Back to Journals » OncoTargets and Therapy » Volume 13
Mesencephalic Astrocyte-Derived Neurotrophic Factor, a Prognostic Factor of Cholangiocarcinoma, Affects Sorafenib Sensitivity of Cholangiocarcinoma Cells by Deteriorating ER Stress
Authors He J , Li G , Liu X , Ma L, Zhang J , Zheng S, Wang J, Liu J
Received 10 January 2020
Accepted for publication 11 August 2020
Published 16 September 2020 Volume 2020:13 Pages 9169—9184
DOI https://doi.org/10.2147/OTT.S245575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Jingyi He,1,2 Guangbing Li,1,2 Xihan Liu,3 Liye Ma,1,2 Jiayao Zhang,1,2 Shunzhen Zheng,1,2 Jianping Wang,1,2 Jun Liu1,2
1Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250021, China; 2Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong 250021, China; 3Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250021, China
Correspondence: Jun Liu
Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, 324, Jing 5 Road, Jinan 250021, Shandong, China
Tel +86 531 87925615
Email [email protected]
Purpose: Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignant tumor characterized by high malignancy and poor prognosis. Although the efficacy of sorafenib against cholangiocarcinoma cell lines has been demonstrated in vivo and in vitro, limited clinical data are available on the efficacy of sorafenib in patients with cholangiocarcinoma. Sorafenib can enhance endoplasmic reticulum (ER) stress-mediated apoptosis, and ER stress and unfolded protein response are also the mechanisms by which cancer cells resist drug therapy. Mesencephalic astrocyte-derived neurotrophic factor (MANF), initially identified as a neurotrophic factor, can be regulated by ER stress activation. There are no available studies on the diagnostic value and therapeutic significance of MANF in ICC. Hence, the purpose of this study was to evaluate the role of MANF in cholangiocarcinoma, investigating the possibility of whether sorafenib could become a reliable strategy for cholangiocarcinoma therapy.
Methods: In this study, the expression level of MANF in ICC patients was investigated by bioinformatic analysis and the results were verified by tissue microarray assay. Cholangiocarcinoma cell lines were also used to determine how MANF regulates the therapeutic effect of sorafenib and to identify the underlying mechanisms.
Results: The results showed that MANF was correlated with poor prognosis and MANF knockdown could facilitate sorafenib-mediated apoptosis and increase the sensitivity of sorafenib treatment by activating excessive ER stress.
Conclusion: MANF is a prognostic marker of cholangiocarcinoma. MANF knockdown increases sorafenib-mediated ER stress and apoptosis in the cholangiocarcinoma cell lines. This mechanism may lead to a new therapeutic strategy in cholangiocarcinoma.
Keywords: intrahepatic cholangiocarcinoma, MANF, sorafenib, prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is one of the most common human malignant digestive system tumors and the second most malignant type of primary liver cancer.1,2 ICC, originating from epithelial cells of the bile duct or hepatic ducts, is characterized by insidious symptoms, high malignancy, early metastasis and a poor curative effect.3,4 Unfortunately, no specific biomarkers exist for ICC.5 Despite improvements in recent therapeutic approaches such as surgery, interventional treatment, ablation therapy, and immune targeting, the prognosis of patients with ICC remains poor,2,6–8 as ICC is insensitive to radiotherapy and chemotherapy.9,10 Therefore, new diagnostic biomarkers and therapeutic strategies are urgently needed.
Sorafenib is a multi-kinase inhibitor, which has exhibited significant safety and efficacy in suppressing tumor proliferation and angiogenesis.11 Although the efficacy of sorafenib against cholangiocarcinoma cell lines has been demonstrated in vivo and in vitro, limited clinical data are available on the efficacy of sorafenib in patients with cholangiocarcinoma.12–15 Sorafenib can activate endoplasmic reticulum (ER) stress-mediated apoptosis in vitro,16,17 and cancer cells can acquire survival advantages and resist drugs by alleviating ER stress.18
Overwhelming cellular demand and a shortage of cellular energy availability lead to the accumulation of wrongly folded proteins which causes ER stress and activates unfolded protein response (UPR) pathways.19 Dysfunction of ER stress and UPR signals underlie the resistance of cancer cells to chemotherapy.20 Studies have shown that ER stress inducers can cause upregulation of MANF in vivo and in vitro.21,22 Mesencephalic astrocyte-derived neurotrophic factor (MANF), initially identified as a survival-promoting factor in the conditioned medium of astrocytes, can also serve as a regulator of ER stress.23,24 Secreted from cells as a canonical neurotrophic factor, MANF is localized intracellularly in the lumen of the ER and can also be secreted into the extracellular space.22,25,26 MANF can reduce ER stress-induced cell death, and studies have shown that ER stress-induced cell death was aggravated by silencing MANF.21,22 Studies showed that MANF could activate the AMPK/mTOR signaling pathway and promote cell proliferation in vitro.27 Activation of the AMPK/mTOR signaling pathway plays a key role in tumorigenesis.28 According to the above-mentioned findings, MANF may be a new therapeutic target in tumors.
