Back to Journals » Journal of Pain Research » Volume 12
Mesotherapy in the treatment of musculoskeletal pain in rehabilitation: the state of the art
Authors Paolucci T, Bellomo RG, Centra MA , Giannandrea N , Pezzi L , Saggini R
Received 21 March 2019
Accepted for publication 29 May 2019
Published 30 July 2019 Volume 2019:12 Pages 2391—2401
DOI https://doi.org/10.2147/JPR.S209610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
T Paolucci,1 RG Bellomo,2 MA Centra,1 N Giannandrea,1 L Pezzi,1 R Saggini1
1University G.d’Annunzio Chieti, Department of Medical and Oral Sciences and Biotechnologies, Chieti-Pescara, Italy; 2University of Study of Urbino Carlo Bo, Department of Biomolecular Sciences, Urbino, Italy
Background: Mesotherapy can be included as an ancillary treatment in the management of localized pain in rehabilitation, but there are no definitive treatment protocols for this approach.
Objectives: The purpose of this review was to examine new indications for more standard protocols of mesotherapy in rehabilitation.
Materials and methods: This systematic review was performed using the following resources: PubMed, Cochrane, PEDro, Scopus, and Google Scholar. The following algorithm was developed, based on the PICO acronym, to evaluate the effects of mesotherapy, with pain as the primary outcome (MESH terms): [mesotherapy AND pain], [mesotherapy AND musculoskeletal], [mesotherapy AND musculoskeletal disorder], [intradermal therapy AND pain], and [intradermal therapy AND musculoskeletal disorder].
Results: Seven articles (N=7) satisfied the inclusion criteria and were considered in the review: two of them treated osteoarthritis of the knee (3 sessions) and pes anserine (9 sessions) emphasizing a good efficacy of mesotherapy. Five studies analyzed spine diseases (specifically, two was about chronic and nonspecific neck pain, two about acute low back pain and one about chronic spinal pain): the results of mesotherapy treatment are encouraging both for the resolution of acute and chronic musculoskeletal vertebral pain from one to five sessions.
Conclusion: Mesotherapy showed a good effect to reduce acute and chronic musculoskeletal pain and, also, it is a well-tolerated treatment. Nonetheless future randomized controlled trials should be desirable for more uniform treatment protocols.
Keywords: mesotherapy, intradermal injection, rehabilitation, pain, musculoskeletal
Introduction
Mesotherapy (or intradermal therapy) can be included as an additional treatment for the management of localized pain, comprising a series of micro-injections in the upper layers of the skin, which allows for slower diffusion of the drug compared with deep administration.1 Mesotherapy is defined as
the use of intra- or subcutaneous injections containing liquid mixture of compounds (pharmaceutical and homeopathic medications, plant extracts, vitamins, and other ingredients) to treat local medical and cosmetic conditions.2
Recommendations for proper medical use of this technique in rehabilitation have been directed toward patients with minor localized musculoskeletal pain syndrome, such as neck pain or low back pain,3 that could benefit from localized treatment that reduces the systemic administration of medications.4
The current mesotherapeutic protocols suggest the administration of a single drug by local injection with needles of 27 Gauge × 4 mm and a low dose of the drug. It has also been recommended that the reflex effect produced by the needles has clinical benefits about pain.1,5,6
In contrast, the original mesotherapy approach involved the use of procaine and many simultaneous injections (from 5 to 18 injections) with needles of 30 or 40 Gauge ×4 mm. Clinical studies have examined the effects of mesotherapy in acute and chronic musculoskeletal pain. Costantino et al reported that the combined administration of conventional NSAIDs and corticosteroids by mesotherapy is an effective and well-tolerated method for managing low back pain in the short term, compared with oral and intramuscular drug therapy.7 Other groups suggested that the response to acupuncture mesotherapy points is greater than that to trigger point mesotherapy at the short-term follow-up. Nevertheless, this technique could be a viable option as an adjunct treatment in the overall treatment of chronic low back pain.8
Regardless of mesotherapy technique, its effects on pain can vary, depending on the points and drugs, for which there remain no definitive treatment protocols. For example, both the mesotherapy with normal saline solution or with a drug cocktail proved efficacy in the short term in improving musculoskeletal neck pain: instead, it would seem that for the maintenance of the good results in a longer period (up to three months from the end of the treatment) the drug cocktail was more effective than saline solution alone.9
The last consensus (2011) of the Italian Society of Mesotherapy (SIM), which convened a panel of experts to review the available evidence and establish recommendations on the use of intradermal therapy in clinical practice, reiterated the need for more large-scale clinical trials to determine the specific benefits and limitations in certain areas of the application of intradermal therapy.1 In particular, a review by Mammucari et al3 identified 2 important areas for further research: 1. confirmation of the efficacy of intradermal NSAIDs for localized pain in reducing the risk of their systemic effects and 2. the use of intradermal opioids to increase our understanding of how to extend the effectiveness of these analgesics in painful musculoskeletal conditions. In patients with arthritis or other musculoskeletal conditions, pain is frequently triggered by inflammation of peripheral tissues (nociceptive pain), but it is also associated with a lesion (or dysfunction) in the nerve pathways (neuropathic pain),10 for which local pharmacological therapy, if it is effective and well tolerated, is an acceptable alternative to systemic NSAIDs.11,12
The purpose of this review was to examine recent clinical studies that have proposed new indications for mesotherapy with more standard protocols in rehabilitation.
