Back to Journals » OncoTargets and Therapy » Volume 8
Merkel cell carcinoma of the head and neck: pathogenesis, current and emerging treatment options
Received 28 April 2015
Accepted for publication 27 July 2015
Published 19 August 2015 Volume 2015:8 Pages 2157—2167
DOI https://doi.org/10.2147/OTT.S72202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Daniele Santini
Alok T Saini, Brett A Miles
Department of Otolaryngology – Head and Neck Surgery, Mount Sinai Hospital, New York, NY, USA
Abstract: Merkel cell carcinoma (MCC) is a relatively uncommon, neuroendocrine, cutaneous malignancy that often exhibits clinically aggressive features and is associated with a poor prognosis. It typically presents as a painless, rapidly enlarging, dome-shaped red or purplish nodule in a sun-exposed area of the head and neck or upper extremities. Our understanding of MCC has increased dramatically over the last several years and the pathogenesis continues to be an area of active research. The etiology is likely multifactorial with immunosuppression, UV-induced skin damage, and viral factors contributing to the development of MCC. The recent discovery of Merkel cell polyomavirus has allowed for at least one aspect of disease development to be much better understood. In most cases, treatment consists of wide local excision with adjuvant radiation therapy. The role of chemotherapeutics is still being defined. The recent advancement of knowledge regarding the pathogenesis of MCC has led to an explosion research into novel therapeutic agents and strategies. This review seeks to summarize the current body of literature regarding the pathogenesis of MCC and potential targets for future therapies.
Keywords: skin cancer, polyomavirus, neuroendocrine cancer, Merkel cells
Introduction
Friedrich Sigmund Merkel first described “Tastzellen”, or touch cells, in the skin in 1875.1 These cells would later become known as Merkel cells. Merkel cells are epithelial cells that form a complex with sensory neurons at the epidermal–dermal junction, but their role as sensory cells has been debated for years. Recent studies confirm that Merkel cells are mechanosensitive and required for appropriate afferent nerve fiber stimulation.2
Merkel cell carcinoma (MCC) is a relatively uncommon, neuroendocrine, cutaneous malignancy that often exhibits clinically aggressive features and is associated with a poor prognosis. Toker, in 1972, described trabecular carcinoma of the skin.3 Six years later, Tang and Toker suggested that trabecular carcinoma originated from Merkel cells, which ultimately gave rise to the term MCC.4 MCC typically presents as a painless, rapidly enlarging, dome-shaped red or purplish nodule in a sun-exposed area of the head and neck or upper extremities.5–7 The acronym AEIOU is used to summarize the classic clinical characteristics of MCC (Asymptomatic, Expanding rapidly, Immune suppression, Older than 50 years of age, UV exposure on fair skin).8 The clinical presentation is frequently mistaken for basal cell carcinoma, amelanotic melanoma, or other cutaneous malignancies. Risk factors include male sex, increased age, fair skin, previous malignancies, UV light exposure, and immunosuppression (specifically, HIV or organ transplantation).9–12 There is a tendency for early and frequent locoregional metastases and recurrence, and the majority of patients die from distant metastases.6 Non-sun-exposed MCC variants have been described, and they tend to be associated with even worse survival.13
The incidence of MCC is estimated to be approximately 0.3–0.6/100,000 in the US, and it appears to have been increasing over the last few decades.12,14–16 Data from the SEER database indicate that in 1986, the incidence was 0.15 cases per 100,000 in the US. However, in 2001, the incidence was noted to be 0.44 cases per 100,000.17 During this time period, the incidence was estimated to increase by 8% annually.18 It is unclear whether this trend is due to an increasingly aged population or increased awareness and diagnosis of the disease.14 It is noted that the introduction of CK-20 as a diagnostic tool for detecting MCC preceded the time period of increasing incidence.14,19 The incidence of MCC in Denmark from 1995 to 2006 appears to be slightly lower at 2.2 cases per million person years.20 Secondary to its rarity, it is difficult, if not impossible, to conduct controlled trials to define optimal treatment regimens for MCC, and prospective, randomized data guiding the management of MCC are lacking.
Pathogenesis
In 2008, Feng et al identified the Merkel cell polyomavirus (MCPyV).21 MCPyV is part of normal skin flora and is a nearly ubiquitous infection in adults.22 Infection likely occurs during childhood via close contact though the exact mode of transmission remains unknown.22,23 The infection appears to be asymptomatic24 and to persist throughout life as antibodies can be detected for decades after infection.25 The level of anti-MCPyV antibodies correlates with the overall viral load in the skin.26 While MCPyV is a common infection in healthy individuals, MCC remains relatively uncommon with an estimated three cases occurring per million people.14,27,28
The oncogenesis of MCC was historically poorly understood; however, more recent technology has allowed viral and molecular oncogenic mechanisms to be elucidated. Our understanding of MCC has increased dramatically over the last several years and continues to be an area of active research. The etiology is likely multifactorial with immunosuppression, UV-induced skin damage, and viral factors contributing to the development of MCC. However, the recent discovery of MCPyV has allowed for at least one aspect of disease development to be much better understood.
MCPyV belongs to the human polyomavirus (HPyV) family. HPyVs share a common morphology, and all are non-enveloped, double-stranded DNA viruses. Generally, the genome of HPyVs can be divided into three functional parts: noncoding control region, early gene region, and late gene region. The noncoding control region contains the transcription start sites and promoter elements. The early gene region encodes small T antigen (ST) and large T antigen (LT). The late gene region encodes capsid proteins VP-1, VP-2, and VP-3.29 Typically, HPyVs cause subclinical infection and only progress to extensive disease in those who are immunocompromised. Among the HPyVs, MCPyV is unique in its association with a cancer, MCC.29 Feng et al found that MCPyV was integrated into the human genome in specimens of MCC, and it is estimated that between 66% and 80% of MCC specimens are positive for MCPyV.21,30 Clonal integration was found in both primary and metastatic specimens, suggesting that integration occurs prior to dissemination supporting the theory that MCPyV is involved in the oncogenesis of MCC. MCPyV encodes LT and ST;21 both are independently required for tumor survival and proliferation.31 LT targets pocket proteins regulating the cell-cycle transit including pRB, p107, and p130. A critical downstream result of this is activation of survivin, an important mediator for cancer cell proliferation.32 Interestingly, MCPyV mutations resulting in premature truncation of LT are demonstrated as a consistent feature in MCPyV-derived MCC. The mutation prevents the inactivation of p53 tumor suppressor but maintains the interaction between LT, HSC-70, and pRB.33 ST activates cap-dependent translation regulator, 4E-BP1, and appears to be the major transforming oncogene.22 These oncogenic mechanisms continue to be investigated to further understanding of this deadly disease.
