Our findings suggest that high lncRNA DANCR expression can predict poor OS, DFS, PHG, HTS, LNM, and DM in cancer patients, implying that high lncRNA DANCR expression may potentially serve as a new indicator for poor prognosis and metastasis in cancer.
Keywords: lncRNA, DANCR, neoplasms, prognosis, metastasis
Introduction
Recent report demonstrates that, the US has witnessed about 1.7 million new cancer cases and 600,000 cancer-related deaths in 2017.1 Nevertheless, the 5-year survival of most cancers remains dismally low, and a large number of scientists are devoting themselves to looking for new biomarkers to determine or diagnose cancer prognosis.
lncRNA, which lacks a meaningful open reading frame, is defined as the transcribed RNA molecule that is >200 nucleotides in length, which has possessed many important functions in disease, such as posttranscriptional, transcriptional, and epigenetic regulation.2,3 In addition, abnormal lncRNA expression is currently recognized to be related to various cancer types.4–7 For instance, some lncRNAs play crucial parts in metastasis, invasion, and proliferation of cancer cells, indicating that lncRNA may serve be a useful marker for predicting cancer prognosis.8–10
Typically, the lncRNA DANCR was discovered by Kretz et al in 2012, which was originally deemed to be essential for the dedifferentiation of epidermal cells.11 Besides, recent studies reveal that DANCR plays a crucial role in the differentiation of periodontal ligament stem cells into osteoblasts, which can also promote tumor cell dissemination and metastasis formation.12–14 Moreover, lncRNA DANCR is also suggested in some studies to be correlated with different tumor biological parameters, such as tumor growth, metastasis, and progression.15–17 Metastasis and prognosis may be affected by lncRNA DANCR; nonetheless, a majority of existing studies are limited by their small sample sizes and discrete outcomes. As a consequence, an updated meta-analysis was performed in this study to determine the prognostic value of lncRNA DANCR in cancer patients.
Materials and methods
Literature collection
In accordance with the standard guidelines for meta-analyses,18,19 related articles that served lncRNA DANCR as a prognostic biomarker for the survival of cancer patients were systemically retrieved from some online databases by two authors independently from inception to November 21, 2018. Meanwhile, text words and Mesh strategies were adjusted based on the databases in this retrieval, including the following terms (“Long non-coding RNA differentiation antagonizing non-protein coding RNA“ or “lncRNA DANCR” or “lncRNA ANCR”) and (“recurrence” or “outcome” or “survival”, “cancer” or “neoplasm” or “tumor” or “carcinoma”, “prognosis” or “prognostic”). Moreover, the reference lists of relevant articles were also manually retrieved during retrieval, so as to avoid missing any potentially eligible studies.
Study selection
All the included studies were then evaluated, and data were extracted by two scholars independently. Typically, the study inclusion criteria were as follows: 1) studies in which all tumors were confirmed by histological or pathological examinations; 2) studies in which the lncRNA DANCR expression levels in human tumor tissues were measured; 3) studies in which patients were grouped in accordance with different lncRNA DANCR expression levels, and the cutoff values of high and low DANCR expression might be the median or mean of all samples in their study; and 4) studies with sufficient original data for statistical analyses of pathological or patient survival parameters with lncRNA DANCR expression.
In addition, the study exclusion criteria were shown below: non-human studies and non-English studies; editorials, reviews, expert opinions as well as letters; database analysis without original data; and studies mentioning functions and molecular structure of lncRNA DANCR only.
Date extraction
Data from the original articles were independently examined and extracted by two reviewers, and any disagreement between them during the process of literature assessment was settled by the consensus with a third reviewer. A series of data were collected in this meta-analysis, including surname of the first author, publication year, country, tumor type, sample size, number of patients with LTS, PHG, HTS, LNM and DM, reference gene and detection method of lncRNA DANCR, as well as HRs and 95% CIs of elevated lncRNA DANCR expression for OS and DFS.
Statistical methods
The Stata version 12.0 software was adopted for all statistical analyses. In addition, the heterogeneity was also measured in this meta-analysis using Q and I2 tests. The test results had indicated the presence of significant heterogeneity in this research (I2≥50%, and P<0.1);20 therefore, the random effect model should be adopted. Besides, the potential publication bias was also assessed by Egger’s test and Begg’s funnel plot. The pooled ORs and HRs should be extracted from the published data; typically, the crude data should be adopted if the HRs could not be obtained directly from the publications. Besides, the survival information extracted from Kaplan–Meier curves should be adopted to estimate the HRs when they were not directly reported in the studies. To make a summary about the outcomes of survival, both SE and the log HR should be collected.21 Moreover, 95% CIs and ORs should be combined to assess the relationship of clinicopathological parameters with lncRNA DANCR.