In the present study, the expression level of MANF in ICC patients was investigated by bioinformatic analysis and the results were verified by tissue microarray assay (TMA). A cholangiocarcinoma cell line was also used to determine how MANF regulates the therapeutic effect of sorafenib and to identify the underlying mechanisms. The results showed that MANF is a prognostic biomarker and MANF knockdown facilitated sorafenib-mediated apoptosis by activating ER stress. A poor prognosis in the MANF overexpression group may be due to reduced drug sensitivity during treatment. This mechanism may be a new therapeutic strategy for cholangiocarcinoma patients. In addition, MANF may be a potential diagnostic and prognostic marker of ICC.
Patients and Methods
Ethics Statement
This study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University (NO:2019–032), and conducted according to the principles stated in the Declaration of Helsinki. Written informed consent was obtained from all patients according to the guidelines of the Ethics Committee. All the datasets were retrieved from the published literature, and all written informed consent was obtained.
Patients and Specimens
246 ICC patients who underwent hepatectomy from January 2011 to December 2014 were enrolled in the study. None of the patients had received chemotherapy or radiotherapy prior to surgery. ICC samples and paired paratumor samples were collected from the patients during surgery. Clinical staging of the tumor was based on the 8th edition Staging System of the American Joint Committee on Cancer.
Immunohistochemical Detection of Tissue Microarray (TMA)
Of 246 ICC patients, 202 had integrated follow-up information for further analysis. For immunohistochemistry, 5-μm sections were prepared from each tissue block. The tissue sections were deparaffinized, rehydrated and rinsed in distilled water. After heating in 10 mmol/L citrate buffer for antigen retrieval, the sections were incubated with primary antibody against ARMET (ab67271, Abcam, CA, USA; dilution 1:100, ARMET is the alternative name for MANF) at 4°C overnight and then secondary antibody for 1 h at room temperature. Stained tissue microarrays are scanned digitally by the tissue microarrays scanner Pannoramic MIDI (3D HISTECH, Hungary) and checked by Pannoramic viewer v. 1.14 (3D HISTECH, Hungary). After setting the diameter and the number of rows, whole slide images are automatically analyzed by the analysis software Quant center (3D HISTECH, Hungary). Quant center automatically recognizes and sets all dark browns on tissue sections as strongly positive, brown-yellow as moderately positive, light yellow as weakly positive, and blue nuclei as negative. According to the brown depth in the images, Quant center automatically classify the target specimen into strong positive, medium positive and weak positive and identify the positive area percentage of each specimen. To assess the average degree of staining within a sample, multiple regions were analyzed. An H-score (minimum 0 and maximum 300) was calculated using the following formula: H-score = (percentage of cells with weak intensity × 1) + (percentage of cells with moderate intensity × 2) + (percentage of cells with strong intensity × 3). “PI” represents the percentage of positive cells in each section and “I” represents the intensity of staining. The scoring was independently assessed by two pathologists who were unaware of the clinical outcomes.
GEO Data Source
Meta-analysis of 4 sets of microarrays from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) including 128 ICC samples and 109 nontumor samples was carried out to evaluate the diagnostic power of MANF. These 4 cohorts included the GSE31370, GSE32958, GSE76311 and GSE81948 datasets. Their characteristics such as cohort ID, RNA-seq platform, sample size (nontumor and tumor samples), publication year, and country are summarized in Table S1.
Cell Culture
The cholangiocarcinoma cell lineS HUCCT1 and QBC939 were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in RPMI-1640 (HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum at 37°C in an incubator with 5% CO2.
Plasmid and Transfections
Small interfering RNA (siRNA) to knockdown MANF and the negative control (NC) were purchased from GenePharma (Shanghai, China). Transient transfections were carried out using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s protocol. Forty-eight hours after transfection, protein expression, cell viability and apoptosis were measured. FLAG-MANF and FLAG-NC plasmids were purchased from GenePharma (Shanghai, China). Plasmids were carried out using EndoFection (GeneCopoeia, USA) following the manufacturer’s protocol. To establish stable transfections, a lentiviral transfer plasmid was purchased from GenePharma (Shanghai, China). The stably transfected cells were incubated in medium containing puromycin, and the puromycin-resistant colonies were observed approximately 2 days after transfection.
