Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Mepolizumab in the treatment of eosinophilic chronic obstructive pulmonary disease
Authors Mkorombindo T , Dransfield MT
Received 5 April 2019
Accepted for publication 10 July 2019
Published 7 August 2019 Volume 2019:14 Pages 1779—1787
DOI https://doi.org/10.2147/COPD.S162781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Takudzwa Mkorombindo, Mark T Dransfield
Lung Health Center, Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Abstract: Despite maximal medical therapy, a subset of patients with chronic obstructive pulmonary disease continue to suffer acute exacerbations. It is also clear that a subset of this population has elevated blood eosinophils. In addition to clearly responding better to inhaled corticosteroids, it is also possible that this subgroup may benefit from biologic treatments targeting eosinophilic inflammation. Mepolizumab, a humanized monoclonal antibody against interleukin-5 (IL-5), may have a therapeutic effect in a subgroup of patients with COPD and eosinophilic airway inflammation. In this review, we discuss the biologic rationale for mepolizumab targeting IL-5 in eosinophilic COPD as well as the results of recently published clinical trials.
Keywords: COPD, acute exacerbations of COPD, eosinophils, mepolizumab
Introduction
The Global Burden of Disease study estimated that chronic obstructive pulmonary disease (COPD) affected 251 million people worldwide and was the third leading cause of death in 2016, causing more than three million deaths.1,2 In addition to the significant impact on mortality, COPD leads to substantial morbidity and loss of productivity and the cost of care in the United States alone is projected to increase from $32 billion in 2010 to $49 billion by 2020.3,4
There is a clear association between expenditures and the severity of the disease5 with hospitalizations for exacerbations being the major driver of costs.4 In addition, exacerbations are associated with accelerated loss of lung function, subsequent cardiovascular events, and prolonged impairments in quality of life, and thus prevention of these events is of paramount importance.6,7
The current Global Initiative for Obstructive Lung Disease (GOLD) recommendations for patients with continued COPD exacerbations despite treatment with a long-acting muscarinic agonist and long-acting β2-agonist (LAMA/LABA) or inhaled corticosteroid (ICS)/LABA combination includes escalation to triple therapy with ICS/LABA/LAMA in those with eosinophils more than 100 cells/μL or the addition of roflumilast in those with chronic bronchitis or azithromycin in former smokers.8–10 Despite the fact that the recent Informing the Pathway of COPD Treatment (IMPACT) study demonstrated a reduction in the risk of moderate or severe exacerbations in patients treated with ICS/LABA/LAMA therapy compared with either dual therapy, and that triple therapy reduced the risk of death compared with LAMA/LABA, approximately 50% of the patients in all study groups suffered an exacerbation during the one-year study.11 This highlights the well-recognized clinical reality that a substantial proportion of patients continue to exacerbate despite optimized medical therapy.12–14 Given the association between acute exacerbations and both short and long-term outcomes,7,15–17 there is an urgent need to advance pharmacotherapies targeting those with continued episodes.11,18,19
Eosinophilic COPD
COPD is heterogeneous with patients demonstrating varied clinical (degree of dyspnea, bronchitis symptoms, exercise capacity, exacerbation risk) and radiographic (CT emphysema, airways disease, bronchiectasis, air trapping) features. These observable phenotypes can be used to select optimal pharmacologic and non-pharmacologic treatments which can, in turn, improve outcomes. For example, lung volume reduction surgery improves survival, quality of life, exercise capacity, and dyspnea for patients with severe upper lobe predominant emphysema.20–22 Endobronchial valve therapy for heterogeneous and homogeneous but collateral-negative emphysema has also been proven to improve lung function (FEV1), dyspnea, quality of life and exercise tolerance23,24 and roflumilast improves lung function and reduces the frequency of exacerbations in patients with severe airflow limitation and chronic bronchitis.25,26 These successes strongly suggest that a phenotype-driven approach to treatment is both feasible and associated with improved outcomes.