Results
Study characteristics
Details about the screening process are shown in Figure 1. In accordance with the exclusion and inclusion criteria, 14 studies involving 1,117 patients were enrolled into this meta-analysis.22–35 Characteristics of the 14 studies included in this meta-analysis are summarized in Table 1. As could be observed, the sample size in the 14 studies ranged from 34 to 135, with an average of 79.57. Besides, all the enrolled studies were published between 2015 and 2018 and were carried out in China. Among these studies, respectively, one study had focused on CVR,25 TNBC,29 RB,30 HCC,34 and BC;35 three concentrated on GC;22,27,28 two focused on OSC;23,32 two on glioma;24,33 and two on CRC.26,31 All clinical pathological parameters were dependent on the pathology. Moreover, it was found that the reference genes of lncRNA DANCR were different among these studies, which had included GAPDH,23–27,29–34 b-actin,22,35 and small nuclear RNA U6.28 Moreover, the thresholds of high and low lncRNA DANCR expression levels, including the median and average lncRNA DANCR expression, were also different among these studies.
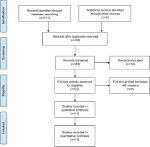  | Figure 1 Flowchart presented the steps of study selection in this meta-analysis. |
Association between the lncRNA DANCR expression level and survival
To assess the role of lncRNA DANCR in OS for cancer patients, cumulative meta-analysis was carried out in this research. As shown, the relationship of OS with lncRNA DANCR was reported in ten studies enrolling 839 patients (Table 2). Meanwhile, the fixed effects model was adopted since there was no significant heterogeneity (I2=0.0%, PQ=0.728). The results suggested that the OS in cancer patients was markedly related to the lncRNA DANCR expression (pooled HR =1.85, 95% CI: 1.56–2.18; Figure 2A). Besides, sensitivity analysis was also carried out, which had confirmed the robustness of these results (Figure 2B). Subsequently, subgroup analyses stratified by cancer type, sample size, NOS score, and HR statistic method were also carried out (Table 3, Figure 3).
  | Table 3 Subgroup analysis of OS by tumor type, sample size, NOS score, and HR statistic method Abbreviations: NOS, Newcastle–Ottawa Scale; OS, overall survival. |
Moreover, cumulative meta-analysis was also performed to determine the role of lncRNA DANCR in DFS among the 330 cancer patients recruited into the eligible studies (Figure 4). The results revealed that lncRNA DANCR was correlated with DFS (pooled HR =2.49, 95% CI: 1.75–3.56) in cancer patients upon statistical analyses. Similarly, the fixed effects model was employed due to the insignificant heterogeneity.
These results suggested that the shorter OS and DFS in cancer patients might be associated with higher lncRNA DANCR expression. As a result, it could be concluded that lncRNA DANCR was an independent factor of the survival for cancer patients.
Association between the lncRNA DANCR expression level and LTS
Figure 5A shows the association between LTS and lncRNA DANCR expression from ten studies involving 757 patients. Specifically, the random-effects model was adopted due to the presence of a significant heterogeneity among the eligible studies (I2=79.4%, PQ=0.000). Our results had revealed a pooled OR of 1.63 (95% CI: 0.80–3.31; high vs low lncRNA DANCR expression). Moreover, sensitivity analysis of all included studies was also performed, and the OR of high to low expression groups was 2.10 (95% CI: 1.25–3.54) after the study by Hao et al22 was excluded (I2=56.6%, PQ=0.018) (Figure 5B and C).
Conforming to the abovementioned results, no significant difference was detected in the LTS incidence between two groups, but additional studies were needed to confirm the association between lncRNA DANCR and LTS in cancer patients.
Association between the lncRNA DANCR expression level and PHG
In this research, data regarding the association between the lncRNA DANCR expression and PHG had been collected from six eligible studies involving 503 cancer patients, and the random-effects model was adopted as a result of the significant heterogeneity (I2=61.4%, PQ=0.024). Besides, the OR of high to low lncRNA DANCR expression groups was 2.10 (95% CI: 1.08–3.75, Figure 6A). Typically, the heterogeneity had disappeared (I2=24.2%, PQ=0.266) after two studies were removed in sensitivity analysis, with the OR of high to low expression groups of 3.14 (95% CI: 1.95–5.05) (Figure 6B and C).
