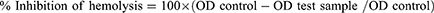Back to Journals » Journal of Experimental Pharmacology » Volume 14
Meriandra dianthera Aqueous Extract and Its Fraction Prevents Blood Coagulation by Specifically Inhibiting the Intrinsic Coagulation Pathway: An in vitro Study
Authors Kiflemariam FK , Tewelde AG, Hamid AM, Beshir BM, Solomon SN, Eman TG, Abraha DM, Kahsu R, Issac J, Kaushik JJ
Received 22 February 2022
Accepted for publication 31 May 2022
Published 29 June 2022 Volume 2022:14 Pages 205—212
DOI https://doi.org/10.2147/JEP.S362258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Filmon Kiflezghi Kiflemariam,1 Abiel Ghebrehiwet Tewelde,1 Ali Mahmud Hamid,1 Bilal Mussa Beshir,1 Samrawit Negasi Solomon,1 Tesfu Gonets Eman,1 Daniel Mebrahtu Abraha,1,2 Russom Kahsu,1 John Issac,1 Jeevan Jyoti Kaushik1
1Department of Clinical Laboratory Sciences, Asmara College of Health Sciences, Asmara, Eritrea; 2Department of Hematology, National Health Laboratory, Asmara, Eritrea
Correspondence: Filmon Kiflezghi Kiflemariam, Department of Clinical Laboratory Sciences, Asmara College of Health Sciences, Asmara, Eritrea, Email [email protected]
Background: Currently, cardiovascular disorders are the primary cause of mortality in the world and constitute a serious medical problem. Blood coagulation is an essential process to prevent excessive blood loss through injured blood vessels; however, abnormal blood clotting in the blood vessels can result in fatal cardiovascular disorders. This study investigated the in vitro anticoagulant activity of Meriandra dianthera crude extract and its fractions and their erythrocyte membrane stabilizing activity.
Methods: The plant leaves were extracted by a decoction method and were further fractionated by a liquid–liquid partition with a solvent of crescent polarity. The in vitro anticoagulant activity of the plant extract and its fractions was assessed by PT and APTT assays, while the membrane stabilizing activity was determined through hypotonic induced hemolysis.
Results: The crude aqueous leaf extract of Meriandra dianthera significantly (P < 0.001) prolonged the intrinsic clotting pathway measured by APTT by specifically acting on the intrinsic coagulation pathway. By using liquid–liquid fractionation, the residual aqueous fraction was identified as the fraction responsible for the anticoagulant activity of the crude extract as it significantly (P< 0.001) prolonged APTT while the other fractions failed. Both the crude extract and its aqueous residue fraction did not affect the extrinsic coagulation pathway measured by PT. In the membrane stabilizing assay, crude extract and aqueous residue fraction showed the highest membrane stabilizing activity.
Conclusion: The crude extract and its aqueous residue fraction showed a potent in vitro anticoagulant and membrane stabilizing activity, which shows the potential of the plant’s leaves as a new source of bioactive molecules for coagulation-related disorders.