Currently, detection of active MCPyV infection is based primarily on polymerase chain reaction (PCR) amplification of viral DNA. However, a recent study demonstrated the use of fluorescence in situ hybridization (FISH) to determine the quality of viral presence within individual MCC cells. The study found similar rates of MCPyV positivity in MCC when comparing FISH with PCR. Using FISH, the authors detected two different intracellular patterns: punctate and diffuse. The punctate pattern correlated with an integrated MCPyV genome, while the diffuse pattern indicated an episomal presence of the genome. The detection of current or past exposure to MCPyV is based on the detection of anti-MCPyV antibodies by enzyme-linked immunosorbent assay.26 Anti-VP1 antibodies are detected in many MCC patients, and anti-LT may be particularly useful for identifying MCPyV within MCC and for detecting tumor recurrence.26,34
Despite the improvement in detection methods for MCPyV infection, the effect of MCPyV positivity on prognosis in MCC has yet to be determined.26 MCPyV-negative and MCPyV-positive MCC may be distinct entities. Recent studies suggest that MCPyV-positive variants are associated with better prognosis,35 and differences in miRNA expression between MCPyV-positive and MCPyV-negative MCC have been described.36 It is possible that MCPyV-negative MCC arises from UV-induced DNA damage37 or that MCPyV-negative MCC is initially induced by MCPyV, but tumor cells lose or eradicate the MCPyV genome after this induction (the “hit-and-run” hypothesis).38 MCC cell lines are divided into two groups (classic and variant) and further categorized based on morphology. It was hypothesized that MCPyV positivity was associated with the classic form of MCC. However, recent studies demonstrate MCPyV negativity in classic cell lines. Additionally, differing levels of MCPyV are seen in various MCC cell lines. There does not appear to be a simple relationship between cell morphology and MCPyV positivity or copy number. Interestingly, all MCPyV-positive cell lines were found to contain a premature stop codon resulting in the aforementioned truncated LT.39 It appears that a sequential number of events may be required for the development of MCC. First, MCPyV integrates into the human domain. Second, expression of T antigens leads to unlicensed viral replication. Third, mutations in the viral domain result in the prevention of viral replication and virion formation conferring protection to the cancer cells from immune targeted destruction.22,40
The molecular mechanisms underlying viral replication and cancer development in MCC continue to be an area of ongoing research. The replication cycle of MCPyV has yet to be elucidated as it has been impossible to cultivate the virus.26 The functional domains of MCPyV LT have been examined. In order for LT to function appropriately, it must be localized in the nucleus. However, a previously identified nuclear localization motif is nonessential to this localization process. Furthermore, LT interaction with HSC-70 is required for growth promotion and induction of E2F target genes.41 LT reduces the expression of Toll-like receptor 9, a key receptor in the innate immune response, via downregulation of the C/EBPβ transactivator providing a mechanism by which MCPyV may subvert the innate immune system.42 ST expression downregulates NF-κB-targeted transcription via interactions with the regulatory protein NEMO, cytoplasmic kinase IκB, and cellular phosphatases PP4c and PP2A Aβ. These interactions prevent the nuclear translocation of NF-κB resulting in the downregulation of a number of genes involved in the innate immune response. These findings may at least in part explain how MCC subverts the immune response, persists, and replicates within host cells.43 Similar to other human tumor viruses, cell-mediated immune response appears to be critical in suppressing MCC. Lending support to the importance of cell-mediated immunity is the increased risk for MCC in HIV infection and post-transplant patients. Additionally, the increased risk seen in elderly patients may be due to age-related loss of cell-mediated immunity.22
ST expression induces microtubule destabilization by affecting the phosphorylation status of stathmin, a microtubule-associated protein, via interactions with cellular phosphorylase subunits. Microtubule destabilization may result in an increasingly mobile and possibly metastatic phenotype. Consequently, chemotherapeutics stabilizing microtubules or targeting stathmin expression may offer novel therapeutic approaches to the treatment of MCC.44
MCPyV has been detected by PCR in other non-melanoma skin cancers including squamous cell carcinoma, basal cell carcinoma, and Bowen disease in immunocompromised patients further confusing the picture in terms of the significance of MCPyV infection in MCC.45 However, MCPyV DNA loads are typically much lower in non-MCC cutaneous neoplasms than in MCC, and MCPyV LT is not detected in non-MCC cutaneous neoplasms.46 The presence of MCPyV has been sought in various other cancers including small cell lung carcinoma,47 melanoma, ovary, breast, bowel,48 but has not been identified. Consequently, MCPyV is only linked to tumorogenesis in MCC.29 There appear to be ethnic and geographical differences in MCPyV infection. MCPyV is seen in MCC in Korean and Japanese patients.49,50 The Japanese strains appear to be distinct from the Caucasian strains.51 Further, there may be geographically related strains of MCPyV spanning five continents.52
Certainly, there remain a large number of unanswered questions regarding the pathogenesis of MCC, and the recognition of multiple variants indicates the need for further research to determine the impact of MCPyV, UV-induced skin damage, cell variants, molecular mechanisms, and immunologic microenvironment in the development, behavior, and prognosis of MCC. This understanding will drive the development of therapeutic strategies for MCC in the future.