The search for the underlying pathobiologic mechanisms, or endotypes, that drive these phenotypes has proven challenging, however, there is increasing evidence that a significant proportion of patients with COPD have eosinophilic airway inflammation, or an eosinophilic endotype, and these patients are exacerbation prone and far more likely to respond to treatment with ICS. Depending on the population studied and the definitions used, airway eosinophilic inflammation is present in between 20% and 40% of all COPD patients.27 Eosinophilic inflammation is associated with increased frequency of total exacerbations,28 and severe exacerbations requiring hospital admission, as well as a higher readmission rate.29 There is also an association between responsiveness to systemic and inhaled corticosteroids and the degree of eosinophilia.28,30–32 Airway eosinophils can be quantified by direct examination of bronchoscopic biopsy samples or bronchoalveolar lavage fluid as well as in induced sputum. Though these methods have been used successfully in research studies and some clinical settings, peripheral blood eosinophilia has been advanced as a more practical surrogate marker for airway eosinophilia.33,34 The correlation between blood and lung eosinophils has been debated with some studies showing a weak correlation35 and others showing a strong relationship between blood eosinophils more than 250 cells/μL and sputum, BAL and tissue eosinophils as well as basement membrane thickness.36 Even though the threshold continues to be debated, the current GOLD document suggests that a peripheral blood eosinophil counts greater than 300 cells/μL or a count greater than 100 cells/μL with two or more moderate or one severe exacerbation be used as a cutoff to consider treatment with ICS. Importantly, even after treatment with ICS, a substantial proportion of patients with COPD and elevated blood eosinophils continue to suffer exacerbations, and there has been substantial interest in the possible application of biologics developed for eosinophilic asthma in this population.11
Regulatory approval of mepolizumab in severe eosinophilic asthma
In 2015 the US Food and Drug Administration (FDA) approved mepolizumab, a monoclonal antibody against interleukin-5 (IL-5) for add-on therapy for the treatment of asthma with severe eosinophilia under trade name NUCALA. Mepolizumab is also available in the European Union, Canada, Japan, and more than ten other countries. FDA approval was based on clinical benefits demonstrated in placebo-controlled trials including Dose Ranging Efficacy and Safety with Mepolizumab (DREAM),37 Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma (MENSA),38 and Steroid Reduction with Mepolizumab Study (SIRIUS).39 In these studies, mepolizumab markedly improved asthma control by showing a significant reduction in dependency on oral steroids,37,38 reduction in exacerbations including those requiring hospitalization,39,40 and improvements in lung function and health-related quality of life.37,38,41
Mechanism of action of mepolizumab
Mepolizumab is a humanized monoclonal antibody that avidly binds circulating IL-5 (IgG1 kappa) preventing it from binding to alpha chain of IL-5 receptor (IL-5 Rα) on the surface of eosinophils. The IL-5 receptor complex causes activation of multiple signaling pathways including the release of cytokines, neuromediators, chemokines, as well as several kinases that promote eosinophil differentiation, proliferation, recruitment, and degranulation. The degranulation is responsible for airway injury through release of toxic granule proteins, cytokines, and reactive oxygen species, all of which promote airway inflammation.42–45 By inhibiting the formation of the IL-5 receptor complex, mepolizumab and the similar antibody reslizumab, block the activation of eosinophils thus causing disruption of normal eosinophil maturation and function resulting in decreased eosinophilic airway inflammation and reduced eosinophil survival.46 Another IL-5 monoclonal antibody being evaluated in COPD, benralizumab directly binds IL-5Rα on mature eosinophils and basophils causing antibody-dependent cytotoxicity (Figure 1).47
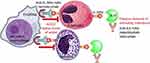 |
Figure 1 Mechanism of Action for monoclonal antibodies targeting IL-5. Mepolizumab and reslizumab bind and neutralize circulating IL-5 preventing interaction of IL5 with IL-5Rα. Benralizumab directly binds IL-5Rα activating NK cells and macrophage to induce antibody-mediated cytotoxicity of eosinophils and basophils. Republished with permission of Dove Medical Press Ltd, from Benralizumab: a unique IL-5 inhibitor for severe asthma, Tan LD, Bratt JM, Godor D, et al, 9, 2016; permission conveyed through Copyright Clearance Center, Inc.47 Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; IL, interleukin; mAB, monoclonal antibody; NK, natural killer. |