In accordance with these results, a significant difference was noted in the incidence of PHG between two groups, indicating that the risk of PHG was remarkably correlated with high lncRNA DANCR expression.
Association between the lncRNA DANCR expression level and HTS
In this meta-analysis, the correlation between HTS and lncRNA DANCR expression was detected in ten eligible studies recruiting 809 patients. Similarly, the random effects model would be adopted (I2=81.4%, PQ=0.000). The results discovered that HTS in cancer patients was notably related to high lncRNA DANCR expression (pooled OR =3.52, 95% CI: 1.67–7.43, Figure 7A). In addition, the heterogeneity had disappeared in sensitivity analysis after the study by Hao et al22 was excluded (I2=0.0%, PQ=0.905), and the OR of high to low lncRNA DANCR expression groups was 4.67 (95% CI: 3.30–6.60) (Figure 7B and C).
According to the analysis results, compared with the low lncRNA DANCR expression group, the tumor stage in high lncRNA DANCR expression group was markedly higher, demonstrating that the risk of HTS was evidently correlated with high lncRNA DANCR expression.
Association between the lncRNA DANCR expression level and LNM
In this research, data collected from eight eligible studies involving 628 cancer patients were also analyzed, and the random effects model had been adopted based on the significant heterogeneity (I2=80.4%, PQ=0.000). Additionally, the OR of to low lncRNA DANCR expression groups was 3.47 (95% CI: 1.42–8.49, Figure 8A). Consistent with the results of previous sensitivity analysis, the heterogeneity had disappeared (I2=0.0%, PQ=0.693) after the study by Hao et al22 was removed (Figure 8B and C).
In accordance with these results, a significant difference was noted between two groups in terms of LNM incidence. As far as cancer patients were concerned, high lncRNA DANCR expression was markedly correlated with greater susceptibility to LNM.
Association between the lncRNA DANCR expression level and DM
In this meta-analysis, the correlation of DM with the lncRNA DANCR expression level was examined in four eligible studies including 241 patients, and the fix effects model was adopted due to the limited heterogeneity (I2=0.0%, PQ=0.666). The OR of high to low lncRNA DANCR expression groups was 4.76 (95% CI: 2.39–9.51, Figure 9). Consistent with these results, the DM incidence was significantly different between two groups, revealing that high lncRNA DANCR expression could remarkably predict a higher tendency to develop DM in cancer patients.
Publication bias
Subsequently, the Begg’s funnel plot was conducted in this study to evaluate the potential publication bias. Figure 10 shows no evidence of obvious asymmetry for DFS (Pr>|z|=0.308), LTS (Pr>|z|=0.283), PHG (Pr>|z|=0.707), LNM (Pr>|z|=0.174), and DM (Pr>|z|=0.734). However, significant publication bias was detected for OS (Pr>|z|=0.004) and HTS (Pr>|z|=0.007).
Discussion
Cancer still poses a serious threat to human health, which is gradually increased in recent years in terms of morbidity.1 Nonetheless, the exact metastasis mechanism in cancer patients remains unclear despite that metastasis is an important indicator of poor prognosis.36,37 Therefore, it is necessary to identify new molecular markers to predict tumor metastasis at present, since they may play critical roles in treating and predicting cancer.38 lncRNAs, one of these molecular markers, can affect tumor initiation, progression, and occurrence, which can easily collect the useful biomarkers for cancer monitoring and diagnosis.39–41
lncRNA DANCR has been verified in previous studies to be an important oncogene in various human cancers, including GC, glioma, CVR, OSC, CRC, RB, HCC, and BC.22–35 Additionally, lncRNA DANCR expression has been confirmed in recent study to be upregulated in CRC tissues, which is correlated with poor survival for CRC patients.26,31 Moreover, according to Li et al, DANCR could positively promote the proliferation and migration of glioma through activating the Wnt/β-catenin signaling pathway.24 Besides, Mao et al also reported that DANCR was upregulated in GC tissues, which could enhance the migration and invasion of GC cells.27 Additionally, Wang et al found that DANCR could strongly suppress HCC proliferation via targeting miR-216a-5p and KLF12.42 Furthermore, Lu et al demonstrated that DANCR was elevated in a broad spectrum of human cancers, and MYC could drive cancer cell proliferation by targeting DANCR.43 These results reveal that lncRNA DANCR may be a crucial prognostic factor for cancer patients. Nevertheless, the underlying mechanisms by which lncRNA DANCR affects cancer remain unknown so far. Therefore, this meta-analysis was performed to examine the prognostic value and clinicopathological significance of lncRNA DANCR in cancer patients.