Histopathology
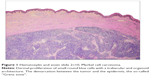
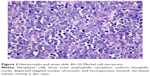
The histopathologic pattern of MCC is a localized, dermal proliferation of uniform, small blue cells with scant cytoplasm and a high mitotic rate (Figures 1 and 2). Ultrastructurally, cells are characterized by neurosecretory (dense core) granules, cytoplasmic processes, and intermediate filaments surrounding medium-sized nuclei.6,15,53 Approximately 80%–90% of MCC specimens are positive for cytokeratin 20 (CK20), which stains in a classic para-nuclear dot-like distribution.15,19,53 CK20, along with other immunohistochemical markers including synaptophysin, chromogranin A, thyroid transcription factor-1 (TTF-1), HMB 45, and S100 help distinguish MCC from other tumors such as melanoma, lymphoma, and cutaneous metastases of small cell carcinoma of the lung. MCC typically stains positively for synaptophysin and chromogranin A and negatively for TTF-1.18,26,54–60
MCC is often difficult to distinguish from other neuroendocrine carcinomas. MCC has been noted to occur in the submandibular gland and to arise from nasal mucosa.61,62 Neuroendocrine salivary carcinomas are rare. They are divided into “Merkel cell type” and “pulmonary type”.61 CK20 can aid in distinguishing between these types as CK20 positivity is a strong predictor of MCC.63 However, cases of primary submandibular MCC negative for CK20 are reported.61 Small cell carcinoma of the parotid is a rare diagnosis that warrants mention as it is extremely difficult to differentiate from MCC. In fact, it may represent a metastasis from an occult or regressed cutaneous MCC. Similar to MCC, small cell carcinoma of the parotid stains positive for CK20 (in a para-nuclear dot-like pattern), neuron-specific enolase, and chromogranin A. While MCC typically stains negative for TTF-1, the staining pattern for this marker in small cell carcinoma of the parotid is less clear. MCPyV positivity was useful as a distinguishing feature, but recently a study demonstrated MCPyV positivity in small cell carcinoma of the parotid. It is unclear whether these cases represent metastasis from occult cutaneous MCC or whether MCPyV plays an oncogenic role in the parotid gland.64
Prognostic factors
Several factors related to MCC are associated with poorer survival outcomes. Advanced age (>75 years), male sex, lip primary site, tumor extension beyond the dermis, increasing tumor size, and positive margins are associated with reduced survival.7,65 Additionally, high mitotic figure count is associated with worse survival, and the immunodetection of mitotic figures in combination with G2+ tumor nuclei with histone-associated mitotic marker H3K79me3T80ph is shown to be a significant predictor of impaired survival when compared to G2+ tumor nuclei with histone-associated mitotic marker phosphohistone H3 and manual mitotic figure count alone.66
Regarding immunosuppression, studies show that worse outcomes are seen with immunosuppressive states and vitamin D deficiency.67,68 Behr et al recently found that the presence of CD3, CD4, CD8, CD8/CD4 ratio, CD68-positive cells, neutrophils, or the presence of PD-L1-positive immune cells within the tumor or in the tumor periphery were not associated with overall or recurrence-free survival. However, they noted that the presence of tertiary lymphoid structures within the tumor was associated with increased recurrence-free survival.69 This is in contrast to a previous study that demonstrated better survival with increased number of infiltrating T-cells.70
As with many head and neck malignancies, both locoregional and distant nodal metastasis are respectively and independently associated with poorer disease-specific survival.7,65 Other work indicates that positive sentinel lymph nodes are associated with increased local recurrence rates.71,72 While Smith et al also found that the presence of multiple positive nodes did not independently predict disease-specific survival when positive nodes were found,7 a recent study found that the number of positive nodes was inversely correlated to the 5-year survival.73 Lack of histopathologic nodal evaluation is also associated with worse survival when compared with pathologically proven negative nodes.7,74 This data indicates that pathologic evaluation of nodal metastasis should be considered in all cases of MCC.
Nodal evaluation
Patients with clinically positive nodal metastases in the setting of MCC should undergo fine needle aspiration for pathological confirmation, and those with confirmed disease should undergo surgical resection of the nodal basins. It is currently recommended that patients who are clinically node negative undergo a sentinel lymph node biopsy (SLNB) in addition to management of the primary lesion.75 SLNB will detect nodal metastases in approximately one-third of patients who are clinically node negative, and locoregional recurrence rate is greatly increased in the event of positive SLNB.76 SLNB is preferable to elective neck dissection as it has decreased postoperative morbidity and superior shoulder function.77
Imaging
There is no standard imaging algorithm recommended for MCC.15 Peloschek et al recommend ultrasonography as the initial imaging modality to assess for nodal metastasis, as it is cost effective.78 However, computed tomography (CT) is routinely used for this purpose as well. CT is more effective in assessing nodal status than magnetic resonance imaging (MRI).79 Positron emission tomography (PET)/CT has similar sensitivity and specificity as conventional imaging when assessing lymph-node involvement78,79 and is recommended by some as first-line imaging for MCC.80 In the event of negative imaging, Enzenhofer et al recommend SLNB and neck dissection as the morbidity of neck dissection is low and the information obtained has high diagnostic and preventive value.80 However, the National Comprehensive Cancer Network (NCCN) guidelines recommend SLNB alone in this circumstance. In patients with positive nodes or suspected metastases, CT, MRI, or PET/CT can be obtained as all have been shown to adequately detect MCC.78,81–86
Enzenhofer et al suggest routine follow-up imaging to include chest radiograph and CT of the head and neck at 3 months posttreatment, chest radiograph and ultrasound at 6, 9, 15, 18, 21, and 30 months posttreatment, and chest radiograph, CT head and neck, and MRI head and neck each year after treatment.80 It would be reasonable to substitute PET/CT for CT at 3 months posttreatment and each year after treatment.78
Staging
In 2010, a consensus staging system for MCC was introduced by the American Joint Commission on Cancer. In summary, stage I includes those with primary tumor size ≤2 cm, while stage II includes those with primary tumor size >2 cm. Stages I and II are further classified as A or B based on pathological evaluation for nodal disease. Cases in which lymph nodes were pathologically evaluated are considered either IA or IIA, while cases in which there was no pathological evaluation are considered either IB or IIB. Positive nodal disease is considered stage III. Stage III is also further classified as A or B based on the method by which nodal disease was discovered. Those discovered on pathological examination alone are considered IIIA, while those appreciated on clinical or radiological evaluation are considered IIIB. Any distant metastasis is considered stage IV.75 A number of potential prognostic factors including antibodies to MCPyV capsid proteins or T-antigen oncoproteins, the presence of lymphovascular invasion, the level of immune suppression, the presence of tumor-infiltrating lymphocytes, unknown primary, and p63 expression may be incorporated into the staging algorithm in the future.87
Treatment