Safety of mepolizumab in asthma and other indications
Mepolizumab is approved for asthma and eosinophilic granulomatosis with polyangiitis (EGPA) and across studies for all indications, over 3000 study patients have received the drug. The most commonly reported adverse events are nasopharyngitis and headache with other reported events being upper respiratory infections, sinusitis, bronchitis, and injection-site pain. Subcutaneous mepolizumab has resulted in slightly higher rates of injection-site reactions compared to placebo and intravenous mepolizumab. The rate of all other events in the treatment groups was comparable to placebo.37,38 Because of the potential impact of mepolizumab on immune surveillance for neoplasia, there has also been concern about the risk of malignancies with the drug. In an open-label multicenter trial for mepolizumab by Rothenberg et al,48 patients with steroid-dependent primary hypereosinophilic syndromes (HES) were treated with mepolizumab 750 mg (every four weeks) with a reduction in blood eosinophils resulting in a steroid-sparing effect. In an open-label extension study, 78 subjects were monitored on mepolizumab for a median duration of 251 weeks.49 Serious adverse events were compared to incidence rates (age-gender-adjusted) from the Surveillance Epidemiology and End Results (SEER) Registry, and the occurrence of neoplasms was higher than the expected number of cases.48,49 During the five-year observation period, seven patients developed neoplasms including two with prostate cancer and another two with basal cell carcinomas. One subject developed multiple myeloma, one mycosis fungoides, and one angioimmunoblastic T cell lymphoma complicated by cardiopulmonary failure. The lack of a true control group and comparison with data from the SEER registry is problematic as it is well established that the risk of malignancy in HES is higher than the general population with a particular increase in the risk of T Cell Lymphoma.50–52 Additionally, HES is a systemic disorder for which several subjects were on other immunosuppressive medications that could place them at higher risk of malignancy.53,54 In the asthma studies DREAM, MENSA, and SIRIUS, malignant neoplasms were only reported in the placebo group of MENSA, occurring in three subjects (5%).37–39
Efficacy of mepolizumab in COPD
Mepolizumab was studied in two Phase III multinational, multicenter, randomized, placebo-controlled studies in patients with COPD (Table 1): 1) Mepolizumab vs Placebo as Add-on Treatment for Frequently Exacerbating COPD Patients (METREX) and 2) Mepolizumab vs Placebo as Add-on Treatment for Frequently Exacerbating COPD Patients Characterized by Eosinophil Level (METREO).55 Both studies examined the efficacy and safety of subcutaneous mepolizumab in patients with moderate to severe COPD and a history of exacerbations despite treatment with triple therapy (ICS/LABA/LAMA). The trials tested the currently approved eosinophilic asthma dose of 100-mg mepolizumab (both studies) as well as a higher a 300-mg dose (METREO) compared with placebo. Patients were required to have had at least two moderate COPD exacerbations (treated with antibiotics, oral steroids or both) or 1 severe exacerbation (requiring hospitalization) in the preceding 12 months and to have been on triple inhaler therapy for at least three months before screening. Patients with a diagnosis of current asthma or non-smokers were excluded. However, a historical diagnosis of asthma was allowed for current and former smokers.
 |
Table 1 Baseline characteristics, efficacy end points, and randomization |
METREX included 836 patients who were randomized to 100-mg of mepolizumab or placebo. Patients were stratified based on blood eosinophil count to the High Stratum defined by an eosinophil count of >150 cells/μL at enrollment or >300 cells/μL in the 12 months preceding enrolment (N=463) or to the Low Statum if neither of these criteria was met (N=374). The study met its primary endpoint for the High Stratum group with an annual rate of severe or moderate exacerbations of 1.40 in the mepolizumab group compared to 1.71 in placebo group (event rate ratio, 0.82; 95% confidence interval [CI], 0.68–0.98; adjusted p= 0.04) (Table 2). There was no significant difference in the primary endpoint in the overall group or the Low Stratum.