In this research, related data collected from the 14 eligible studies involving 1,117 cancer patients were analyzed, and a fixed or a random effects model had been adopted based on the heterogeneity analysis results. For cancer patients, high lncRNA DANCR expression could potentially serve as an indicator of poor prognosis. Besides, significant differences were found in OS and DFS between the two groups after combining HRs from the Cox multivariate analyses, and it was found that poor OS and DFS in various cancer kinds were associated with high lncRNA DANCR expression. Moreover, high lncRNA DANCR expression in cancer patients was also remarkably related to some clinicopathological parameters, including PHG, HTS, DM, and LNM. To sum up, findings of this meta-analysis indicated that lncRNA DANCR might serve as a valuable biomarker for the poor prognosis of most cancers.
Limitations
Several limitations should be taken into consideration when interpreting the conclusion of this meta-analysis. First, data in this meta-analysis might not be applicable for countries all over the world, since all the included studies were from China. Second, in spite of the best effort made to search for all relevant studies only 14 studies were ultimately enrolled in this study; the relatively small sample size might reduce the stringency of our conclusion. Third, the criterion of high expression was not consistent among all articles, making it difficult to obtain the same value. Last but not least, there were other factors that might affect cancer prognosis, such as comorbidities and therapies, but related information was not available in the analyzed enrolled articles, which had therefore become an inherent shortcoming of this systematic review and meta-analysis. As a consequence, the role of lncRNA DANCR in cancer should be further confirmed by more high-quality and well-designed studies.
Conclusion
To sum up, our findings suggest that high lncRNA DANCR expression in a series of cancers is remarkably correlated with poor OS, DFS, PHG, HTS, DM, and LNM. As a result, lncRNA DANCR may potentially serve as a biomarker to determine metastasis and predict the prognosis for cancer patients.
Abbreviations
BC, bladder cancer; CRC, colorectal cancer; CVR, cervical cancer; DFS, disease-free survival; DM, distant metastasis; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GC, gastric cancer; HCC, hepatocellular carcinoma; HTS, high tumor stage; lncRNA DANCR, long non-coding RNA anti-differentiation noncoding RNA; LNM, lymph node metastasis; LTS, larger tumor size; NOS, Newcastle–Ottawa Scale; OS, overall survival; OSC, osteosarcoma; PHG, poor histological grade; RB, retinoblastoma; TNBC triple negative breast cancer.
Acknowledgment
This article was supported by the Science and Technology Development Program of Shandong Province (No. 2015GSF118110).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Johnsson P, Lipovich L, Grander D, Morris KV. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 1840;2014(3):1063–1071. | ||
Fan YH, Fang H, Ji CX, Xie H, Xiao B, Zhu XG. Long noncoding RNA CCAT2 can predict metastasis and poor prognosis: a meta-analysis. Clin Chim Acta. 2017;466:120–126. | ||
Dai W, Tian C, Jin S. Effect of lncRNA ANRIL silencing on anoikis and cell cycle in human glioma via microRNA-203a. Onco Targets Ther. 2018;11:5103–5109. | ||
Zheng Y, Gao Y, Li X, et al. Long non-coding RNA NAP1L6 promotes tumor progression and predicts poor prognosis in prostate cancer by targeting inhibin-β A. Onco Targets Ther. 2018;11:4965–4977. | ||
Liu Q, Liu H, Cheng H, Li Y, Li X, Zhu C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. Onco Targets Ther. 2017;10:2461–2471. | ||
Hua JT, Ahmed M, Guo H, et al. Risk SNP-Mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19. Cell. 2018;174(3):564–575.e18. | ||
Ma Z, Huang H, Xu Y, et al. Current advances of long non-coding RNA highly upregulated in liver cancer in human tumors. Onco Targets Ther. 2017;10:4711–4717. | ||
Fan Y, Yan T, Chai Y, Jiang Y, Zhu X. Long noncoding RNA HOTTIP as an independent prognostic marker in cancer. Clinica Chimica Acta. 2018;482:224–230. | ||
Guo H, Ahmed M, Zhang F, et al. Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat Genet. 2016;48(10):1142–1150. | ||
Kretz M, Webster DE, Flockhart RJ, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26(4):338–343. | ||