Cell Proliferation Assay
Cells were plated in 96-well plates at a concentration of 2500 cells/well and transfected with siRNAs, plasmids or lentiviral. A 10-μL aliquot of cell counting kit-8 (CCK8) reagent (CK04-3000T, Dojindo, Japan) was added to each well, and the cells were incubated at 37°C for 2 h. Absorbance values at OD 450 nm were measured using a microculture plate reader (Bio Tek).
Invasion Assay
For the migration assay, Boyden chambers (pore size, 8 μm) (BD Biosciences) were used. The cells (5×104 cells in 0.2 mL of medium) were placed in the upper chamber, and 0.70 mL of DMEM containing 15% FBS was placed in the lower chamber. After 24-h incubation, cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were fixed in methanol for 10 min and stained with 0.05% crystal violet. Cells underneath the filters were observed and counted under a microscope. For the invasion assay, the same conditions were applied as described for the migration assay except that the invasion chambers were coated with the biocoat Matrigel.
Apoptosis Analysis
Cell apoptosis was detected using the Annexin V-APC/7-AAD apoptosis detection kit (BioGems International, CA, USA) according to the manufacturer’s instructions. The cells were harvested by EDTA-free trypsin. After washing twice with PBS, the cells were resuspended in binding buffer at a concentration of 0.1–1 × 107 cells/mL. 100 μL cell suspension (0.1–1 × 106 cells) was transferred and mixed with 5 μL Annexin V-APC and 10 μL 7-AAD. After incubation in the dark for 15 min at room temperature and the addition of 150 μL binding buffer, the stained cells were analyzed by BD FACSCanto II flow cytometry (Beckman Coulter, CA, USA). Apoptotic cells were calculated as the sum of early and late apoptotic cells. Each sample was analyzed in triplicate.
Western Blotting
Liquid nitrogen frozen liver tissues were immersed in RIPA-added phenylmethylsulfonyl fluoride (100: 1) (Beyotime, China) supplemented with protease and phosphatase inhibitors, and sonicated on ice to obtain a homogenate. Specimens were centrifuged at 15,000 x g for 15 min and the supernatant was used for Western blotting. Protein concentration was assessed by the BCA protein assay kit (Beyotime, China). Proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes. After incubation with horseradish-peroxidase-conjugated secondary antibodies for 2 h at room temperature, signals were detected by chemiluminescent reagents (Millipore, USA) and β-actin served as an internal control. The primary antibodies used in this study were obtained from Cell Signaling Technology (Beverly, MA, USA): BiP (3177, 1:1000), IRE1α (3294, 1:1000), CHOP (2895, 1:1000), PERK (5683, 1:1000), Bcl-2 (4223, 1:1000), Bax (5023, 1:1000), Anti-rabbit IgG (7074, 1:5000), Anti-mouse IgG (7076, 1:5000); and Abcam (Cambridge, MA, USA): ARMET (ab67271, 1:1000), XBP1 (ab37152, 1:1000).
Mouse Xenograft Models
The animal experiments were approved by the Animal Research Committee of Shandong Provincial Hospital Affiliated to Shandong University (NO:2019–032), and all procedures strictly followed the NIH Guide for Care and Use of Laboratory Animals. Five-six week old female nude mice were purchased from the Beijing Vital River Laboratory Animal Technology Co. (Beijing, China). Approximately 5.0×106 HUCCT1/sh-MANF or HUCCT1/Vector cells were suspended in 100 μL PBS and subcutaneously injected into the right side of the posterior flank. After 4 weeks, sorafenib (20 mg/kg) was administered via intraperitoneal injection every other day. All mice were killed after 2 weeks.
Statistics for Meta-Analysis
Stata 12.0 was used to analyze the pooled diagnostic value of MANF with the data from the GEO datasets. I2 was used to evaluate the heterogeneity of these studies, and significant heterogeneity was indicated when I2>50%. The random effect model and subgroup analysis were performed to assess the sources of heterogeneity, when heterogeneity was quite conspicuous between the studies. Publication bias was determined by Begg’s funnel plot and Egger’s test.
Data Analysis and Statistics
Statistical analyses were performed using SPSS software (version 22.0; IBM Corporation, USA). The relationships between MANF expression and the clinicopathological parameters were examined using the Chi-squared test. Survival analysis was evaluated by the Kaplan–Meier method. The relationships between different variables and survival were determined by the multivariate Cox proportional hazards method. Pearson’s correlation was used to assess the linear association between two variables. P-values less than 0.05 were considered statistically significant. All the sample data are presented as mean ± standard deviation (SD). The differences between tumor and nontumor samples were determined by the non-parametric test. All experiments were performed in three independent trials with duplicates. Differences between statistical comparisons were determined by the Student’s t-test and one-way analysis of variance (ANOVA). In all cases, P<0.05 was considered statistically significant.