 |
Table 2 Primary and secondary efficacy end points |
METREO enrolled only subjects with an eosinophilic phenotype defined as a peripheral blood eosinophil count of at least 150 cells/μL at screening or greater than 300 cells/μL during the year prior to screening. Six hundred seventy-four patients were randomized to three arms (placebo, 100 mg mepolizumab, or 300 mg mepolizumab). Like METREX the primary endpoint was the annual rate of moderate or severe exacerbations, but no significant difference between study arms was observed.
For secondary endpoints, there was an increase in time to first moderate or severe exacerbation in METREX 192 days vs 141 days (hazard ratio, 0.75; 95% CI, 0.60 to 0.94; adjusted p=0.04). This effect was not reproduced in METREO at either dose. There was no significant difference between placebo and mepolizumab on all other endpoints including the rate of severe exacerbations requiring hospitalization or emergency department visit, COPD Assessment Test (CAT), and St. George’s Respiratory Questionnaire (SGRQ-C) scores at week 52 compared to baseline.
Meta-analysis of METREO & METREX
A pre-specified meta-analysis using data from both studies was performed to increase power and to more precisely determine the effect of mepolizumab on exacerbation endpoints. A total of 1136 patients from METREO and METREX were included [Mepolizumab 100 mg: N=456; Mepolizumab 300 mg: N=225, Placebo: N=455]. Analyses were conducted based on eosinophil profile (Figure 2). For patients with eosinophil counts of 300 cells/μL or greater the mean annual rate of moderate or severe exacerbations was 23% lower in mepolizumab 100 mg vs placebo (rate ratio, 0.77; 95% CI, 0.63–0.94). The meta-analysis also suggested a dose–response relationship between treatment effect and increasing eosinophil count, based on both screening and historical counts. There was a non-significant trend toward greater exacerbation rate in patients with screening counts less than 150 cells/μL and no historical count greater than 300 cells/μL.
 |
Figure 2 Exacerbations (moderate or severe) by screening eosinophil count. The rate of moderate or severe exacerbations quantified based on peripheral blood eosinophil count. At the time of screening, data are from a pre-specified meta-analysis from METREO and METREX. From The New England Journal of Medicine, Pavord ID, Chanez P, Criner GJ, et al, Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease, 377, 1613-1629. Copyright © (2017) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.55 |
Safety of mepolizumab in COPD
Mepolizumab was generally well tolerated in both METREX and METREO, similar to prior trials for other indications. The incidence of adverse events and serious adverse events was similar in Mepolizumab (100 and 300 mg) compared to placebo. There were no significant differences between groups in nasopharyngitis (14–21%), headache (9–15%), or pneumonia (9–11%) though the rate of pneumonia was relatively high compared with prior trials of dual and triple ICS-containing therapy in COPD11,56 Injection-site reactions across both trials occurred at similar rates in the mepolizumab 100 mg (2%), mepolizumab 300 mg (5%), and placebo (4%). No patients developed parasitic infections; however, screening for these before initiation of therapy for asthma or EGPA remains a recommendation. There were no cases of herpes zoster reported, but vaccination before initiation of therapy is also recommended. Less than 1% of the patients were diagnosed with neoplasms and no difference in this was detected across treatment groups.