Keywords: blood coagulation, in vitro, anticoagulant, fractionation, medicinal plant, prothrombin time, APTT, Meriandra dianthera
Introduction
The critical mechanism to maintain the delicate equilibrium between hemorrhage and thrombosis through anticoagulants and pro-coagulants and thereby to retain blood in the blood vessels during injury is achieved through the process of hemostasis. Hemostasis is attained through primary and secondary mechanisms. Primary hemostasis is the formation of a platelet plug at the site of damage in response to vascular injury. The secondary hemostasis includes a proteolysis process in which precursors of the coagulation factors are activated to accelerate the formation of the subsequent clotting factor,1 which is accomplished via the intrinsic and extrinsic pathways,2 which converge in the common pathway to convert soluble fibrinogen into insoluble fibrin polymers. The polymers result in the formation of a clot by creating a meshwork that is reinforced by platelets and other blood components.3 This coagulation process is vital to avoid excessive blood loss through the injured blood vessels; however, any abnormal blood clotting in the blood vessels can result in fatal cardiovascular disorders.4 Currently, cardiovascular disorders are the primary cause of mortality in the world and constitute a serious medical problem and it is predicted that these disorders will cause mortality of almost 23.6 million people by 2030.5
Anticoagulants are a class of drugs that inhibit the formation of a clot in the blood vessels by affecting either the availability or activation of blood coagulation factors and platelets. However, these treatments frequently result in life-threatening side effects like thrombocytopenia, bleeding events, and osteopenia.6 Therefore, the alarming limitations of the currently used anticoagulants have stimulated the search for alternative new agents from natural sources.7
Plants, due to their effect on the biological process, could be a new source for the development of novel anticoagulant agents. Medicinal plant-based drugs in addition to being simple and effective, they also show a broad spectrum of activity with special attention on preventive action. There is compelling scientific evidence that indicates the intake of dietary anticoagulants or phytochemicals with anticoagulant activity can eventually diminish or eliminate the risk of thromboembolic diseases.8 Herbs like garlic are commonly sold in the market as blood thinners and their anticoagulant activity has been shown by studies.8 However, despite the popular use of Meriandra dianthera in traditional medicine no study has been done, to the best of the author’s knowledge, to scientifically determine its in vitro anticoagulant activity.
Meriandra dianthera (or its synonym name Meriandera bengalensis) is a plant belonging to the Lamiaceae family, locally known as Nihba/Mezaguf. Traditionally, its leaves are reported to be used to treat hypertension, malaria, gastritis, bronchial asthma, and infectious hepatitis.9
Materials and Methods
Plant Collection and Authentication
Fresh leaves of Meriandra dianthera were collected from Adi-nfas, North of Asmara, Capital city of Eritrea. The botanical identification and authentication of the plant was done by a botanist from the Eritrean Institute of Technology (EIT). A voucher specimen (No MD-R-0012) was placed in the herbarium, Asmara College of Health Sciences, Department of Biochemistry.
Plant Preparation
The collected plant leaves were thoroughly washed with distilled water to remove dirt, mud, or filthy particles present on the surface and were shade dried at room temperature for 2 weeks. The shade dried plants were then powdered using mortar and pestle for further extraction purposes.
Hot Aqueous Extraction (Decoction)
The hot water extraction from the leaves of Meriandra dianthera was performed according to the method described by a previous study7 with some modifications. A total of 100 g of powdered Meriandra dianthera leaves were extracted for 5 h at 100°C with 1 L distilled water. Then, the hot extract was filtered using grade No. 1 filter paper. Rotary evaporator was used to concentrate the filtrate under reduced pressure. Finally, the semi-liquid product was further dried in a water bath at 60°C, and was stored at −20°C until further analysis.
Fractionation of Meriandra dianthera Hot Crude Extract
The leave crude extract was further fractionated by a liquid–liquid partition with increasing polarity of solvents (ie, chloroform, ethyl acetate, butanol) to separate the crude extract compounds according to their solubility in their respective solvents to obtain four different fractions of chloroform, ethyl acetate, butanol, and aqueous residue fractions.
Blood Sample Collection and Preparation of Pooled Plasma
Blood was obtained from 10 healthy individuals by using sterile syringes from the median cubital vein on their left arm. Volunteers who were suffering from any cardiovascular diseases, diabetes, obesity, dyslipidemic disorders, or smokers and those who recently used nonsteroidal anti-inflammatory drugs, oral contraceptives, or anticoagulant therapy were excluded from the blood sample collection. The collected blood sample was transferred into a vacutainer containing 3.2% trisodium citrate to prevent the clotting process, and centrifugation was done at a rate of 3000 rpm for 15min using the HERMLE benchtop centrifuge to obtain platelet-poor plasma. The obtained plasma sample of each individual was pooled in a plain tube using a pipette and was analyzed immediately.
In vitro Blood Coagulation Study
In this study, the action of the plant extracts in the intrinsic and common blood coagulation pathways was evaluated by the Activated Partial Thromboplastin Time (APTT) assay and that of the extrinsic pathway was determined by the Prothrombin Time (PT) assay.