Materials and methods
Study selection
A systematic review was performed using PubMed, Cochrane, PEDro, Scopus, and Google Scholar per the guidelines of the PRISMA statement.13 The following algorithm was developed, based on the PICO acronym,14 to evaluate the effects of mesotherapy, using pain as the primary outcome, in patients with musculoskeletal problems (MESH terms): [mesotherapy AND pain], [mesotherapy AND musculoskeletal], [mesotherapy AND musculoskeletal disorder], [intradermal therapy AND pain], and [intradermal therapy AND musculoskeletal disorder]. Bibliographies of the retrieved studies were searched to identify other relevant studies; country, author, affiliated institutions, and enrollment periods were extracted and reviewed to identify and exclude duplicate publications from the same cohort.
Data extraction
Three investigators (AMC, NG, and LP) carried out the research autonomously and subsequently crossed the data to screen titles and abstracts and independently assessed the risk of bias. Disputes were resolved by consensus. An evaluative score has been assigned to each research included in the manuscript according to the Pedro-Score.23
Eligibility criteria
The inclusion criteria were articles that had been published in the last 6 years (from January 2012), randomized and nonrandomized clinical trials, retrospective studies, mean age of patients between 18 and 70 years, full English text, in the area of rehabilitation, and at least 15 patients who were treated in each group. Exclusion criteria were other observational studies, case reports, articles without an abstract or full text, and those that did not deal with human subjects. Articles that were published between 2012 and 2018 were included. When incomplete data were reported, the corresponding author of a study was contacted to obtain the missing data. The flow diagram that shows the selection of studies is presented in Figure 1.
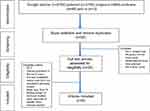 |
Figure 1 Flowchart of study identification and selection.Abbreviation: RCT, randomized controlled trial. |
Types of intervention
Patients were treated through mesotherapy with drugs, such as NSAIDs, myorelaxants, and steroids, with or without anesthetic or saline solution. The studies in this review considered the following diseases: neck pain, low back pain, knee osteoarthritis, and nonspecific spinal disease.
Outcome measures
The primary outcome was the reduction of pain in acute and chronic conditions. The secondary outcomes were efficacy of rehabilitation, recovery of function, and quality of life.
Results
Seven articles (N=7) satisfied the inclusion criteria and were considered in the review: 2 articles treated osteoarthritis of the knee,16,17 and 5 articles treated spine diseases 2 on neck pain,18,19 2 on low back pain,20,21 and 1 on chronic spinal pain.22 The PEDro scores in these studies ranged from 5 to 8 (Table 1).23 A total of 643 patients were studied, comprising 517 females and 126 males, with an average age of 45.43±15.48 years.