Jia Q, Jiang W, Ni L. Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch Oral Biol. 2015;60(2):234–241. | ||
Zhu L, Xu PC. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun. 2013;432(4):612–617. | ||
Jia J, Li F, Tang XS, et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget. 2016;7(25):37868–37881. | ||
Ma X, Wang XIN, Yang C, et al. DANCR acts as a diagnostic biomarker and promotes tumor growth and metastasis in hepatocellular carcinoma. Anticancer Res. 2016;36(12):6389–6398. | ||
Zhen Q, Gao LN, Wang RF, et al. LncRNA DANCR promotes lung cancer by sequestering miR-216a. Cancer Control. 2018;25(1):107327481876984. | ||
Xu D, Yu J, Gao G, Lu G, Zhang Y, Ma P. LncRNA DANCR functions as a competing endogenous RNA to regulate Rab1a expression by sponging miR-634 in glioma. Biosci Rep. 2018;38(1):BSR20171664. | ||
Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216. | ||
Harris AL. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):385–386. | ||
Fan YH, Ji CX, Xu B, Fan HY, Cheng ZJ, Zhu XG. Long noncoding RNA activated by TGF-beta in human cancers: a meta-analysis. Clin Chim Acta. 2017;468:10–16. | ||
Ma PJ, Guan QK, Xu DW, Zhao J, Qin N, Jin BZ. LncRNA PANDAR as a prognostic marker in Chinese cancer. Clin Chim Acta. 2017;475:172–177. | ||
Hao YP, Qiu JH, Zhang DB, Yu CG. Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol. 2017;39(6):1010428317699798. | ||
Jiang N, Wang X, Xie X, et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating Axl via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55. | ||
Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/β-catenin signaling. Biomed Pharmacother. 2018;102:602–607. | ||
Liang H, Zhang C, Guan H, Liu J, Cui Y. LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J Cell Physiol. 2019;234(5):7266–7278. | ||
Liu Y, Zhang M, Liang L, Li J, Chen YX. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2015;8(9):11480–11484. | ||
Mao Z, Li H, Du B, et al. LncRNA DANCR promotes migration and invasion through suppression of lncRNA-LET in gastric cancer cells. Biosci Rep. 2017;37(6):BSR20171070. | ||
Pan L, Liang W, Gu J, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9(2):1915–1930. | ||
Sha S, Yuan D, Liu Y, Han B, Zhong N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open. 2017;6(9):1310–1316. | ||
Wang JX, Yang Y, Li K. Long noncoding RNA DANCR aggravates retinoblastoma through miR-34c and miR-613 by targeting MMP-9. J Cell Physiol. 2018;233(10):6986–6995. | ||
Wang Y, Lu Z, Wang N, et al. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp Mol Med. 2018;50(5):57. | ||
Wang Y, Zeng X, Wang N, et al. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol Cancer. 2018;17(1):89. | ||
Yang JX, Sun Y, Gao L, Meng Q, Yang BY. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma. 2018;65(5):790–798. | ||
Yuan SX, Wang J, Yang F, et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63(2):499–511. | ||
Zhan Y, Chen Z, Li Y, et al. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J Exp Clin Cancer Res. 2018;37(1):273. | ||
Guan H, Mei Y, Mi Y, et al. Downregulation of lncRNA ANRIL suppresses growth and metastasis in human osteosarcoma cells. Onco Targets Ther. 2018;11:4893–4899. | ||
Liu H, Pan Y, Han X, Liu J, Li R. MicroRNA-216a promotes the metastasis and epithelial-mesenchymal transition of ovarian cancer by suppressing the PTEN/Akt pathway. Onco Targets Ther. 2017;10:2701–2709. | ||
Fan YH, Ye MH, Wu L, et al. Overexpression of miR-98 inhibits cell invasion in glioma cell lines via downregulation of IKKε. Eur Rev Med Pharmacol Sci. 2015;19(19):3593–3604. | ||
Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2):155–165. | ||
Gao P, Wei GH. Genomic insight into the role of lncRNA in cancer susceptibility. Int J Mol Sci. 2017;18(6):E1239. | ||
Gao P, Xia JH, Sipeky C, et al. Biology and clinical implications of the 19q13 aggressive prostate cancer susceptibility locus. Cell. 2018;174(3):576–589.e18. | ||
Wang J, Pu J, Zhang Y, et al. DANCR contributed to hepatocellular carcinoma malignancy via sponging miR-216a-5p and modulating KLF12. J Cell Physiol. 2019;234(6):9408–9416. | ||
Lu Y, Hu Z, Mangala LS, et al. Myc targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res. 2018;78(1):64–74. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.











