Back to Journals » Cancer Management and Research » Volume 13
Meningeal “Lazarus Response” to Lorlatinib in a ROS1-Positive NSCLC Patient Progressing to Entrectinib
Authors Facchinetti F, Levy A, Ammari S, Naltet C, Lavaud P, Aldea M, Vasseur D, Planchard D, Besse B
Received 6 January 2021
Accepted for publication 17 March 2021
Published 26 March 2021 Volume 2021:13 Pages 2805—2810
DOI https://doi.org/10.2147/CMAR.S292730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Francesco Facchinetti,1,2 Antonin Levy,3,4 Samy Ammari,5 Charles Naltet,6 Pernelle Lavaud,6 Mihaela Aldea,6 Damien Vasseur,7 David Planchard,6 Benjamin Besse2,6
1Predictive Biomarkers and Novel Therapeutic Strategies in Oncology, Inserm U981, Gustave Roussy Cancer Center, Villejuif, France; 2Université Paris-Saclay, Faculté de Médecine, Le Kremlin-Bicêtre, France; 3Department of Radiation Oncology, Institut d’Oncologie Thoracique (IOT), Gustave Roussy Cancer Center, Villejuif, France; 4INSERM U1030, Molecular Radiotherapy, Gustave Roussy, Université Paris-Saclay, Villejuif, France; 5Department of Radiology, Gustave Roussy Cancer Center, Villejuif, France; 6Department of Medical Oncology, Institut d’Oncologie Thoracique (IOT), Gustave Roussy Cancer Center, Villejuif, France; 7Department of Medical Biology and Pathology, Gustave Roussy Cancer Center, Villejuif, France
Correspondence: Benjamin Besse
Department of Medical Oncology, Institut d’Oncologie Thoracique (IOT), Gustave Roussy Cancer Center, Villejuif, France
Email [email protected]
Background: ROS1 tyrosine kinase inhibitors (TKIs) have showed activity and efficacy in ROS1-rearranged non-small cell lung cancer (NSCLC). In the clinical practice, besides the utilization of crizotinib, less is known about the best treatment strategies involving additional, new-generation TKIs for the sequential treatment of ROS1-positive NSCLC patients.
Case Presentation: A patient suffering from a ROS1-rearranged lung adenocarcinoma, after receiving cisplatin-pemetrexed chemotherapy, was treated with entrectinib, a new-generation ALK/ROS1/NTRK inhibitor. After 16 months, central nervous system (CNS) metastases appeared, without extra-cerebral disease progression. Stereotactic brain radiotherapy was performed and entrectinib was maintained, due to the global systemic disease control. Approximately one month after radiotherapy, thoracic and meningeal progressions were detected, the latter highly symptomatic with neurocognitive disorders, visual hallucinations and worsening of psycho-motor impairment. A lumbar puncture was positive for tumor cells and for an EZR-ROS1 fusion. The administration of lorlatinib (a third-generation ALK/ROS1 inhibitor) prompted an extremely rapid improvement of clinical conditions, anticipating the positive results observed at radiologic evaluation that confirmed the disease response still ongoing after nine months since treatment start.
Discussion: With the expanding availability of targeted agents with differential activity on resistance mechanism and on CNS disease, choosing wisely the best treatment strategies is pivotal to assure the best clinical outcomes in oncogene-addicted NSCLC patients. Here we have reported lorlatinib reverted an almost fatal meningeal carcinomatosis developing during entrectinib in a ROS1-positive NSCLC patient.
Keywords: tyrosine kinase inhibitors, TKI, central nervous system, CNS, brain, lung cancer, radiotherapy
Introduction
The current availability of several tyrosine kinase inhibitors (TKIs) for each oncogenic molecular alterations (eg EGFR mutations, ALK and ROS1 rearrangements) allows the significant improvement of survival outcomes in non-small cell lung cancer (NSCLC) patients. ROS1 (whose rearrangement is present in 1–2% of NSCLC patients) is a receptor tyrosine kinase (RTK) structurally similar to ALK (4–7% of NSCLC), and since the first-generation TKI crizotinib, several other inhibitors are active against both RTKs.1,2 Novel-generation ROS1 TKIs include ceritinib, entrectinib, cabozantinib, brigatinib, talactrectinib (DS-6051b), lorlatinib, repotrectinib.3
Notably, besides crizotinib, entrectinib has recently received the approval by EMA, FDA and the Japanese agency PMDA for the treatment of ROS1-driven lung cancer patients, while lorlatinib, a third-generation ALK/ROS1 TKI, is the treatment of choice at crizotinib progression.4,5 Besides the sequence crizotinib-lorlatinib, less is known concerning treatment strategies involving TKIs other than crizotinib administered as upfront targeted agents, considering the relative rarity of ROS1 positivity detection in NSCLC patients.
Here, we report the history of a patient suffering from ROS1-rearranged NSCLC developing meningeal progression while undergoing entrectinib. Switching to lorlatinib engendered an impressive clinic-radiological disease response still ongoing.