Multidisciplinary treatment is essential to deliver optimal care to patients with MCC.75,88 Treatment consists of wide local excision with or without adjuvant therapy depending on the size of the primary lesion and stage of disease. There is controversy regarding the ideal margin width, and the NCCN guidelines recommend 1–2 cm margins when feasible. Studies show no difference in recurrence-free survival based on the size of surgical margin.89,90 Mohs micrographic surgery has been proposed as an alternative to wide local excision.91–93 Among the benefits of Mohs micrographic surgery are tissue conservation and identification of tumors that would otherwise require extremely wide excision margins.94 Nevertheless, surgical resection of MCC with negative margins is the preferred primary modality of therapy when possible. Positive nodal disease should be treated with neck dissection and adjuvant radiotherapy.89,95 In the event of pathologically confirmed negative nodes, patients at high risk for locoregional recurrence should have neck dissection and/or radiotherapy of nodal basins.75
Radiation therapy can be considered for primary therapy in patients who are not surgical candidates.96,97 Veness and Howle reported a 5-year overall survival of 40% using radiotherapy alone (50–55 Gy) in a cohort of 41 patients.98 With positive margins, definitive radiotherapy is an alternative to re-resection and results in less treatment delay.99
Postsurgical adjuvant radiation is often indicated in the treatment of MCC and is shown to improve outcomes. In the only randomized, prospective trial concerning adjuvant radiation, Jouary et al found that adjuvant radiation significantly reduced the probability of regional recurrence but did not affect overall survival.100 A number of other studies note either lower recurrence rates90,101–105 or improved survival with adjuvant radiation.65,101,106,107 A systematic review of literature found that adjuvant radiation resulted in significantly higher 3-year local control rate, decreased recurrence rate, and improved 1- and 3-year overall survival rates, and that adjuvant chemotherapy did not offer any added benefit to adjuvant radiation.108 Fang et al using data from the SEER database, found similar cause-specific survival in patients with MCC <1 cm with no nodal metastases when comparing surgery alone to surgery with adjuvant radiation.109 Additionally, Ellis and Davis propose that adjuvant radiotherapy be considered optional in patients with the lowest risk of locoregional recurrence (immunocompetent patients with primary tumor ≤1 cm with no adverse histologic features, clear margins, and pathologically negative nodes). The relevant studies related to radiation therapy for MCC are summarized in Table 1. However, more research needs to define specific prognostic factors that determine ideal candidates for withholding adjuvant radiotherapy.94
  | Table 1 Key studies assessing recurrence and survival with adjuvant radiation therapy for Merkel cell carcinoma |
The use of chemotherapy is less well defined for MCC. Chemotherapy is currently used in advanced stage MCC and as palliative therapy. There is no standard choice of chemotherapeutic agent. A variety of groups have used chemotherapy regimens based on treatments for lung small cell carcinoma as it is noted to have similar neuroendocrine properties to MCC. Often, there is initial regression, but recurrences develop within 4–15 months.107,110–118 A recent retrospective study found that adjuvant chemoradiotherapy resulted in improved overall survival when compared with adjuvant radiotherapy in patients with positive margins, tumor size at least 3 cm, and male sex.65 Other studies suggest that the effect of adjuvant chemotherapy on recurrence is unclear or that there is no significant improvement in survival when compared with adjuvant radiation therapy.96,119–121 Eng et al retrospectively reviewed 85 cases of MCC. They found that adjuvant therapy did not affect survival, and those treated with adjuvant radiation had a similar recurrence rate as those treated with adjuvant chemoradiotherapy. They concluded that the role of adjuvant chemotherapy was unclear, though they only had a small number of patients in the chemotherapy group.119 One study found that adjuvant chemotherapy was actually associated with worse survival.121 Currently, the literature on chemotherapy use in MCC is inadequate to suggest routine use.120–122 However, palliative brachytherapy offers good palliation without affecting disease or overall survival.123 The relevant studies related to chemoradiotherapy for MCC are summarized in Table 2.
  | Table 2 Key studies assessing recurrence rate and survival with adjuvant chemoradiotherapy for Merkel cell carcinoma |
Potential therapies
With the recent discovery of MCPyV and the elucidation of molecular pathways instrumental in the development of MCC, there has been an explosion of research into new therapeutic options for the disease. A recent gene expression analysis comparing MCPyV-positive MCC, MCPyV-negative MCC, and normal Merkel cells identified a number of differences in the gene expression profile. Downregulated genes were primarily involved in immune function. One of the few distinguishing factors between MCPyV-negative and MCPyV-positive MCC was the increased expression of cell adhesion molecules seen in MCPyV-negative MCC.124 No definitive conclusions could be drawn; however, the study identifies a number of genes and pathways that can be further evaluated in the search for novel treatment strategies. Both antiviral and immune-modulating therapeutic options are being explored. A mouse model demonstrates the potential benefit of vaccination for MCPyV.125 MCC specimens show upregulated vascular endothelial growth factor receptor,126 platelet-derived growth factor receptor (PDGFR),127 and KIT,128–131 a tyrosine kinase receptor similar to PDGFR, which have been shown to stimulate growth in MCC in vitro.132 Pazopanib, a tyrosine kinase inhibitor acting against vascular endothelial growth factor receptor and PDGFR, is being explored as a potential treatment option. Similarly, imatinib mesylate, a tyrosine kinase inhibitor targeting KIT, has been investigated. Unfortunately, a phase II clinical trial was prematurely discontinued as imatinib failed to show sufficient effects on progression-free and overall survival (only one of 23 patients showed partial response, and many patients demonstrated rapid progression).128 Somatostatin receptors are also upregulated in MCC. Unfortunately, somatostatin analogs have shown poor results in terms of response or time to recurrence.133–135 As mentioned previously, survivin is upregulated in MCC. As a result, YM155, a survivin inhibitor, is currently being tested for efficacy in MCC and has improved survival in mice with MCC.32
There have been reports of spontaneous regression of MCC136,137 and case reports of MCC development during tumor necrosis factor-alpha inhibitor use.138,139 Reconstitution of cell-mediated immunity and loss of cell-mediated immunity may be responsible for these events, respectively.22 T-cell infiltration may play a role in spontaneous regression as an increased number of lymphocytes have been noted surrounding tumor nests in regressing MCC compared with non-regressing MCC.140 Previously, it was considered that a biopsy might induce a T-cell response resulting in regression; however, more recent studies fail to demonstrate an increase in CD8 T-cell infiltration after biopsy.141 The role of the immune system has led to research into immune-modulating drugs as potential therapeutic options for MCC. Interferon therapy has been reported but to date has been unsuccessful.142,143 Imiquimod has been topically applied in conjunction with radiotherapy resulting in a complete response lasting 7 months.144 A potential area of interest is cytokine-induced inflammatory responses, where fusion proteins containing antibodies and cytokines bind to their corresponding antigen on tumor cells to produce an immune response.145 Inhibition of T antigens is another area of potential interest, and there is currently a phase II trial under way examining the results of intratumoral injection of interleukin-12 in attempt to incite an immune response against tumor cells.122
A recent study associated MCC with a number of mutations in various cancer-related genes. Among these genes are PDE4DIP, MLL3, ERCC5, AURKB, ATR, TSHR, and BCL2L2.146 Further studies including larger cohorts will be required to clarify the significance of and potentially identify therapeutic options targeted toward these gene mutations.