 |
Table 1 Summary of articles |
Regarding the primary outcome, pain was evaluated with the Visual Analogue Scale (VAS)15 in most studies,17–22 except for Chel et al,16 which only presented The Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores to quantify pain and recovery of function of the knee.24 The Visual Analogue Scale (VAS) is a measurement instrument usually used in clinical research to measure the intensity of musculoskeletal pain while WOMAC is a scale used to evaluate the condition of patients with osteoarthritis of the knee and hip, including pain, stiffness, and physical functioning of the joints. Paolucci et al18 used the Verbal Rating Scale (VRS), a 5-category verbal scale with which pain is assessed by asking patients to indicate which of 5 words best describe their current pain.25
Moreover, Ferrara et al22 quantified pain with the short-form McGill Pain Questionnaire to control the effects of therapies and pain relief in patients along the treatment time.26
With respect to the assessment of pain and function of the spine, the authors used specific scales and, specifically, the Neck Disability Index (NDI), a valid and sensitive tool for measuring changes in pain and disability in patients with musculoskeletal neck pain,18,19,27 and the Patient-Specific Functional Scale (PSFS), a patient-specific outcome measure that examines the functional status of the spine.21,28 The Knee Injury and Osteoarthritis Outcome Score (KOOS) is used to quantify short-term and long-term symptoms and function in subjects with knee injury and osteoarthritis.17,29
Finally, to evaluate the increase in quality of life that is perceived by patients, the Short Form-12 Health Survey (SF-12), a 12-item questionnaire that assesses generic health outcomes from the patient’s perspective, was administered.18,30 The self-reported Patient Global Impression of Change (PGIC) scale quantifies how the patients’ health change in painful condition.19,31
Discussion
The purpose of this review was to determine whether there are new indications for mesotherapy treatment with specific standard protocols in rehabilitation, particularly with respect to reductions in pain in musculoskeletal disorders, as chronic and acute spinal pain and OA. This technique was effective and well tolerated in patients with comorbidities, wherein the administration of NSAIDs was a contraindication.11,32,33 In our review, we analyzed 7 studies that assessed the efficacy of mesotherapy and its tolerability in the four common musculoskeletal pathologies (OA, low-back pain, neck pain and pes anserine).
Knee pain
Chen et al16 reported an improvement in pain and function in patients who were treated with mesotherapy and concluded that this approach is safe and effective in OA. Their study comprised a control group treated with oral diclofenac 75 mg (twice per day) for the first 3 months and 2 mesotherapy groups with different protocols. The first mesotherapy group included patients with acute keen pain according to the following protocol: 2 mL 1% lidocaine, 40 mg piroxicam in 2 mL, and 100 units of calcitonin in 1 mL at 1, 8, and 15 days. Instead, the second mesotherapy group included patients with chronic keen pain and the protocol was: 2 mL 2% procaine, 2 mL organic silica (Conjonctyl), and 100 units calcitonin at 1, 15, 30, and 60 days. In this study, the physician used 2 injection techniques: profound intradermic injection (IDP) (injection depth 2–4 mm) and superficial intradermic injection (IDS) (injection depth 1–2 mm) respectively with sterile single-use needles of 0.26 mm ×4 mm and 0.3 mm ×13 mm. The therapeutic efficacy and safety of mesotherapy were evaluated with the WOMAC Scale at the beginning of the treatment (T0) and at 6-month follow-up (T1). Side effects and correlating symptoms, such as allergy, dyspepsia, heartburn, nausea, bloating, were recorded at T0 and T1. Chen et al observed better results and minor side effects in the mesotherapy group, with good adherence to the protocol.
A limitation of this study was the impossibility to measure the plasma levels of NSAIDs in the mesotherapy group; thus, the authors failed to compare therapies (oral vs mesotherapy) at equal concentration. Moreover they reported the “acupuncture reflexological effects” as a possible confounding factor but the study failed to differentiate between the effects of mesotherapy and reflexotherapy. The injection of the dry-needle could simulate the acupuncture effect that improves function and provides pain relief in patients with OA.34,35
Another confounding factor is the mechanical distension of tissue after the injection of the drug, that could activates the cutaneous and subcutaneous receptors able to mediate the production of endorphins, molecules with a powerful analgesic effect.3
Saggini et al17 treated bursitis of the pes anserinus with intradermal therapy in patients with grade II Kellgren-Lawrence knee osteoarthritis, noting a significant reduction in pain and improved articular function. In this research, the mesotherapy group was treated with sodium diclofenac at 25 mg/mL (1 mL) 3 times/week for 3 weeks, whereas the control group was treated with diclofenac 50 mg/day for 3 weeks. The primary outcome measures were pain intensity, as assessed by VAS,15 and the ability to perform activities of daily living, based on the KOOS scale.29
The evaluation scales were administered before mesotherapy treatment and at the follow-up times 30, 60 and 90 days after the last treatment.