Case Presentation
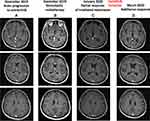
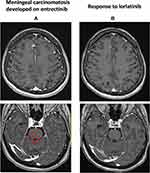
In February 2018, a 62-year-old woman with a previous light smoking history (five packs/year) was diagnosed with lung adenocarcinoma with pleural, pericardial, lymph nodal and bone metastases. After two cycles of first-line chemotherapy with cisplatin and pemetrexed (best response stable disease) and the detection of an EZR-ROS1 fusion on liquid biopsy (InVisionFirst-Lung amplicon-based assay),6 confirmed by FISH on tumor sample, the patient received entrectinib 600 mg daily since July 2018. No relevant side effects were recorded and partial response was achieved after two months. In November 2019, multiple brain lesions were detected (Figure 1A), without extra-cerebral progression. Patient complained the recent onset of right harm and gait impairment, conditioning an ECOG performance status (PS) of 2. Entrectinib was suspended and fractionated stereotactic brain radiotherapy was performed on the five largest metastases (the remaining ones being sub-centimetric) (Figure 1B). In January 2020, after entrectinib resumption, new brain MRI and CT scan respectively detected meningeal carcinomatosis (Figure 2A), with partial regression of the irradiated metastases (Figure 1C), and thoracic progression (carcinomatous lymphangitis and parenchymal lesions increase, pleural effusion onset, Figure 3A). A lumbar puncture was positive for tumor cells and for an EZR-ROS1 fusion was detected in the cerebrospinal fluid (CSF) by the Oncomine™ Lung cfDNA Assay (Thermo Fisher Scientific), without additional mutations across the kinase domain, potentially implicated in resistance. Meanwhile, neurocognitive disorders worsened, with the onset of visual hallucinations and psycho-motor deficiency (ECOG PS 3). After having received the approval within a “temporary authorisation for use” program, third line lorlatinib 100 mg daily was initiated at the end of January 2020 and led to a rapid neurological improvement in the very next days, leading to patient’s discharge. CT scan and brain MRI of March 2020, performed six weeks after treatment start, detected thoracic response (Figure 3B), with major regression of brain and meningeal involvement (Figure 1D, Figure 2B). At the last follow-up of October 2020 after nine months since lorlatinib initiation, the patient is in good clinical conditions (ECOG PS 1), intra- and extra-cranial disease response is maintained, and the TKI is well tolerated without toxic effects (eg hypercholesterolemia, hypertriglyceridemia, weight increase, neurocognitive disorders).7
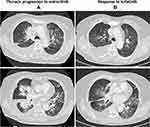 |
Figure 3 Thoracic response to lorlatinib. (A) Progression to entrectinib. (B) Response to lorlatinib after six weeks of treatment. |
Discussion
Several inhibitors are currently available for the treatment of ROS1-positive NSCLC. Targeted therapy is recommended as the first-line treatment of choice.8,9 The evidence sustaining these recommendations is low, considering that only Phase I–II, non-randomized trials have been conducted in this molecular subset of patients.4,5,10–16 Upfront chemotherapy can indeed be considered, especially in the case of low-burden disease without relevant symptoms, waiting for complete molecular information. Targeted first-line treatment options are represented by crizotinib and entrectinib, with a potential preference for the second in case of brain metastases.3 Ceritinib and entrectinib failed in showing activity after progression on crizotinib.14,17 Lorlatinib is active after crizotinib, while its administration at entrectinib progression has been far less evaluated (only one patient across prospective study and retrospective series).5,18–22 Albeit lorlatinib is not licensed yet for the treatment of ROS1-positive NSCLC according to health authorities, its administration at progression to a previous ROS1 TKI is mentioned in both ESMO and NCCN guidelines.8,23
Central nervous system (CNS) metastases are common in ROS1-rearranged lung adenocarcinoma patients, documented in 20–30% and 35–50% of treatment-naïve and post-crizotinib settings, respectively.24,25 Albeit lorlatinib is known to act in CNS disease at crizotinib progression in ALK- and ROS1-driven lung cancers, only one recent case report described a radiological dimensional decrease of brain metastases in a ROS1-positive patient receiving lorlatinib at entrectinib progression.26 Entrectinib is deemed to harbor a better CSF penetration compared to crizotinib, potentially explaining better CNS activity.4 In the present case, the absence of the resistance mutation is the CSF, in particular G2032R, suggest that the progressive disease might be due to an insufficient CNS exposure to the TKI. Lorlatinib was preferred to an attempt of entrectinib dose escalation, given its well-known CNS activity and the fact that a fast response was required considering critical patient’s conditions. The impressive CSF penetration of lorlatinib (75% of which pass through the blood-brain-barrier) has probably contributed both to the extremely rapid disease response and to the prolonged CNS disease control observed.27,28 As a result, rapid and dramatic improvement of neurological condition, such as in our patient, is expected, before any formal response by imaging assessment.
In the clinical situation here presented, lorlatinib was likely the only inhibitor (together with repotrectinib)15 to tame disease aggressiveness at the CNS level at the moment of entrectinib progression. The recent availability of several targeted agents for ROS1-rearranged NSCLC requires a careful process of decision-making in order to guarantee the best patients’ outcomes.