Discussion
The overall results of METREX/METREO suggest that there may be a role for mepolizumab in reducing the risk of exacerbations in COPD patients with an elevated blood eosinophil level. METREX met its primary endpoint with a significant reduction in the rate of moderate-to-severe exacerbations in the high eosinophil stratum. However, this was not replicated in METREO raising questions about the robustness of the result. The pre-specified pooled analysis did show that in the population with blood eosinophils above 300 cells/μL there was a 23% reduction in mean annual rate of exacerbations with mepolizumab 100 mg vs placebo and that there appeared to be a linear relationship between treatment effect and the degree of blood eosinophilia. The meta-analysis also suggested a trend toward harm with a higher annual rate of exacerbations with mepolizumab compared with placebo in patients with a screening eosinophil count <150 cells/μL and no documented historical eosinophil count >300 cells/μL though mechanisms for this potential chance observation are uncertain.
While these data suggest a possible role for mepolizumab in COPD, the magnitude of benefit does not appear as significant or as consistent compared to that observed in the asthma drug development program. The rate ratio for exacerbations for mepolizumab compared to placebo in the DREAM and MENSA trials combined was 0.51 (95% CI 0.42, 0.62) compared to 0.77 (95% CI, 0.63–0.94) in the METREX/METREO analysis. In addition to the clear and consistent demonstration of benefits on overall exacerbation risk, the registration trials in asthma also showed improvements in a number of other key outcomes including oral steroid dose, hospitalizations, lung function, and quality of life.37–39,57 For example, SIRIUS demonstrated glucocorticoid sparing effects of mepolizumab in asthma (50% reduction in daily oral steroid use) and improved symptoms and quality of life as evidenced by improvement in Asthma Control Questionnaire 5 (ACQ5) and St. George’s Respiratory Questionnaire (SGRQ)39 scores. In MENSA, there was a significant reduction (53%) in the rate of clinically significant exacerbations in subjects on high dose ICS and additional controller medication, and a very impressive reduction (61%) in exacerbations requiring hospitalizations or emergency department visits.38 There are several possible explanations for the differences in results between asthma and COPD trials. First, the entry criteria for eosinophilic asthma were defined differently and perhaps more stringently than the criteria for eosinophilic COPD. In DREAM, subjects were required to meet one or more of an induced sputum eosinophil count of 3% or greater, a peripheral blood eosinophil count of 300 cells/μL or more, an exhaled nitric oxide concentration (FENO) of 50 parts per billion (ppb) or more, or prompt deterioration of asthma control after a 25% or less reduction in regular maintenance inhaled or oral corticosteroids. Similarly, all patients in MENSA were required to have a screening peripheral blood eosinophils count greater than 150 cells/μL or a historical value >300 cells/μL. Second, the heterogeneity of exacerbation mechanisms in COPD is likely greater than that in asthma, even among those with an eosinophilic phenotype, with older age, comorbidities, and bacterial infections playing a more significant role. As such, the biological impact of IL-5 targeted treatment in COPD might be expected to be less.
The United States FDA’s Pulmonary Allergy Drugs Advisory Committee voted against approval of mepolizumab as add-on therapy for COPD on July 25, 2018. The committee cited concerns about efficacy and questioned patient selection. In particular, the committee noted the failure to replicate the exacerbation reduction finding in the two studies, the lack of benefit on key secondary outcomes, and was concerned that the inclusion of patients with concomitant asthma might have biased the findings toward benefit. With a wholesale acquisition cost (not inclusive of rebates, allowances, and returns) of approximately USD 2500 per 100-mg dose, equaling an annual cost of around USD 30,000,58 there were also concerns about cost-effectiveness given the limitations in the efficacy data. The FDA panel did feel the trials had demonstrated that mepolizumab was likely safe in COPD but voted against efficacy and against approval stating more clinical trial data were needed. GlaxoSmithKline indicated they would continue to work with the FDA to determine the next steps to provide the appropriate clinical data.59
Other IL-5 targeting drugs have also been approved for eosinophilic asthma including benralizumab, a monoclonal antibody against the alpha-chain of IL-5 Receptor, and reslizumab, a monoclonal anti-IL-5 antibody similar to mepolizumab but delivered intravenously. Benralizumab has also been tested explicitly in COPD patients. In a Phase II trial, benralizumab did not show a decrease in the annual rate of exacerbations compared with placebo in patients with moderate to severe COPD with at least one exacerbation and a sputum eosinophil count >3% within previous 12 months or at screening.60 In two phase three multicenter, double-blinded, placebo-controlled trials 1) Benralizumab Efficacy in Moderate to Very Severe COPD With Exacerbation History (GALATHEA) and 2) Efficacy and Safety of Benralizumab in Moderate to Very Severe COPD With Exacerbation History (TERRANOVA) Benralizumab again failed to meet its primary endpoint of reduction of rate of exacerbations though the full results have not been published.61,62 Taken together, the results of the mepolizumab and benralizumab development programs suggest more work is needed to establish IL-5 targeted treatment as a viable therapeutic approach in COPD.