Another area of research involves telomerase reverse transcriptase (TERT). Activation of telomerase is a key step in malignant transformation in a number of cancers. TERT, the catalytic component of telomerase, expression is an important part of the activation process. TERT expression and telomerase activation are prominent in MCC. Additionally, TERT promoter mutations are seen in MCC, occur more often in sun-exposed areas, and are more common in MCPyV-negative tumors. Increased TERT mRNA expression is associated with worse survival.147 Once again, studies with larger cohorts will need to confirm these findings and determine whether novel therapeutic approaches targeting TERT expression and telomerase activity would be beneficial in treating MCC. Desch and Kunstfeld also calls for the implementation of an MCC network that will allow collection of a sufficient number of patients in an MCC registry and multicenter clinical trials to explore treatment options.122
Conclusion
MCC is a relatively uncommon, neuroendocrine, cutaneous malignancy. Traditional therapy involves surgical resection with negative margins, when feasible, with or without adjuvant radiation depending on the size of the primary lesion and stage of disease. The role of chemotherapy needs to be further defined. With the discovery of MCPyV and the elucidation of molecular pathways involved in the oncogenesis of MCC, there has been an increase in research into new targeted and immunologic therapeutic options. These efforts are likely to yield improved treatment strategies for patients afflicted with MCC.
Disclosure
The authors report no conflicts of interest in this work.
References
Merkel F. Tastzellen and Tastkörperchen bei den Hausthieren und beim Menschen. Arch Mikrosk Anat. 1875;11:636. | ||
Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol. 2015;25(2):74–81. | ||
Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. | ||
Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978;42(5):2311–2321. | ||
Hitchcock CL, Bland KI, Laney RG 3rd, Franzini D, Harris B, Copeland EM 3rd. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988;207(2):201–207. | ||
Koljonen V. Merkel cell carcinoma. World J Surg Oncol. 2006;4:7. | ||
Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope. 2012;122(6):1283–1290. | ||
Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–381. | ||
Kempf W, Mertz KD, Hofbauer GF, Tinguely M. Skin cancer in organ transplant recipients. Pathobiology. 2013;80(6):302–309. | ||
Koljonen V, Kukko H, Tukiainen E, et al. Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transplant. 2009;24(10):3231–3235. | ||
Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–498. | ||
Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832–841. | ||
Marcoval J, Ferreres JR, Penín RM, Pérez D, Viñals JM. Merkel cell carcinoma: differences between sun-exposed and non-sun-exposed variants – a clinical analysis of 36 cases. Dermatology. 2014;229(3):205–209. | ||
Agelli M, Clegg LX, Becker JC, Rollison DE. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer. 2010;34(1):14–37. | ||
Huber GF. Modern management of Merkel cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):109–115. | ||
Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–27. | ||
Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. | ||
Becker JC. Merkel cell carcinoma. Ann Oncol. 2010;21(Suppl 7):vii81–vii85. | ||
Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140(2):427–447. | ||
Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801. | ||
Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. | ||
Arora R, Chang Y, Moore PS. MCV and Merkel cell carcinoma: a molecular success story. Curr Opin Virol. 2012;2(4):489–498. | ||
Martel-Jantin C, Pedergnana V, Nicol JT, et al. Merkel cell polyomavirus infection occurs during early childhood and is transmitted between siblings. J Clin Virol. 2013;58(1):288–291. | ||
Tolstov YL, Knauer A, Chen JG, et al. Asymptomatic primary Merkel cell polyomavirus infection among adults. Emerg Infect Dis. 2011;17(8):1371–1380. | ||
Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7(6):509–515. | ||
Coursaget P, Samimi M, Nicol JT, Gardair C, Touzé A. Human Merkel cell polyomavirus: virological background and clinical implications. APMIS. 2013;121(8):755–769. | ||
Signorini L, Belingheri M, Ambrogi F, et al. High frequency of Merkel cell polyomavirus DNA in the urine of kidney transplant recipients and healthy controls. J Clin Virol. 2014;61(4):565–570. | ||
Zhang C, Liu F, He Z, et al. Seroprevalence of Merkel cell polyomavirus in the general rural population of Anyang, China. PLoS One. 2014;9(9):e106430. | ||
Dalianis T, Hirsch HH. Human polyomaviruses in disease and cancer. Virology. 2013;437(2):63–72. | ||
Mangana J, Dziunycz P, Kerl K, Dummer R, Cozzio A. Prevalence of Merkel cell polyomavirus among Swiss Merkel cell carcinoma patients. Dermatology. 2010;221(2):184–188. | ||
Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121(9):3623–3634. | ||
Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4(133):133ra56. | ||
Houben R, Schrama D, Becker JC. Molecular pathogenesis of Merkel cell carcinoma. Exp Dermatol. 2009;18(3):193–198. | ||
Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–8397. | ||
Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938–945. | ||
Veija T, Sahi H, Koljonen V, Bohling T, Knuutila S, Mosakhani N. miRNA-34a underexpressed in Merkel cell polyomavirus-negative Merkel cell carcinoma. Virchows Arch. 2015;466(3):289–295. | ||
Kuwamoto S. Recent advances in the biology of Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063–1077. | ||