The authors concluded that mesotherapy is effective and safe, yielding the same results as conventional therapy but with minor adverse effects. The efficacy of mesotherapy has been demonstrated by echographic evaluation: higher drug concentrations in the subcutaneous tissue have local effects near inflammatory cells, sensory fibers, and vascular mediators that orchestrate inflammation and pain.1,36
The 2 studies16,17 that assessed the effectiveness of mesotherapy for knee pain could not be compared because they used different protocols (eg, with regard to pharmacological dosage and times of administration). Also, 1 study concerned a specific condition in knee pain (pes anserine bursitis).17
The drugs that are administered through mesotherapy have different pharmacokinetic (distribution and diffusion) onset, and duration before and after activity, based on the site of injection.3 Intradermal therapy with NSAIDs or other drugs increases their concentration in the targeted region (skin, tendon and muscle) compared with intramuscular administration. This effect has been confirmed by the immunogenic effect of tetanus toxoid after intradermal or intramuscular administration.37 NSAIDs act through COX inhibition and the consequent reduction in the levels of prostaglandin and other inflammatory mediators.38 In addition, nitric oxide levels are higher, enhancing the peripheral antinociception effect.39 The sodium channel blocker, lidocaine, is often used in association with NSAIDs, and its pain-relieving effects appear to be related to the generation of ectopic activity in afferent neurons without conduction block an effect that decreases ongoing and evoked neuropathic pain.40,41 By local application, the depressive effects of lidocaine are greater in A-fibers than in C-fibers in muscle cutaneous afferents.
Clinical practice guidelines for the management of osteoarthritis recommend pharmacological and no pharmacological approaches in knee OA. Oral drugs provide the best benefits in improving pain but induce adverse effects, such as renal dysfunction and gastrointestinal toxicity, especially in patients with such co-morbidities as cardiovascular disease.42
Low back pain
Two studies20,21 analyzed the efficacy of mesotherapy in low back pain (LBP). Cui et al21 performed intracutaneous sterile water injection (ISWI) to relieve acute LBP: experimental group patients received the intracutaneous sterile water injection in the lumbosacral region whereas control group received the intracutaneous of isotonic saline. The primary outcome was the reduction in pain intensity using the VAS15 at 10, 45, and 90 min and 1 day after the injections. However, no other specific functional scales were considered as secondary outcomes, except for the global rating of change and satisfaction. The mean VAS score was significantly lower in patients who received ISWI at 45 and 90 min and 1 day after treatment, compared to control group.
Koyucu et al20 were given ISWIs to women with LBP during pregnancy. The study included a placebo-treated patient group that received dry injections. The pain scores were assessed at 10, 30, 60, 90, 120, and 180 mins after injection. VAS scores at 30 min after injection, the primary outcome of the study, were significantly lower in the study versus control group. Secondary outcomes where the need for epidural analgesia, mode of delivery and Apgar scores, a satisfaction questionnaire on the pain relief method, and the IBFAT43 score for infant breastfeeding. The need for epidural analgesia, time of delivery, mode of delivery, Apgar scores, and breastfeeding scores were similar in both groups.
The authors concluded that sterile water injection is a simple, cost-effective, and promising method for treating LBP during labor. ISWI does not interfere with labor and can decrease the need for epidural analgesia. Possible limitations of this study were the lack of the dose of sterile water that was used for each injection and the absence of long-term follow-up.
Other studies have demonstrated that ISWI provides statistically and clinically significant pain relief for neck and shoulder pain in whiplash syndrome patients, cervicogenic headache, acute attacks of urolithiasis and chronic myofascial pain syndrome, and women who experience continuous lower back pain during labor.44–46
Sterile water injection is safe to administer (even to women in labor), does not interact with other drugs, is inexpensive, and controls acute pain.