Activated Partial Thromboplastin Time (APTT) Assay
In vitro intrinsic anticoagulant pathway was measured by Activated Partial Thromboplastin Time (APTT). Human normal pooled plasma (200 µL) was mixed with 100 µL of different concentrations of Meriandra dianthera crude extract and fractions (500 μg/mL, 1 mg/mL, 2 mg/mL, 5 mg/mL, and 10 mg/mL). Meanwhile, APTT automated reagent was reconstituted and subjected to APTT assay, together with 0.025 M calcium chloride. The clotting time was then recorded by an automated coagulometer (ACL elite coagulation analyzer, Instrumentation Laboratory Company). Plasma with vehicle (0.9% normal saline) was used as a negative control (absence of anticoagulant activity) and heparin (1 IU/mL) was used as a positive control. The tests were done in triplicate for each concentration.
Prothrombin Time (PT) Assay
In vitro extrinsic anticoagulant pathway was measured by Prothrombin Time (PT) assay. Human normal pooled plasma (200 µL) was mixed with 100 µL of different concentrations of Meriandra dianthera crude extract and fractions (500 μg/mL, 1 mg/mL, 2 mg/mL, 5 mg/mL, and 10 mg/mL). The PT automated reagent was reconstituted and subjected to PT assay, together with 0.025 M calcium chloride. The clotting time was then recorded by an automated coagulometer (ACL elite coagulation analyzer, Instrumentation Laboratory Company). Plasma with vehicle (0.9% normal saline) was used as a negative control and heparin (1 IU/mL) was used as a positive control. The tests were also done in triplicate for each concentration.
Membrane Stabilizing Assay
The membrane stabilizing activity of the extract and its fractions was evaluated by using hypotonic-solution induced human erythrocyte hemolysis, as previously done with slight modification.10 The assay mixture consisting of 0.5 mL of 2% (v/v) human erythrocyte suspension mixed with 5 mL of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate buffered saline (pH 7.4) containing either the extract at different concentrations or the reference drug, acetylsalicylic acid (0.1 mg/mL). The mixtures were incubated for 10 minutes at room temperature and centrifuged at 3000 rpm for 10 minutes. The absorbance (OD) of the supernatant was measured at 560 nm using a spectrophotometer. The percentage inhibition of hemolysis was calculated according to the following equation:
Qualitative Phytochemical Analysis
The crude extract and all its fractions were subjected to preliminary phytochemical screening for the presence of alkaloids, flavonoids, anthraquinone, C-glycosides, O-glycosides, saponin, phenol, tannin, sterols, Terpenoids following the procedure.11,12
Data Analysis
All experiments were performed in triplicate, and the results were expressed as mean ± SEM The data were analyzed by using one-way analysis of variance (ANOVA) with Tukey’s HSD as the post hoc in SPSS software version 20. Significant differences for all data sets were measured and designated as *P < 0.05 and **P < 0.01.
Ethical Consideration
This research received ethical clearance from the Asmara College of Health Science research ethical committee with Approval Number of ACHS/REC/2571. The volunteers that participated in the study were ahead informed regarding the full scope of the study and verbal consent followed by written informed consent was obtained.
Result and Discussion
The study was designed to investigate the in vitro anticoagulant activity of hot aqueous extract of Meriandra dianthera and its fractions. The percent extraction yield for crude extract and fractions of the plant is shown in Table 1. A total of 300 g leaf part of Meriandra dianthera was extracted by decoction method and gave a yield percent of 16.88% crude extract. Thirty grams of crude extract were then used for liquid–liquid partition to obtain a different percent of the four fractions.