Results
MANF Overexpression is Correlated with Poor Prognosis in ICC
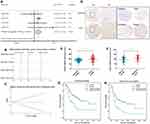
Four ICC microarrays from the GEO database were analyzed by meta-analysis to determine the MANF expression level in ICC tissues compared with normal controls. As shown in the forest plot (Figure 1A), expression of MANF was significantly increased in ICC samples (pooled OR=2.05, 95% CI=1.21–3.48, I2 = 0%, P=0.641). All data were generated by a random effect model. The Chi-squared test was used to analyze study heterogeneity. As shown in the sensitivity analysis, there were no significant differences between the microarrays (Figure 1B). There was no significant publication bias or heterogeneity by Begg’s test (Figure 1C). Thus, the meta-analysis showed that MANF was overexpressed in ICC tissues compared with paired nontumor tissues.
To confirm the results of the bioinformatic analysis, MANF expression in ICC specimens was assessed using IHC staining of ICC tissue microarrays. As shown in Figure 1, MANF was significantly upregulated in ICC tissues compared with adjacent nontumor controls (n=246) (P<0.01) (Figure 1D and E). Of 246 patients, and patients were grouped based on TNM stage. The patients were divided into early stage (TNM I–II) and late stage (TNM III–IV). MANF expression level in patients with late stage (n=87) was significantly higher than that in patients with early stage (n=159) (P<0.01) (Figure 1F).
The association between MANF and overall survival (OS) of ICC patients was then analyzed. Of the 246 ICC patients, 202 had complete follow-up data. According to MANF expression level, 101 patients were divided into the high expression group and 101 into the low expression group. The results showed that patients in the high expression group had a shorter OS (P<0.01; Figure 1G) and disease-free survival (DFS) (P<0.01; Figure 1H) compared with the low expression group.
The association between MANF and clinical characteristics was further analyzed using the Chi-squared test. MANF overexpression was associated with higher TNM stage (P<0.05), lymphatic metastasis (P<0.01), larger tumor size (P<0.05) and a high level of carcinoembryonic antigen (CEA) (P<0.05), carbohydrate antigen 19–9 (CA19-9) (P<0.05), and alpha-fetoprotein (AFP) (P<0.05) (Table 1). In terms of age, gender, hepatitis B virus (HBV) infection, cirrhosis, tumor number, differentiation grade, and venous invasion, there were no significant differences between the different MANF expression groups.
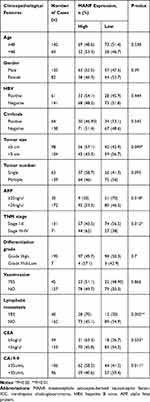 |
Table 1 The Relationship Between MANF Status and Clinicopathological Features of ICC |
Univariate Cox regression analyses of OS demonstrated that MANF expression in tumor tissues (P<0.01), CEA (P<0.01), CA19-9 (P<0.05), tumor size (P<0.01), tumor number (P<0.01), TNM stage (P<0.01) and lymphatic metastasis (P<0.01) were independent prognostic factors for ICC patients and multivariate Cox regression analyses of OS characterized by univariate analysis (P<0.05) demonstrated that MANF expression in tumor tissues (P<0.01), tumor size (P<0.05) and tumor number (P<0.05) were independent prognostic factors for ICC patients (Table 2). Univariate analysis and multivariate analysis of DFS obtained the same results (Table 3). The remaining clinical features were not found to be prognostic indicators, which could be due to an insufficient number of cases. Hence, in relation to tumor progression, MANF was overexpressed in ICC and MANF overexpression was correlated with poor prognosis of ICC patients.