Houben R, Grimm J, Willmes C, Weinkam R, Becker JC, Schrama D. Merkel cell carcinoma and Merkel cell polyomavirus: evidence for hit-and-run oncogenesis. J Invest Dermatol. 2012;132(1):254–256. | ||
Fischer N, Brandner J, Fuchs F, Moll I, Grundhoff A. Detection of Merkel cell polyomavirus (MCPyV) in Merkel cell carcinoma cell lines: cell morphology and growth phenotype do not reflect presence of the virus. Int J Cancer. 2010;126(9):2133–2142. | ||
Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105(42):16272–16277. | ||
Houben R, Angermeyer S, Haferkamp S, et al. Characterization of functional domains in the Merkel cell polyoma virus Large T antigen. Int J Cancer. 2015;136(5):E290–E300. | ||
Shahzad N, Shuda M, Gheit T, et al. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J Virol. 2013;87(23):13009–13019. | ||
Griffiths DA, Abdul-Sada H, Knight LM, et al. Merkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signaling. J Virol. 2013;87(24):13853–13867. | ||
Knight LM, Stakaityte G1, Wood JJ, et al. Merkel cell polyomavirus small T antigen mediates microtubule destabilization to promote cell motility and migration. J Virol. 2015;89(1):35–47. | ||
Kassem A, Technau K, Kurz AK, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009;125(2):356–361. | ||
Scola N, Wieland U, Silling S, Altmeyer P, Stücker M, Kreuter A. Prevalence of human polyomaviruses in common and rare types of non-Merkel cell carcinoma skin cancer. Br J Dermatol. 2012;167(6):1315–1320. | ||
Wetzels CT, Hoefnagel JG, Bakkers JM, Dijkman HB, Blokx WA, Melchers WJ. Ultrastructural proof of polyomavirus in Merkel cell carcinoma tumour cells and its absence in small cell carcinoma of the lung. PLoS One. 2009;4(3):e4958. | ||
Sastre-Garau X, Peter M, Avril MF, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218(1):48–56. | ||
Chun SM, Yun SJ, Lee SC, Won YH, Lee JB. Merkel cell polyomavirus is frequently detected in Korean patients with Merkel cell carcinoma. Ann Dermatol. 2013;25(2):203–207. | ||
Hattori T, Takeuchi Y, Takenouchi T, et al. The prevalence of Merkel cell polyomavirus in Japanese patients with Merkel cell carcinoma. J Dermatol Sci. 2013;70(2):99–107. | ||
Matsushita M, Iwasaki T, Kuwamoto S, et al. Merkel cell polyomavirus (MCPyV) strains in Japanese Merkel cell carcinomas (MCC) are distinct from Caucasian type MCPyVs: genetic variability and phylogeny of MCPyV genomes obtained from Japanese MCPyV-infected MCCs. Virus Genes. 2014;48(2):233–242. | ||
Martel-Jantin C, Filippone C, Tortevoye P, et al. Molecular epidemiology of Merkel cell polyomavirus: evidence for geographically related variant genotypes. J Clin Microbiol. 2014;52(5):1687–1690. | ||
Ramahi E, Choi J, Fuller CD, Eng TY. Merkel cell carcinoma. Am J Clin Oncol. 2013;36(3):299–309. | ||
Becker J, Mauch C, Kortmann RD, et al. Short German guidelines: Merkel cell carcinoma. J Dtsch Dermatol Ges. 2008;6(Suppl 1):S15–S16. | ||
Knoepp SM, Hookim K, Placido J, Fields KL, Roh MH. The application of immunocytochemistry to cytologic direct smears of metastatic Merkel cell carcinoma. Diagn Cytopathol. 2013;41(8):729–733. | ||
Dancey AL, Rayatt SS, Soon C, Ilchshyn A, Brown I, Srivastava S. Merkel cell carcinoma: a report of 34 cases and literature review. J Plast Reconstr Aesthet Surg. 2006;59(12):1294–1299. | ||
Bobos M, Hytiroglou P, Kostopoulos I, Karkavelas G, Papadimitriou CS. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28(2):99–104. | ||
Kontochristopoulos GJ, Stavropoulos PG, Krasagakis K, Goerdt S, Zouboulis CC. Differentiation between Merkel cell carcinoma and malignant melanoma: an immunohistochemical study. Dermatology. 2000;201(2):123–126. | ||
Agaimy A, Erlenbach-Wünsch K, Konukiewitz B, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod Pathol. 2013;26(7):995–1003. | ||
Kao CS, Warren S, Idrees MT. Merkel cell carcinoma exhibiting cytoplasmic OCT4 staining: a potential new diagnostic immunohistochemical marker. Am J Dermatopathol. 2014;36(3):274–276. | ||
Lombardi D, Accorona R, Ungari M, Melocchi L, Bell D, Nicolai P. Primary Merkel cell carcinoma of the submandibular gland: when CK20 status complicates the diagnosis. Head Neck Pathol. 2015;9(2):309–314. | ||
Snow SN, Larson PO, Hardy S, et al. Merkel cell carcinoma of the skin and mucosa: report of 12 cutaneous cases with 2 cases arising from the nasal mucosa. Dermatol Surg. 2001;27(2):165–170. | ||
Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21(2):226–234. | ||
Fisher CA, Harms PW, McHugh JB, et al. Small cell carcinoma in the parotid harboring Merkel cell polyomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):703–712. | ||
Chen MM, Roman SA, Sosa JA, Judson BL. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: an analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg. 2015;141(2):137–141. | ||
Henderson SA, Tetzlaff MT, Pattanaprichakul P, et al. Detection of mitotic figures and G2+ tumor nuclei with histone markers correlates with worse overall survival in patients with Merkel cell carcinoma. J Cutan Pathol. 2014;41(11):846–852. | ||
Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425–432. | ||
Samimi M, Touzé A, Laude H, et al. Vitamin D deficiency is associated with greater tumor size and poorer outcome in Merkel cell carcinoma patients. J Eur Acad Dermatol Venereol. 2014;28(3):298–308. | ||
Behr DS, Peitsch WK, Hametner C, et al. Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol. 2014;7(11):7610–7621. | ||
Sihto H, Joensuu H. Tumor-infiltrating lymphocytes and outcome in Merkel cell carcinoma, a virus-associated cancer. Oncoimmunology. 2012;1(8):1420–1421. | ||
Kouzmina M, Leikola J, Böhling T, Koljonen V. Positive sentinel lymph node biopsy predicts local metastases during the course of disease in Merkel cell carcinoma. J Plast Surg Hand Surg. 2013;47(2):139–143. | ||