 |
Table 1 Percent Yield of Extraction and Fractions of Meriandra dianthera Leaves |
The in vitro anticoagulant activity was measured by APTT and PT assays. APTT is able to measure the inhibition of intrinsic factors of the blood coagulation pathway such as factor XII, XI, IX, VIII, and V, while the PT assay gives a clear picture of the extrinsic pathway factors such as factors I, II, V, X and VII.13
The crude extract significantly prolonged the intrinsic clotting time to 52.63±0.26 s and 59.16±4.7 s at a concentration of 5 mg/mL and 10 mg/mL respectively compared with the control of 30.76±1.87 s (Figure 1). Aqueous residue fraction showed the most potent anticoagulant activity as it significantly prolonged (P < 0.01) the intrinsic clotting time even at the lowest concentrations of 500 μg/mL, 1mg/mL and 2 mg/mL into 42.8±1.77 s, 49.73±4.09 s, 52.8±7.31 s, respectively. At higher concentrations of 5 mg/mL and 10 mg/mL, the aqueous residue fraction also showed a highly significant increase (P < 0.001) in the intrinsic clotting time into 74.7±3.41 s and 125±13.98 s, respectively (Figure 2).
In the APTT assay, it is noteworthy to highlight that the aqueous residue fraction was more active compared to the crude extract. This indicates that the active anticoagulant constituents were concentrated in the aqueous residue fraction after the crude extract was purified by a liquid–liquid partition. The above explanation was substantiated by the fact that the remaining fractions, ie, the chloroform, ethyl acetate, and n-butanol fractions failed to increase the clotting time significantly even at higher concentrations (Figures 3–5). A similar result was obtained from a previous study conducted on aqueous leaf extract of Jatropha gossypiifolia which also found the residual aqueous fraction to be the fraction responsible for the anticoagulant activity in the crude extract of the plant.14 Considering the fact that prothrombin time was not affected by the crude extract and its fractions rule out the effect of the plant’s active constituents on the extrinsic and common pathway, clotting factors and also calcium chelating activity. Heparin, the standard drug used in this study, in the existence of antithrombin prolongs the APTT assay by inactivating factor XII, XI and IX.2 Although further study is required to identify the exact mechanism of action of the plant and its effect on individual coagulation factors, this study shows that the plant Meriandra dianthera exerts its potent anticoagulant activity by specifically acting/inhibiting on any of the factors involved in the intrinsic pathway.
In addition to their anticoagulant activity, the crude extract and its fractions were also evaluated for their red blood cell membrane stabilizing activity. The anti-inflammatory activity of the Meriandra dianthera extract and its fractions were assessed by the hypotonic solution-induced RBC membrane lysis inhibition assay. The osmotic fragility of the red blood cell is usually used to assess the effects of chemicals on the cell membrane as an in vitro assay.15 The erythrocyte membrane also functions as a model for lysosomal membrane and the RBC membrane stabilizing assay serves as a technique for the rapid screening of potential anti-inflammatory compounds. This is due to the observation that numerous agents capable of damaging the erythrocytes also result in releasing hydrolytic enzymes from lysosomes.16 In this study, as can be seen in Figure 6, the aqueous residue fraction followed by crude extract showed the highest membrane stabilizing activity of 76% and 73% respectively at a concentration of 3 mg/mL indicating their potency in preventing erythrocyte and lysosomal membrane lysis and also showing that the plant leaves also possess anti-inflammatory activity. The other fraction showed different levels of protection.
In the preliminary phytochemical screening, the crude extract and aqueous residue fraction which showed the highest membrane stabilizing activity were found to contain flavonoids, tannin, and saponins which is in line with previous studies that showed the role of these phytochemicals in stabilizing the erythrocyte and lysosomal membrane.17,18 The preliminary phytochemical test results of the crude and its fractions indicate the varying presence of flavonoids, phenols, saponins, sterols, terpenoids, tannins, anthraquinone, c-glycosides, and o-glycosides (Table 2). The presence of this wide range of phytochemical constituents indicates that the Meriandra dianthera leaf possesses numerous biologically active compounds, which could serve as a potential source of drugs in herbal medicine.
 |
Table 2 Result of the Preliminary Phytochemical Analysis on Meriandra dianthera Crude Extract and Its Fractions |
Conclusion
This study has clearly shown that the hot aqueous leaf extract of Meriandra dianthera has a potent in vitro anticoagulant activity. Further purification of the crude extract showed that the residual aqueous fraction was the fraction with the highest anticoagulant activity by specifically acting on the intrinsic coagulation pathway. This shows the plants promising potential as a new source of bioactive molecules for therapeutic purposes. In addition to the anticoagulant activity, the crude extract and residual aqueous fraction also showed the highest activity in the inhibition of hemolysis in red blood cells. We, therefore, recommend further identification and biological characterization of the anticoagulant potent molecules from this plant and to identify the specific clotting factors in the coagulation cascade that are most sensitive to the action of this plant.