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Variables for Overall Survival in ICC Patients |
 |
Table 3 Univariate and Multivariate Analyses of Prognostic Variables for Disease-Free Survival in ICC Patients |
MANF Knockdown Inhibits Cell Proliferation by Suppressing the Phosphorylation of mTOR
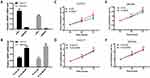
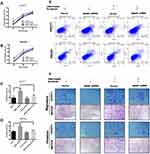
To determine if MANF knockdown can be used to regulate the biological behavior of cholangiocarcinoma cells, the cells were transfected with si-NC and si-MANF. Knockdown and overexpression efficiency of MANF were tested by RT-qPCR in HUCCT1 and QBC939, respectively (Figure 2A and B). Then, CCK8 and flow cytometry assays were performed to determine whether MANF knockdown affected the proliferation and apoptosis of HUCCT1 and QBC939 cells. CCK8 analysis showed that cholangiocarcinoma cells transfected with siRNA-581 had a lower rate of proliferation than NC-treated cells (P < 0.05; Figure 2C and D). However, no significant difference in apoptosis levels was noted following flow cytometry assays (P > 0.05; Figure 3A and B). Western blotting revealed that downregulation of MANF made no difference to the expression of CHOP, PERK, IRE1α and GRP78, but reduced the expression levels of p-mTOR (P < 0.05; Figure 3E and F). These results suggested that MANF knockdown inhibits cell proliferation by suppressing the phosphorylation of mTOR.
Overexpression of MANF Has No Effect on the Biological Behavior of Cholangiocarcinoma Cells
The above data suggested that downregulation of MANF inhibited the proliferation of HUCCT1 and QBC939 cells. To further confirm the effect of MANF on the biological behavior of cholangiocarcinoma cells, MANF was transiently overexpressed in HUCCT1 and QBC939 cells via transfection with the FLAG-MANF plasmid. No statistically significant results following CCK8 (P > 0.05; Figure 2E and F) and flow cytometry assays were observed (P > 0.05; Figure 3C and D). Western blotting revealed no significant differences between CHOP, PERK, IRE1α, GRP78 and p-mTOR/mTOR (P > 0.05; Figure 3E) in HUCCT1. These results suggested that overexpression of MANF had no significant effect on the biological behavior of cholangiocarcinoma cells.
MANF Knockdown Increases Sorafenib Sensitivity of ICC Cells
The above clinical data suggested that MANF overexpression in cholangiocarcinoma was an adverse prognostic factor, but the current results showed limited support for this. It has been reported that ER stress is involved in the sensitivity of drug therapy.29,30 The CCK8, Transwell and flow cytometry assays were then carried. HUCCT1 and QBC939 cells with stable MANF or NC silencing were successfully established, and treated with sorafenib for 24, 48, and 72 h. The CCK8 assay results demonstrated that cholangiocarcinoma cells transfected with MANF shRNA had a lower rate of proliferation than cells transfected with vector (P<0.01; Figure 4A and B), when treated with 10 μM sorafenib. Flow cytometry assays consistently demonstrated that MANF silencing significantly increased HUCCT1 and QBC939 cell apoptosis at the sorafenib concentration of 10 μm (P<0.01; Figure 4C and E). Cell migration and invasion ability were found to be significantly decreased by MANF knockdown (Figure 4F). The above results showed that MANF knockdown induced lower cell viability and increased apoptosis in cholangiocarcinoma cells after sorafenib treatment.
MANF Knockdown Increases Sorafenib-Mediated ER Stress and Apoptosis
To elucidate the mechanism by which downregulation of MANF promoted sorafenib sensitivity in ICC, Western blot analysis was performed. The results showed that cells with MANF knockdown treated with 10 μm sorafenib expressed more ER stress and apoptosis markers such as PERK, GRP78, PDI, IRE1α, XBP1, CHOP, cleaved-caspase-3 and Bax than NC knockdown cells, but decreased the expression of Bcl-2 (Figure 5A). These results suggested that cells with MANF knockdown had a more severe ER stress response and increased apoptosis, when treated with sorafenib, compared with the control group.
MANF Knockdown Facilitates Sorafenib-Mediated Apoptosis by Activating GRP78 Signaling
GRP78 is known to play a key role in stimulating death signals and provoking apoptosis in hepatocellular carcinoma, when excessive and prolonged ER stress occurs.31 Similarly, VER155008 an inhibitor of GRP78 was used to confirm whether GRP78 is involved in sorafenib-mediated cell apoptosis and stimulation of death signals in cholangiocarcinoma. CCK8 and flow cytometry assays suggested that VER155008 reduced the cell inhibition and apoptosis rate of cholangiocarcinoma cells, when used together with sorafenib, compared to treatment with sorafenib alone (P<0.05; Figure 4A and B). These findings demonstrated that VER155008 significantly decreased the effect of sorafenib compared with sorafenib alone (P<0.05; Figure 4B and C). Cell migration and invasion ability were increased by VER155008 (Figure 4F). Western blot assays indicated that cells treated with 15 μg/mL VER155008 showed significant downregulated expression of CHOP, BAX and cleaved-caspase-3, and upregulated Bcl-2 expression (Figure 5B). These results demonstrated that sorafenib-mediated apoptosis was involved in excessive activation of GRP78.