A path forward for these drugs might include targeting patients with more significantly elevated eosinophils at enrolment (or historically) as suggested by the METREO/METREX meta-analysis, though the prevalence of eosinophils counts greater than 300 cells/μL in these studies was only 36%, a rate comparable to those observed in other populations of exacerbating patients.11 This will make recruitment for such clinical trials very challenging and markedly increase costs. An alternative approach would be to test the utility of anti-IL5 drugs at the time of hospitalization for an eosinophilic exacerbation as a means to accelerate recovery and delay the time to re-exacerbation or readmission. These studies also present significant logistic challenges but the value of an effective treatment in that setting is clear.
Disclosure
Mark T Dransfield reports grants from the American Lung Association, NIH, Department of Defense and Department of Veterans Affairs; consulting fees from AstraZeneca, GlaxoSmithKline, Mereo, Pulmonx, PneumRx/BTG, and Quark; contracted clinical trial support from AstraZeneca, Boehringer Ingelheim, Boston Scientific, GALA, GlaxoSmithKline, Nuvaira, Pulmonx, and PneumRx/BTG. The authors report no other conflicts of interest in this work.
References
1. Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi:10.1016/S2213-2600(17)30293-X
2. World Health Organization. Projections of mortality and causes of death, 2016 to 2030. Available from: https://www.who.int/healthinfo/global_burden_disease/projections/en/ Accessed December 1, 2018.
3. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi:10.1016/S0140-6736(96)07492-2
4. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228. doi:10.3109/15412555.2010.481697
5. Hilleman DE, Dewan N, Malesker M, Friedman M. Pharmacoeconomic evaluation of COPD. Chest. 2000;118(5):1278–1285. doi:10.1378/chest.118.5.1278
6. Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi:10.1164/rccm.201711-2239OC
7. Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi:10.1164/rccm.201605-1014OC
8. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017
9. Kardos P, Mokros I, Sauer R, Vogelmeier CF. Health status in patients with COPD treated with roflumilast: two large noninterventional real-life studies: DINO and DACOTA. Int J Chron Obstruct Pulmon Dis. 2018;13:1455–1468. doi:10.2147/COPD.S159827
10. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
11. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
12. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
13. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
14. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
15. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
16. Lange P, Halpin DM, O’Donnell DE, MacNee W. Diagnosis, assessment, and phenotyping of COPD: beyond FEV(1). Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss:3–12. doi:10.2147/COPD.S85976
17. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
18. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24(4):457–463. doi:10.1007/s11606-009-0907-y
19. Sahin H, Varol Y, Naz I, Aksel N, Tuksavul F, Ozsoz A. The effect of pulmonary rehabilitation on COPD exacerbation frequency per year. Clin Respir J. 2018;12(1):165–174. doi:10.1111/crj.12507
20. Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):2018–2027. doi:10.1164/ajrccm.160.6.9902117
21. Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi:10.1056/NEJMoa030287
22. Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg. 2012;143(1):47–54, 54 e41. doi:10.1016/j.jtcvs.2011.09.050
23. Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145(5):981–991. doi:10.1378/chest.13-1579
24. Donohue JF, Niewoehner D, Brooks J, O’Dell D, Church A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15:78. doi:10.1186/1465-9921-15-78
25. Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694. doi:10.1016/S0140-6736(09)61255-1
26. Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast–the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi:10.1186/1465-9921-12-122
27. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47.
28. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi:10.1164/rccm.201108-1553OC
29. Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi:10.1016/j.chest.2016.10.003
30. Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27(5):964–971. doi:10.1183/09031936.06.00072105
31. Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014;44(3):789–791. doi:10.1183/09031936.00062614
32. Martinez FJ, Rabe KF, Calverley PMA, et al. Determinants of response to roflumilast in severe chronic obstructive pulmonary disease. Pooled analysis of two randomized trials. Am J Respir Crit Care Med. 2018;198(10):1268–1278. doi:10.1164/rccm.201712-2493OC
33. Kolsum U, Damera G, Pham TH, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol. 2017;140(4):1181–1184 e1187. doi:10.1016/j.jaci.2017.04.027
34. Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349. doi:10.2147/COPD.S152291
35. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0
36. Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
37. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
38. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
39. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
40. Yancey SW, Ortega HG, Keene ON, et al. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139(4):1167–1175 e1162. doi:10.1016/j.jaci.2016.08.008
41. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X
42. Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EA. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61(5):589–597. doi:10.1111/j.1398-9995.2006.01060.x
43. Possa SS, Leick EA, Prado CM, Martins MA, Tiberio IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 2013;4:46. doi:10.3389/fphar.2013.00046
44. Pazdrak K, Stafford S, Alam R. The activation of the Jak-STAT 1 signaling pathway by IL-5 in eosinophils. J Immunol. 1995;155(1):397–402.
45. Sano M, Leff AR, Myou S, et al. Regulation of interleukin-5-induced beta2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol. 2005;33(1):65–70. doi:10.1165/rcmb.2005-0076OC
46. Keating GM. Mepolizumab: first global approval. Drugs. 2015;75(18):2163–2169. doi:10.1007/s40265-015-0513-8
47. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71–81. doi:10.2147/JAA.S78049
48. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–1228. doi:10.1056/NEJMoa070812
49. Roufosse FE, Kahn JE, Gleich GJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131(2):
50. Simon HU, Plotz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999;341(15):1112–1120. doi:10.1056/NEJM199910073411503
51. Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):389–413. doi:10.1016/j.iac.2007.07.002
52. Schwartz RS. The hypereosinophilic syndrome and the biology of cancer. N Engl J Med. 2003;348(13):1199–1200. doi:10.1056/NEJMp030019
53. Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21(5):852–858. doi:10.1681/ASN.2009101043
54. Vial T, Descotes J. Immunosuppressive drugs and cancer. Toxicology. 2003;185(3):229–240.
55. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208
56. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7
57. Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–993. doi:10.1056/NEJMoa0805435
58. Review IfCaE. Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. Available from: https://icer-review.org/wp-content/uploads/2018/04/Asthma-Revised-Report-FOR-PUBLICATION-11.13.2018.pdf. Accessed December 1, 2018.
59. GSK reports on outcome of the FDA advisory committee on mepolizumab for the treatment of COPD patients on maximum inhaled therapy. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-reports-on-outcome-of-the-fda-advisory-committee-on-mepolizumab-for-the-treatment-of-copd-patients-on-maximum-inhaled-therapy/. Accessed December 1, 2018.
60. Brightling CE, Bleecker ER, Panettieri RA
61. AstraZeneca. Efficacy and safety of benralizumab in moderate to very severe chronic obstructive pulmonary disease (COPD) with exacerbation history (TERRANOVA). Available from: https://clinicaltrials.gov/ct2/show/NCT02155660NLMidentifier:NCT02155660
62. AstraZeneca. Benralizumab efficacy in moderate to very severe chronic obstructive pulmonary disease (COPD) with exacerbation history (GALATHEA). Available from: https://clinicaltrials.gov/ct2/show/NCT02138916NLMidentifier:NCT02138916
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