Chronic spinal pain
Chronic spinal pain (CSP) includes chronic low back pain, failed back surgery, chronic whiplash-associated disorders, and chronic non-traumatic neck pain.47 Ferrara et al22 performed a retrospective study to compare the effects of mesotherapy, using a drug mixture, versus normal saline solution in CSP. Patients received 1 mesotherapy session/week for 5 weeks using a drug cocktail that was composed of normal saline solution (1 ml), lidocaine hydrochloride 2% (0.5 ml), and lysine acetylsalicylate (0.5 ml) or only normal saline solution. However, the authors did not describe the site of injection, failing to differentiate between the neck and back regions. Patients were assessed at baseline (T0), at the end of the 5-week treatment (T1), and at 4 weeks (T2) and 12 weeks (T3) using the VAS, the short-form McGill Pain Questionnaire, and Present Pain Intensity. Both groups experienced a significant improvement in all outcome measures after mesotherapy at T1. At T3, only VAS showed a significant difference, improving more in the study group. The authors concluded that normal saline solution in mesotherapy alleviated the intensity of CSP equal to drug cocktail respect a short follow up but respect long term outcome improvement the drug cocktail showed a better result.
Only 2 studies used a drug cocktail,16,22 although the use of a mixture of drugs has not received full consensus among experts1 due to the increased risk of pharmacological interactions, even if two active drugs, specifically lidocaine and NSAIDs have been reported to be safe. Moreover, with drug mixtures, it is not possible to identify the effects of individual drugs with regard to efficacy and tolerability. Saggini et al17 examined the combination of NSAIDs and anesthetics, and Yang et al19 combined a steroid and anesthetic. The use of a single drug appears to reduce the risk of drug-drug interactions and local side effects.1
Neck pain
Mesotherapy is also applied in cervical pain under acute and chronic conditions. Neck pain is a major public health problem with a high prevalence in the general population; its lifetime prevalence varies widely, from 14% to 71%.48
Neck pain can be classified in several ways—for example, acute versus chronic and neuropathic versus non-neuropathic because this distinction affects the diagnostic assessment and treatment at all levels of care. A study of the cervical region found that 43% of 100 patients had non-neuropathic pain, 7% had predominantly neuropathic pain, and 50% had mixed pain.49 In patients with chronic pain due to musculoskeletal conditions, pain is triggered by inflammation and dysfunction of the nerve pathways (neuropathic pain).
Paolucci et al18 described the efficacy of mesotherapy with lidocaine versus dry mesotherapy on trigger points in patients with chronic neck pain and showed a reduction of neck pain at the end of the therapy sessions respect baseline. In the treatment group, the average reduction in pain, by VAS, was 2.26 points, compared with 0.79 points in the control group and also for NDI in the treatment group the results showed an improvement in pain equal to 24.64 points instead in the control group was only 7.50. Moreover, also the quality of life improved significantly in the treated group compared to the control group for SF-12 scale30 (p=0.000 at T1and at T-follow-up). Certain evidence suggests that trigger point injections with a variety of fluids including water, saline, local anesthetics, vitamin B solutions, long-acting corticosteroids, acetylsalicylate, ketorolac, and botulinum toxin are better tolerated and more effective than dry needling.18,50,51
Yang et al19 demonstrated that intracutaneous injection (MLB) of local anesthetics and steroid into the affected paravertebral area is sufficient to relieve acute nonspecific neck pain and that the analgesic effect is more potent than that of oral ibuprofen (IBP). VAS scores at various time points (3 h, 1 day, and 3 days) declined from 7 to 4 in the IBP group and 6 to 3 in the MLB group. To evaluate disability the NDI was measured,27 indicating improved function in the IBP group from 38 to 24 and in the MLB group from 36 to 13. Moreover, to measure the patient’s belief of the efficacy of treatment, the self-reported Patient Global Impression of Change (PGIC) scale was administered,31 which was lower in MLB compared with IPB patients.
Conclusion
The data respect this systematic review suggest that mesotherapy can be recommended in the rehabilitation treatment of musculoskeletal pain. In summary, mesotherapy showed good results in reducing pain and improving function in musculoskeletal pain disorders of the spine (neck pain and low nack pain): the best result is obtained in acute pain compared to chronic pain.