MANF Knockdown Potentiates the Sensitivity to Sorafenib in Cholangiocarcinoma Cells in vivo
The above data suggested that MANF potentiates sensitivity to sorafenib in HUCCT1 cells. To confirm this, we investigated the effect of MANF downregulation using ICC xenografts in nude mice. HUCCT1 cells with stable MANF silencing were subcutaneously inoculated into the right iliac fossa of 6-week-old female athymic nude mice for the indicated number of days. After 4 weeks, all mice were given sorafenib (10 mg/kg b.w.). As shown in Figure 6, tumors derived from mice injected with MANF knockdown cells were more sensitive to sorafenib therapy than tumors derived from mice injected with the NC (P<0.05; Figure 6A–C). Western blotting of xenograft tumor tissues showed that MANF protein levels were successfully decreased (P<0.05; Figure 6D). These results suggested that MANF knockdown enhanced the therapeutic efficacy of sorafenib in vivo.
Discussion
ICC is the second most common histologic type of primary malignant liver tumors.32 ICC accounts for approximately 3% of all primary hepatobiliary malignancies and 5‑10% of all cases of cholangiocarcinoma.33 The prognosis of ICC is poor. More than 70% of patients with ICC are unresectable or have advanced disease requiring systemic therapies such as chemotherapy at the time of diagnosis.34,35 However, ICC is not sensitive to radiotherapy and chemotherapy.9,10 Therefore, new therapeutic targets and strategies are urgently needed to prolong patients’ survival.
MANF, a secreted protein localized in the ER, is widely expressed in mammalian tissues, and can be upregulated by ER stress activation.23,36 Elina et al demonstrated that the protective effect of MANF was associated with inhibition of the NF-κB signaling pathway and alleviation of ER stress. MANF also has a direct link with human beta cell proliferation.37 These molecules are key targets in malignant tumor treatment. Thus, MANF may be involved in the process of cancer initiation and progression. In the current study, we determined MANF expression and evaluated the prognostic role of MANF in ICC. The results suggested that MANF was always overexpressed in ICC, indicating the significance of MANF in tumorigenesis. Furthermore, high expression of MANF was associated with higher TNM stage, larger tumor size and excess tumor markers indicating a poor prognosis. In addition, we found that OS and DFS in patients with overexpression of MANF were significantly reduced, when the follow-up and prognostic data of ICC patients were analyzed. Multivariate Cox regression analyses showed that overexpression of MANF was one of the significant risk factors predicting OS and DFS, and MANF could be utilized as a prognostic marker to predict ICC and its prognosis.
When MANF was artificially upregulated or downregulated in vivo to investigate the biological behavior of cholangiocarcinoma cells, the results suggested that MANF knockdown inhibited cell proliferation by suppressing the phosphorylation of mTOR. The mTOR signaling pathway is frequently activated in several human cancers, regulating a variety of cellular activities, such as proliferation, differentiation, growth, metabolism, angiogenesis and metastasis.38 Moreover, MANF also plays a protective role against ER stress-induced cell death, and MANF overexpression can protect neuronal cells against ER stress-induced cell death. MANF overexpression activates the PI3K/Akt/mTOR pathway and downregulates CHOP, and caspase-3.22,39 Conversely, MANF knockdown suppressed ICC cell growth, reduced the phosphorylation of mTOR, but had no effect on the expression of CHOP, GRP78, PERK, and IRE1α. However, no significant differences in these parameters were found in the MANF overexpression group.
The above clinical data suggested that MANF overexpression in cholangiocarcinoma is an adverse prognostic factor, but the current results provide limited support for this. It has been reported that MANF is regulated by ER stress which is involved in the sensitivity of drug therapy.29,30,40 Sorafenib, a multi-kinase inhibitor, has been shown to have antitumor activity in human ICC cell lines in vitro and in vivo.11 However, there is limited evidence to support the therapeutic effectiveness of sorafenib treatment for ICC patients in clinical practice,13–15 probably because the therapeutic sensitivity of sorafenib is unsatisfactory. ER stress plays a key role in tumor pathogenesis, and recent studies have shown that ER stress promotes the process of tumor progression and resistance to chemoradiotherapy.41,42 When cancer cells were treated with drugs such as cisplatin or sorafenib, ER stress-induced UPR signaling was seen as a compensatory mechanism, whereas severe and prolonged ER stress damaged the biological function of cells and mediated cell apoptosis.43 A recent study showed that MANF can regulate the expression of caspase-3 via regulation of CHOP.39 The expression of CHOP, a stress-inducible nuclear protein, is upregulated under ER stress and its expression is low under physiological conditions.44,45 Upregulation of CHOP leads to cell cycle arrest and apoptosis, and ER stress cannot be alleviated.46 Cancer cells can alleviate ER stress to acquire survival advantages to drive cancer occurrence, progression and drug resistance.18 High multiplication potentiality and drug resistance of tumor cells ultimately result in poor prognosis. Based on our findings, we confirm that MANF knockdown potentiated sensitivity to sorafenib, deteriorated sorafenib-mediated ER stress and induced apoptosis in ICC cells both in vivo and in vitro. These results may contribute to improving the value of sorafenib in the clinical treatment of cholangiocarcinoma.