Santamaria-Barria JA, Boland GM, Yeap BY, Nardi V, Dias-Santagata D, Cusack JC Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol. 2013;20(4):1365–1373. | ||
Iyer JG, Storer BE2, Paulson KG, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8,044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(4):637–643. | ||
Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5,823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–761. | ||
Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. | ||
Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142(6):685–690. | ||
Murer K, Huber GF, Haile SR, Stoeckli SJ. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck. 2011;33(9):1260–1264. | ||
Peloschek P, Novotny C, Mueller-Mang C, et al. Diagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur J Radiol. 2010;73(2):317–323. | ||
Colgan MB, Tarantola TI, Weaver AL, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol. 2012;67(6):1250–1256. | ||
Enzenhofer E, Ubl P, Czerny C, Erovic BM. Imaging in patients with Merkel cell carcinoma. J Skin Cancer. 2013;2013:973123. | ||
Zager JS, Brodsky S, Berman CG. Imaging of Merkel cell carcinoma. Curr Probl Cancer. 2010;34(1):65–76. | ||
Anderson SE, Beer KT, Banic A, et al. MRI of Merkel cell carcinoma: histologic correlation and review of the literature. AJR Am J Roentgenol. 2005;185(6):1441–1448. | ||
Gollub MJ, Gruen DR, Dershaw DD. Merkel cell carcinoma: CT findings in 12 patients. AJR Am J Roentgenol. 1996;167(3):617–620. | ||
Treglia G, Kakhki VR, Giovanella L, Sadeghi R. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysis. Am J Clin Dermatol. 2013;14(6):437–447. | ||
Concannon R, Larcos GS, Veness M. The impact of (18)F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: results from Westmead Hospital, Sydney, Australia. J Am Acad Dermatol. 2010;62(1):76–84. | ||
Hawryluk EB, O’Regan KN, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68(4):592–599. | ||
Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J Natl Compr Canc Netw. 2014;12(9):1255–1262. | ||
Schneider S, Thurnher D, Erovic BM. Merkel cell carcinoma: interdisciplinary management of a rare disease. J Skin Cancer. 2013;2013:189342. | ||
Morand G, Vital D, Pézier T, et al. Merkel cell carcinoma of the head and neck: a single institutional experience. J Skin Cancer. 2013;2013:325086. | ||
Gillenwater AM, Hessel AC, Morrison WH, et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001; 127(2):149–154. | ||
O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. Comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23(10):929–933. | ||
Gollard R, Weber R, Kosty MP, Greenway HT, Massullo V, Humberson C. Merkel cell carcinoma: review of 22 cases with surgical, pathologic, and therapeutic considerations. Cancer. 2000;88(8):1842–1851. | ||
Boyer JD, Zitelli JA, Brodland DG, D’Angelo G. Local control of primary Merkel cell carcinoma: review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiation. J Am Acad Dermatol. 2002;47(6):885–892. | ||
Ellis DL, Davis RS. Evidence-based management of primary and localized Merkel cell carcinoma: a review. Int J Dermatol. 2013;52(10):1248–1258. | ||
Balakrishnan V, Berry S, Stew B, Sizeland A. Benefits of combined modality treatment of Merkel cell carcinoma of the head and neck: single institution experience. J Laryngol Otol. 2013;127(9):908–916. | ||
Poulsen M, Rischin D. Merkel cell carcinoma–current therapeutic options. Expert Opin Pharmacother. 2003;4(12):2187–2192. | ||
Tuskada A, Fujimura T, Hashimoto A, et al. Successful local control of cutaneous Merkel cell carcinoma on the eyelid with CyberKnife radiosurgery. Eur J Dermatol. 2013;23(5):725–726. | ||
Veness M, Howle J. Radiotherapy alone in patients with Merkel cell carcinoma: the Westmead Hospital experience of 41 patients. Australas J Dermatol. 2015;56(1):19–24. | ||
Hruby G, Scolyer RA, Thompson JF. The important role of radiation treatment in the management of Merkel cell carcinoma. Br J Dermatol. 2013;169(5):975–982. | ||
Jouary T, Leyral C, Dreno B, et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol. 2012;23(4):1074–1080. | ||
Kokoska ER, Kokoska MS, Collins BT, Stapleton DR, Wade TP. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997;174(6):688–693. | ||
Meeuwissen JA, Bourne RG, Kearsley JH. The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1995;31(2):325–331. | ||
Lewis KG, Weinstock MA, Weaver AL, Otley CC. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142(6):693–700. | ||
Jabbour J, Cumming R, Scolyer RA, Hruby G, Thompson JF, Lee S. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease–results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14(6):1943–1952. | ||
Medina-Franco J, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1,024 cases. Ann Surg Oncol. 2001;8(3):204–208. | ||
Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25(9):1043–1047. | ||
Veness MJ, Perera L, McCourt J, et al. Merkel cell carcinoma: improved outcome with adjuvant radiotherapy. ANZ J Surg. 2005;75(5):275–281. | ||
Hasan S, Liu L, Triplet J, Li Z, Mansur D. The role of postoperative radiation and chemoradiation in Merkel cell carcinoma: a systematic review of the literature. Front Oncol. 2013;3:276. | ||
Fang L, Parvathaneni U, Nghiem P. Surgery with or without radiotherapy for Merkel cell carcinoma. Int J Radiat Oncol. 2008;72(1). Supplement:S508. | ||
George TK, Di Sant’agnese PA, Bennett JM. Chemotherapy for metastatic Merkel cell carcinoma. Cancer. 1985;56(5):1034–1038. | ||
McAfee WJ, Morris CG, Mendenhall CM, Werning JW, Mendenhall NP, Mendenhall WM. Merkel cell carcinoma: treatment and outcomes. Cancer. 2005;104(8):1761–1764. | ||