Abbreviations
APTT, activated partial thromboplastin time; PT, prothrombin time; OD, optical density; ASA, acetylsalicylic acid; mg, milligram; mL, milliliter.
Acknowledgments
We acknowledge the faculty of Asmara College of Health Sciences, the National Health Laboratory and the Eritrean National Drug Quality Control Laboratory for their support in conducting this study.
Funding
The authors received no financial support for the research.
Disclosure
The authors report no conflict of interest in this work.
References
1. Cordier W, Cromarty AD, Botha E, et al. Effects of selected South African plant extracts on haemolysis and coagulation. Hum Exp Toxicol. 2011;31:250–257. doi:10.1177/0960327111398675
2. Hillman R, Ault KA, Leporrier M, Rinder HM. Hematology in Clinical Practice.
3. Anthony M, Yastik J. In vitro anti-platelet aggregation activity of the extract of Protorhus Iongifolia. The Journal of Nursing Education. 2011;50:140. doi:10.3928/01484834-20110131-04
4. Klausner A. Activating the body’s blood clot dissolvers: biotech’s new role. Bio/Technol. 1983;1(4):330–336.
5. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
6. Kumar S, Joseph L, George M, Sharma A. A review on anticoagulant/antithrombotic activity of natural plants used in traditional medicine. Int J Pharm Sci Rev Res. 2011;8:70–74.
7. Manicam C, Abdullah JO, Tohit ER, Seman Z, Chin SC, Hamid M. In vitro anticoagulant activities of Melastoma malabathricum Linn. aqueous leaf extract: a preliminary novel finding. J Med Plants Res. 2010;4:1464–1472.
8. Al-Saadi N. In vitro study of the anticoagulant activity of some plant extracts. Indian J Appl Res. 2011;3:120–122. doi:10.15373/2249555X/JULY2013/32
9. Bein E, Sweden MR. Regionala, Useful Trees and Shrubs in Eritrea: Identification, Propagation, and Management for Agricultural and Pastoral Communities. Technical Handbook; No. 12. Nairobi: Regional Soil Conservation Unit, RSCU/SIDA; 1996.
10. Shinde UA, Phadke AS, Nair AM, et al. Membrane stabilizing activity — a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70(3):251–257. doi:10.1016/S0367-326X(99)00030-1
11. Harborne JB. Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall Ltd; 1998.
12. Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. India: Nirali Prakashan; 2008.
13. Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112(4):e53–e60. doi:10.1161/CIRCULATIONAHA.104.478222
14. Félix-Silva J, Souza T, Camara RB, et al. In vitro anticoagulant and antioxidant activities of Jatropha gossypiifolia L. (Euphorbiaceae) leaves aiming therapeutical applications. BMC Complement Altern Med. 2014;14(1):405. doi:10.1186/1472-6882-14-405
15. Mohseni M, Chishti AH. The headpiece domain of dematin regulates cell shape, motility, and wound healing by modulating RhoA activation. Mol Cell Biol. 2008;28(15):4712–4718. doi:10.1128/MCB.00237-08
16. Brown JH, Mackey HK, Riggilo DA. A novel in vitro assay for anti-inflammatory agents based on stabilization of erythrocytes. Proc Soc Exp Biol Med. 1967;125(3):837–843. doi:10.3181/00379727-125-32219
17. El-Shabrawy O, El-Gindi OD, Melek FR, Abdel-Khalik SM, Haggag MY. Biological properties of saponin mixtures of Fagonia cretica and Fagonia mollis. Fitoterapia. 1997;68:219–222.
18. Middleton JM. Biological properties of plant flavonoids: an overview. Int J Pharmacogn. 1996;34(5):344–348. doi:10.1076/phbi.34.5.344.13245
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.