Yang et al19 and Cui et al21 performed a single mesotherapy session in acute musculoskeletal pain (neck and low back pain), with acceptable pain control and rapid recovery of function. Instead, in chronic musculoskeletal pain were dosed 3 or 5 mesotherapy sessions.3 Mesotherapy has found limited use but with good results even in knee pain: Saggini et al17 administered 9 sessions of mesotherapy (3 times per week) for pes anserine bursitis.
In conclusion, mesotherapy could be a good option in rehabilitation field for musculoskeletal pain with good advantages and few or no side effects. Future randomized controlled trials should be desirable for have more uniform treatment protocols.
Key Points
- Mesotherapy is a good option in rehabilitation that allows the physiotherapist to propose exercises without pain and can accelerate healing.
- The modality for inserting the needle and the dose of drug that is injected into the trigger point or painful area: In mesotherapy, the needles are 4-mm, 27 gauge or 13-mm, 30/31 gauge, positioned at 30–45 degrees with respect to the skin surface. Only 0.10–0.20 mL of drug is injected at each point, 2–3 cm apart. Two mesotherapy infiltration techniques are recognized: IDP (profound intradermal injection) and IDS (superficial intradermic injection), for which the injection is made at depths of 2–4 mm and 1–2 mm, respectively.
- Number of sessions: From 1 session for acute pain to a maximum of 9 sessions for chronic pain (Table 2).
- Drugs used: The most common medications in mesotherapy are NSAIDs and lidocaine/procaine with sterile water solution or isotonic saline solution. The use of corticosteroids is limited and is not suggested in mesotherapy. The drugs that are used in mesotherapy should have specific indications in the data sheet, because many are used off label.
 |
Table 2 Number of mesotherapy sessions |
Abbreviation list
WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; VAS, visual analog scale; IBFAT, Infant Breastfeeding Assessment Tool; NDI, Neck Disability Index; VRS, Verbal Rating Scale; SF-12, Short Form-12 Health Survey; PGIC, Patient Global Impression of Change; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; LBP, low back pain; CNP, chronic neck pain; ANP, acute neck pain; CSP, chronic spinal pain; ALBP, acute low back pain; N, number of samples; E, experimental; C, control; M, male; F, female; LBP*, low back pain in labor; RCT, randomized controlled trial; NCT, nonrandomized controlled trial; RNCT, retrospective nonrandomized controlled trial.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mammucari M, Gatti A, Maggiori S, Bartoletti CA, Sabato AF. Mesotherapy, definition, rationale and clinical role: a consensus report from the Italian Society of Mesotherapy. Eur Rev Med Pharmacol Sci. 2011;15:682–694.
2. Sarkar R, Garg VK, Mysore V. Position paper on mesotherapy. Indian J Dermatol Venereol Leprol. 2011;77(2):232–237. doi:10.4103/0378-6323.77479.)
3. Mammucari M, Gatti A, Maggiori S, Sabato AF. Role of mesotherapy in musculoskeletal pain: opinions from the Italian Society of Mesotherapy. Evid Based Complement Altern Med. 2012;2012:436959. doi:10.1155/2012
4. Mammucari M, Maggiori E, Lazzari M, Natoli S. Should the general practitioner consider mesotherapy (intradermal therapy) to manage localized pain? Pain Ther. 2016;5(1):123–126. doi:10.1007/s40122-016-0052-3
5. Tringali G, Navarra P. Optimal solubility of diclofenac β-cyclodextrin in combination with local anaesthetics for mesotherapy applications. Evid Based Complement Alternat Med. 2017;2017:8321325. doi:10.1155/2017/8321325
6. Crenna P, Mancia P. Reflex actions in mesotherapy. J Mesother. 1981;1:29–40.
7. Costantino C, Marangio E, Coruzzi G. Mesotherapy versus systemic therapy in the treatment of acute low back pain: a randomized trial. Evid Based Complement Alternat Med. 2011;317183. doi:10.1155/2011/317183
8. Di Cesare A, Giombini A, Di Cesare M, Ripani M, Vulpiani MC, Saraceni VM. Comparison between the effects of trigger point mesotherapy versus acupuncture points mesotherapy in the treatment of chronic low back pain: a short term randomized controlled trial. Complement Ther Med. 2011;19(1):19–26. doi:10.1016/j.ctim.2010.11.002)
9. Viscito R, Ferrara PE, Ljoka C, et al. Mesotherapy as a treatment of pain and disability in patients affected by neck pain in spondylartrosis. Ig Sanita Pubbl. 2018;74(1):95–101.