Redmond J 3rd, Perry J, Sowray P, Vukelja SJ, Dawson N. Chemotherapy of disseminated Merkel-cell carcinoma. Am J Clin Oncol. 1991;14(4):305–307. | ||
Azagury M, Chevallier B, Atlan D, Graic Y, Dayot JP, Thomine E. VP-16, cisplatin, doxorubicin, and bleomycin in metastatic Merkel cell carcinoma. Report of a case with long-term remission. Am J Clin Oncol. 1993;16(2):102–104. | ||
Voog E, Biron P, Martin JP, Blay JY. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999;85(12):2589–2595. | ||
Wobser M, Kürzinger N, Ugurel S, Bröcker EB, Becker JC. Therapy of metastasized Merkel cell carcinoma with liposomal doxorubicin in combination with radiotherapy. J Dtsch Dermatol Ges. 2009;7(6):521–525. | ||
Schlaak M, Podewski T, Von Bartenwerffer W, et al. Induction of durable responses by oral etoposide monochemotherapy in patients with metastatic Merkel cell carcinoma. Eur J Dermatol. 2012;22(2):187–191. | ||
Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, Gilchrist J. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18(12):2493–2499. | ||
Pollack SV, Goslen JB. Small-cell neuroepithelial tumor of skin: a Merkel-cell neoplasm? J Dermatol Surg Oncol. 1982;8(2):116–122. | ||
Eng TY, Boersma MG, Fuller CD, Cavanaugh SX, Valenzuela F, Herman TS. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27(5):510–515. | ||
Eng TY, Naguib M, Fuller CD, Jones WE 3rd, Herman TS. Treatment of recurrent Merkel cell carcinoma: an analysis of 46 cases. Am J Clin Oncol. 2004;27(6):576–583. | ||
Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–2309. | ||
Desch L, Kunstfeld R. Merkel cell carcinoma: chemotherapy and emerging new therapeutic options. J Skin Cancer. 2013;2013:327150. | ||
Garibyan L, Cotter SE, Hansen JL, et al. Palliative treatment for in-transit cutaneous metastases of Merkel cell carcinoma using surface-mold computer-optimized high-dose-rate brachytherapy. Cancer J. 2013;19(4):283–287. | ||
Mouchet N, Coquart N, Lebonvallet N, et al. Comparative transcriptional profiling of human Merkel cells and Merkel cell carcinoma. Exp Dermatol. 2014;23(12):928–930. | ||
Gomez B, He L, Tsai YC, Wu TC, Viscidi RP, Hung CF. Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen. Cell Biosci. 2013;3(1):29. | ||
Fernández-Figueras MT, Puig L, Musulén E, et al. Expression profiles associated with aggressive behavior in Merkel cell carcinoma. Mod Pathol. 2007;20(1):90–101. | ||
Kartha RV, Sundram UN. Silent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesis. Mod Pathol. 2008;21(2):96–104. | ||
Samlowski WE, Moon J, Tuthill RJ, et al. A phase II trial of imatinib mesylate in Merkel cell carcinoma (neuroendocrine carcinoma of the skin): a Southwest Oncology Group study (S0331). Am J Clin Oncol. 2010;33(5):495–499. | ||
Feinmesser M, Halpern M, Kaganovsky E, et al. c-kit expression in primary and metastatic Merkel cell carcinoma. Am J Dermatopathol. 2004;26(6):458–462. | ||
Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. CD117 (KIT receptor) expression in Merkel cell carcinoma. Am J Dermatopathol. 2002;24(4):289–293. | ||
Strong S, Shalders K, Carr R, Snead DR. KIT receptor (CD117) expression in Merkel cell carcinoma. Br J Dermatol. 2004;150(2):384–385. | ||
Krasagakis K, Fragiadaki I, Metaxari M, et al. KIT receptor activation by autocrine and paracrine stem cell factor stimulates growth of Merkel cell carcinoma in vitro. J Cell Physiol. 2011;226(4):1099–1109. | ||
di Bartolomeo M, Bajetta E, Buzzoni R, et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77(2):402–408. | ||
Meier G, Waldherr C, Herrmann R, Maecke H, Mueller-Brand J, Pless M. Successful targeted radiotherapy with 90Y-DOTATOC in a patient with Merkel cell carcinoma. A case report. Oncology. 2004;66(2):160–163. | ||
Fakiha M, Letertre P, Vuillez JP, Lebeau J. Remission of Merkel cell tumor after somatostatin analog treatment. J Cancer Res Ther. 2010;6(3):382–384. | ||
Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34(6):815–822. | ||
Wooff JC, Trites JR, Walsh NM, Bullock MJ. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32(6):614–617. | ||
Krishna SM, Kim CN. Merkel cell carcinoma in a patient treated with adalimumab: case report. Cutis. 2011;87(2):81–84. | ||
Linn-Rasker SP, van Albada-Kuipers GA, Dubois SV, Janssen K, Zweers PG. Merkel cell carcinoma during treatment with TNF-alpha inhibitors: coincidence or warning? Ned Tijdschr Geneeskd. 2012;156(22):A4464. | ||
Inoue T, Yoneda K, Manabe M, Demitsu T. Spontaneous regression of Merkel cell carcinoma: a comparative study of TUNEL index and tumor-infiltrating lymphocytes between spontaneous regression and non-regression group. J Dermatol Sci. 2000;24(3):203–211. | ||
Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS One. 2012;7(7):e41465. | ||
Biver-Dalle C, et al. Use of interferon-alpha in two patients with Merkel cell carcinoma positive for Merkel cell polyomavirus. Acta Oncol. 2011;50(3):479–480. | ||
Willmes C, Adam C, Alb M, et al. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012;72(8):2120–2128. | ||
Balducci M, De Bari B, Manfrida S, D’Agostino GR, Valentini V. Treatment of Merkel cell carcinoma with radiotherapy and imiquimod (Aldara): a case report. Tumori. 2010;96(3):508–511. | ||
Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–159. | ||
Graves CA, Jones A, Reynolds J, et al. Neuroendocrine Merkel cell carcinoma is associated with mutations in key DNA repair, epigenetic and apoptosis pathways: a case-based study using targeted massively parallel sequencing. Neuroendocrinology. 2015;101(2):112–119. | ||
Xie H, Liu T, Wang N, et al. TERT promoter mutations and gene amplification: promoting TERT expression in Merkel cell carcinoma. Oncotarget. 2014;5(20):10048–10057. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.