GRP78 is a chief regulator of ER function, and PERK, IRE1, and ATF6 were activated while GRP78 was released from these complexes.47 A recent study showed that downregulation of GRP78 suppressed proliferation, induced apoptosis, and increased the sensitization to chemotherapy-induced apoptosis of cancer cells.48,49 However, under pathological conditions, excessive UPR activation and severe ER stress lead to abnormal protein secretion and apoptosis.50 One study suggested that upregulation of GRP78 and excessive stress stimulated by psoralen leads to increased apoptosis and expression of CHOP, the p-JNK/JNK ratio, and Bax/Bcl-2 ratio.31 In the present study, when MANF knockdown cells were treated with sorafenib, excessive ER stress-mediated apoptosis was alleviated by artificial inhibition of GRP78. ER stress was alleviated and the expression of XBP1, CHOP, cleaved caspase-3 and Bax/Bcl-2 was decreased.
Our study confirmed that MANF was upregulated in ICC tissues which indicated a poor prognosis. MANF overexpression might help tumor cells to survive during drug therapy by alleviating ER stress and enhancing ER adaptive capacity, leading to a poor prognosis. Moreover, low expression of MANF may increase sorafenib sensitivity in cholangiocarcinoma cell lines, which is beneficial in the clinical application of sorafenib and could improve the prognosis of patients with cholangiocarcinoma. MANF is a molecular marker in the treatment and prognosis of ICC. We provide a new perspective for further examination of the biological functions of MANF. However, more fundamental experiments are needed.
Conclusion
MANF is a molecular marker in the treatment and prognosis of ICC. MANF knockdown increases sorafenib-mediated ER stress and apoptosis in the cholangiocarcinoma cell lines.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81373172, 81770646 and 26020105131715).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
2. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289.
3. Sirica AE, Gores GJ, Groopman JD, et al. Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology. 2019;69(4):1803–1815.
4. Chun YS, Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729241.
5. Li Y, Huang Y, Chen J. Diagnostic value of serum biomarkers for intrahepatic cholangiocarcinoma. J Coll Physicians Surg Pak. 2019;29(10):962–966.
6. Zhang YM, Zhou ZT, Liu GM. Is recurrent pyogenic cholangitis an independent poor prognostic indicator for resectable intrahepatic cholangiocarcinoma? HPB (Oxford). 2019;21(1):132.
7. Wang X, Yan Y, Chen X, et al. The antitumor activities of marsdenia tenacissima. Front Oncol. 2018;8:473.
8. Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015;157(4):666–675.
9. Bisello S, Buwenge M, Palloni A, et al. Radiotherapy or chemoradiation in unresectable biliary cancer: a retrospective study. Anticancer Res. 2019;39(6):3095–3100.
10. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111.
11. Chen J, Jin R, Zhao J, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367(1):1–11.
12. Sugiyama H, Onuki K, Ishige K, et al. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol. 2011;46(6):779–789.
13. Poddubskaya EV, Baranova MP, Allina DO, et al. Personalized prescription of tyrosine kinase inhibitors in unresectable metastatic cholangiocarcinoma. Exp Hematol Oncol. 2018;7:21.
14. Luo X, Jia W, Huang Z, et al. Effectiveness and safety of sorafenib in the treatment of unresectable and advanced intrahepatic cholangiocarcinoma: a pilot study. Oncotarget. 2017;8(10):17246–17257.
15. Chakunta HR, Sunderkrishnan R, Kaplan MA, Mostofi R. Cholangiocarcinoma: treatment with sorafenib extended life expectancy to greater than four years. J Gastrointest Oncol. 2013;4(4):E30–2.
16. Holz MS, Janning A, Renné C, Gattenlöhner S, Spieker T, Bräuninger A. Induction of endoplasmic reticulum stress by sorafenib and activation of NF-kappaB by lestaurtinib as a novel resistance mechanism in Hodgkin lymphoma cell lines. Mol Cancer Ther. 2013;12(2):173–183.