10. Freynhagen R, Baron R, Tölle T, Stemmler E, Gockel U, Stevens M. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT). Curr Med Res Opin. 2006;22(3):529–537. doi:10.1185/030079906X89874
11. Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical nsaids for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004;5:28. doi:10.1186/1471-2474-5-27
12. Derry S, Wiffen PJ, Kalso EA, et al. Topical analgesics for acute and chronic pain in adults an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;12(5):CD008609. doi:10.1002/14651858.CD008609.pub2 Review.
13. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.137/journal.pmed.1000097
14. van Loveren C, Aartman IH. The PICO (Patient-Intervention-Comparison-Outcome) question. Ned Tijdschr Tandheelkd. 2007;114(4):172–178.
15. Polly BE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emergency Med. 2001;8(12):1153–1157.
16. Chen L, Li D, Zhong J, Qiu B, Wu X. Therapeutic effectiveness and safety of mesotherapy in patients with osteoarthritis of the knee. Evidence-Based Compl Altern Med. 2018;4:6513049.
17. Saggini R, Di Stefano A, Dodaj I, Scarcello L, Bellomo RG. Pes anserine bursitis in symptomatic osteoarthritis patients: a mesotherapy treatment study. J Altern Compl Med. 2015;21(8):480–484. doi:10.1089/acm.2015.0007
18. Paolucci T, Piccinini G, Trifan PD, Zangrando F, Saraceni VM. Efficacy of trigger points mesotherapy for the treatment of chronic neck pain: a short term retrospective study. Int J Phys Ther Rehab. 2016;2:113. doi:10.15344/2455-7498/2016/113
19. Yang XN, Geng ZS, Zhang XL, et al. Single intracutaneous injection of local anesthetics and steroids alleviates acute nonspecific neck pain: A CONSORT-perspective, randomized, controlled clinical trial. Medicine (Baltimore). 2018;97(28):e11285. doi:10.1097/MD.0000000000011285
20. Genç Koyucu R, Demirci N, Ender Yumru A, et al. Effects of intradermal sterile water injections in women with low back pain in labor: a randomized, controlled, clinical trial. Balkan Med J. 2018;35:148–154. doi:10.4274/balkanmedj.2016.0879
21. Cui JZ, Geng ZS, Zhang YH, Feng JY, Zhu P, Zhang XB. Effects of intracutaneous injections of sterile water in patients with acute low back pain: a randomized, controlled, clinical trial. Braz J Med Biol Res. 2016;49(3). doi:10.1590/1414-431X20155092
22. Ferrara PE, Ronconi G, Viscito R, et al. Efficacy of mesotherapy using drugs versus normal saline solution in chronic spinal pain: a retrospective study. Int J Rehabil Res. 2017;40(2):171–174. doi:10.1097/MRR.0000000000000214
23. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133.
24. Ornetti P, Dougados M, Paternotte S, Logeart I, Gossec L. Validation of a numerical rating scale to assess functional impairment in hip and knee osteoarthritis: comparison with the WOMAC function scale. Ann Rheum Dis. 2011;70(5):740–746. doi:10.1136/ard.2010.135483
25. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi:10.1016/j.jpainsymman.2010.08.016
26. Melzack R. The short-form Mcgill pain questionnaire. Pain. 1987;30:191–197.
27. Saltychev M, Mattie R, McCormick Z, Laimi K. Psychometric properties of the neck disability index amongst patients with chronic neck pain using item response theory. Disabil Rehabil. 2018;40(189):2116–2121. doi:10.1080/09638288.2017.1325945
28. Bailliea L, Baconb CJ, Hewitta CM, Moran RW. Predictors of functional improvement in people with chronic low back pain following a graded Pilates-based exercise programme. J Bodyw Mov Ther. 2019;23(1):211–218. doi:10.1016/j.jbmt.2018.06.007
29. Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24(8):1317–1329. doi:10.1016/j.joca.2016.03.010
30. Ware J
31. Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10(4):S73. doi:10.1016/j.jpain.2009.03.011
32. Katz JD, Shah T. Persistent pain in the older adult: what should we do now in light of the 2009 american geriatrics society clinical practice guideline? Pol Arch Med Wewn. 2009;119(12):795–800.
33. Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–132. doi:10.1016/j.bpg.2009.11.005
34. Lin X, Huang K, Zhu G, Huang Z, Qin A, Fan S. The effects of acupuncture on chronic knee pain due to osteoarthritis: A meta-analysis. J Bone Joint Surg- Am Vol. 2016;98(18):1578–1585. doi:10.2106/JBJS.15.00620
35. Vas J, Perea-Milla E, Mendez C. Acupuncture and moxibustion as an adjunctive treatment for osteoarthritis of the knee a large case series. Acupunct Med. 2004;22(1):23–28. doi:10.1136/aim.22.1.23
36. Xiao WH, Bennett GJ. C-fiber spontaneous discharge evoked by chronic inflammation is suppressed by a long-term infusion of lidocaine yielding nanogram per milliliter plasma levels. Pain. 2008;137(1):218–228. doi:10.1016/j.pain.2008.02.018
37. Pitzurra M, Marconi P. Immunogenesis and mesother- apy: the immunoresponse to antigens inoculated intradermally. Giornale Di Mesoterapia. 1981;1:9–14.
38. Gøtzsche P. Non-steroidal anti-inflammatory drugs. Clin Evid. 2002;8:1203–1211.
39. Romero TRL, Resende LC, Duarte IDG. The neuronal NO synthase participation in the peripheral antinociception mechanism induced by several analgesic drugs. Nitric Oxide. 2011;25(4):431–435. doi:10.1016/j.niox.2011.08.002
40. Bach FW, Jensen TS, Kastrup J, Stigsby B, Dejgard A. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1990;40:29–34.
41. Boas RA, Covino BG, Shahnarian A. Analgesic responses to i.v. lignocaine. Br J Anaesth. 1982;54:501–505. doi:10.1093/bja/54.5.501
42. Hochberg MC, Altman RD, April KT, et al. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–474. doi:10.1002/acr.21596
43. Altuntas N, Turkyilmaz C, Yildiz H, et al. Validity and reliability of the infant breastfeeding assessment tool, the mother baby assessment tool, and the LATCH scoring system. Breastfeed Med. 2014;9(4):191–195. doi:10.1089/bfm.2014.0018
44. Lee N, Coxeter P, Beckmann M, et al. A randomised non-inferiority controlled trial of a single versus a four intradermal sterile water injection technique for relief of continuous lower back pain during labour. BMC Pregnancy Childbirth. 2011;11:21. doi:10.1186/1471-2393-11-21
45. Derry S, Straube S, Moore RA, Hancock H, Collins SL. Intracutaneous or subcutaneous sterile water injection compared with blinded controls for pain management in labour. Cochrane Database Syst Rev. 2012;1:CD009107. doi:10.1002/14651858.CD009107
46. Byrn C, Lindh M, Hösterey U, Fogelberg M, Linder LE, Bunketorp O. Subcutaneous sterile water injections for chronic neck and shoulder pain following whiplash injuries. Lancet. 1993;341:449–452. doi:10.1016/0140-6736(93)90204-T
47. Malflieta A, Krege J, Meeus M, et al. Applying contemporary neuroscience in exercise interventions for chronic spinal pain: treatment protocol. Braz J Phys Ther. 2017;21(5):378–387. doi:10.1016/j.bjpt.2017.06.019
48. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834–848. doi:10.1007/s00586-004-0864-4
49. Liu R, Kurihara C, Tsai HT, Sylvestri PJ, Bennett MI, Cohen SP. Classification and treatment of chronic neck pain: a longitudinal cohort study. Reg Anesth Pain Med. 2017;42:52–61. doi:10.1097/AAP.0000000000000505
50. Scott NA, Guo B, Barton PM, Gerwin RD. Trigger point injections for chronic non-malignant musculoskeletal pain: a systematic review. Pain Med. 2009;10:54–69. doi:10.1111/j.1526-4637.2008.00526.x
51. Criscuolo CM. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5:407–411. doi:10.1007/s11916-001-0051-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