17. Fan L, He Z, Head SA, et al. Clofoctol and sorafenib inhibit prostate cancer growth via synergistic induction of endoplasmic reticulum stress and UPR pathways. Cancer Manag Res. 2018;10:4817–4829.
18. Mujtaba T, Dou QP. Advances in the understanding of mechanisms and therapeutic use of bortezomib. Discov Med. 2011;12(67):471–480.
19. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–335.
20. Jing D, Zhou W, Shen L, et al. RIG-I promotes IFN/JAK2 expression and the endoplasmic reticulum stress response to inhibit chemoradiation resistance in nasopharyngeal carcinoma. Cancer Med. 2019;8(14):6344–6357.
21. Tadimalla A, Belmont PJ, Thuerauf DJ, et al. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008;103(11):1249–1258.
22. Apostolou A, Shen Y, Liang Y, Luo J, Fang S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res. 2008;314(13):2454–2467.
23. Matlik K, Yu LY, Eesmaa A, et al. Role of two sequence motifs of mesencephalic astrocyte-derived neurotrophic factor in its survival-promoting activity. Cell Death Dis. 2015;6:e2032.
24. Yu YQ, Liu LC, Wang FC, et al. Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab. 2010;30(1):79–91.
25. Tseng KY, Anttila JE, Khodosevich K, et al. MANF promotes differentiation and migration of neural progenitor cells with potential neural regenerative effects in stroke. Mol Ther. 2018;26(1):238–255.
26. Hartley CL, Edwards S, Mullan L, et al. Armet/Manf and Creld2 are components of a specialized ER stress response provoked by inappropriate formation of disulphide bonds: implications for genetic skeletal diseases. Hum Mol Genet. 2013;22(25):5262–5275.
27. Zhang J, Cai Q, Jiang M, et al. Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDA-induced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol. 2017;89:45–56.
28. Liu G, Kuang S, Cao R, Wang J, Peng Q, Sun C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019;33:fj201802619RR.
29. Zhou B, Lu Q, Liu J, et al. Melatonin increases the sensitivity of hepatocellular carcinoma to sorafenib through the PERK-ATF4-Beclin1 pathway. Int J Biol Sci. 2019;15(9):1905–1920.
30. Liu D, Fan Y, Li J, et al. Inhibition of cFLIP overcomes acquired resistance to sorafenib via reducing ER stressrelated autophagy in hepatocellular carcinoma. Oncol Rep. 2018;40(4):2206–2214.
31. Yu Y, Yu R, Men W, et al. Psoralen induces hepatic toxicity through PERK and ATF6 related ER stress pathways in HepG2 cells. Toxicol Mech Methods. 2019;30:1–9.
32. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
33. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599.
34. Puhalla H, et al. Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg. 2005;189(2):173–177.
35. Yedibela S, Demir R, Zhang W, Meyer T, Hohenberger W, Schönleben F.Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol. 2009;16(2):404–412.
36. Lindholm P, Peränen J, Andressoo JO, et al. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci. 2008;39(3):356–371.
37. Hakonen E, Chandra V, Fogarty CL, et al. MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia. 2018;61(10):2202–2214.
38. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156.
39. Xu S, Di Z, He Y, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Abeta toxicity via attenuating Abeta-induced endoplasmic reticulum stress. J Neuroinflammation. 2019;16(1):35.
40. Mizobuchi N, Hoseki J, Kubota H, et al. ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct. 2007;32(1):41–50.
41. Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6(1):55–69.
42. Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4(7):423–436.
43. Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4(11):e374.
44. Oida Y, Izuta H, Oyagi A, et al. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008;1208:217–224.
45. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389.
46. Park SJ, Kim Y, Yang SM, et al. Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc Natl Acad Sci U S A. 2019;116(28):14154–14163.
47. Gorman AM, Healy SJ, Jäger R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther. 2012;134(3):306–316.
48. Xing X, Li Y, Liu H, Wang L, Sun L. Glucose regulated protein 78 (GRP78) is overexpressed in colorectal carcinoma and regulates colorectal carcinoma cell growth and apoptosis. Acta Histochem. 2011;113(8):777–782.
49. Mhaidat NM, Alali FQ, Matalqah SM, et al. Inhibition of MEK sensitizes paclitaxel-induced apoptosis of human colorectal cancer cells by downregulation of GRP78. Anticancer Drugs. 2009;20(7):601–606.
50. Rao J, Zhang C, Wang P, et al. C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1alpha signalling in acute liver failure. Biochem J. 2015;466(2):369–378.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.