Ethical Considerations
The patient provided written consent to publish her clinical history.
Disclosure
Dr Facchinetti declares editorial activities sponsored by BMS and Roche. Dr Lavaud declares travel accommodations: Astellas-Pharma, Astra Zeneca, Ipsen, Janssen Oncology, Mundi Pharma. Dr Planchard declares consulting: advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Clinical trials research: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmun, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo. Travel, Accommodations, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer. Pr. Besse declares sponsored research at Gustave Roussy Cancer Center, 4DPharma, Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Ignyta, IPSEN, Inivata, Janssen, Merck KGaA, MSD, Nektar, Onxeo, OSE immunotherapeutics, Pfizer, Pharma Mar, Roche-Genentech, Sanofi, Servier, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma, and Tolero Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Facchinetti F, Rossi G, Bria E, et al. Oncogene addiction in non-small cell lung cancer: focus on ROS1 inhibition. Cancer Treat Rev. 2017;55:83–95. doi:10.1016/j.ctrv.2017.02.010
2. Sehgal K, Piper-Vallillo AJ, Viray H, Khan AM, Rangachari D, Costa DB. Cases of ROS1-rearranged lung cancer: when to use crizotinib, entrectinib, lorlatinib, and beyond? Precis Cancer Med. 2020;3:17. doi:10.21037/pcm-2020-potb-02
3. Facchinetti F, Friboulet L. Profile of entrectinib and its potential in the treatment of ROS1-positive NSCLC: evidence to date. Lung Cancer Targets Ther. 2019;10:87–94. doi:10.2147/LCTT.S190786
4. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three Phase 1–2 trials. Lancet Oncol. 2020;21(2):261–270. doi:10.1016/S1470-2045(19)30690-4
5. Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691–1701. doi:10.1016/S1470-2045(19)30655-2
6. Mezquita L, Swalduz A, Jovelet C, et al. Clinical relevance of an amplicon-based liquid biopsy for detecting ALK and ROS1 fusion and resistance mutations in patients with non–small-cell lung cancer. JCO Precis Oncol. 2020;4:
7. Nagasaka M, Ge Y, Sukari A, Kukreja G, Ou SHI. A user’s guide to lorlatinib. Crit Rev Oncol Hematol. 2020;151:102969. doi:10.1016/j.critrevonc.2020.102969
8. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv192–iv237. doi:10.1093/annonc/mdy275
9. Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040–1091. doi:10.1200/JCO.20.03570
10. Shaw AT, Riely GJ, Bang Y-J, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126. doi:10.1093/annonc/mdz131
11. Wu YL, Yang JCH, Kim DW, et al. Phase II study of crizotinib in east asian patients with ROS1-positive advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi:10.1200/JCO.2017.75.5587
12. Michels S, Massutí B, Schildhaus HU, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14(7):1266–1276. doi:10.1016/j.jtho.2019.03.020
13. Landi L, Chiari R, Tiseo M, et al. Crizotinib in MET deregulated or ROS1 rearranged pretreated non-small-cell lung cancer (METROS): a Phase II, prospective, multicentre, two-arms trial. Clin Cancer Res. 2019;25(24):7312–7319. doi:10.1158/1078-0432.CCR-19-0994
14. Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. doi:10.1200/JCO.2016.71.3701
15. Cho BC, Drilon A, Doebele R, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J Clin Oncol. 2019;37(suppl):abstr9011. doi:10.1200/JCO.2019.37.15_suppl.9011
16. Papadopoulos KP, Borazanci E, Shaw AT, et al. U.S. Phase I first-in-human study of taletrectinib (DS-6051b/AB-106), a ROS1/TRK inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2020;26(18):4785–4794. doi:10.1158/1078-0432.CCR-20-1630
17. Drilon A, Siena S, Ou SHI, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7(4):400–409. doi:10.1158/2159-8290.CD-16-1237
18. Frost N, Christopoulos P, Kauffmann-Guerrero D, et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol. 2021;13:1758835920980558. doi:10.1177/1758835920980558
19. Hochmair MJ, Fabikan H, Illini O, et al. Later-line treatment with lorlatinib in ALK-and ROS1-rearrangement-positive nsclc: a retrospective, multicenter analysis. Pharmaceuticals. 2020;13(11):371. doi:10.3390/ph13110371
20. Peled N, Gillis R, Kilickap S, et al. GLASS: global lorlatinib for ALK(+) and ROS1(+) retrospective study: real world data of 123 NSCLC patients. Lung Cancer. 2020;148:48–54. doi:10.1016/j.lungcan.2020.07.022
21. Zhu VW, Lin YT, Kim DW, et al. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor–refractory ALK-positive or ROS1-positive NSCLC. J Thorac Oncol. 2020;15(9):1484–1496. doi:10.1016/j.jtho.2020.04.019
22. Lee J, Sun JM, Lee SH, et al. Efficacy and safety of lorlatinib in Korean non–small-cell lung cancer patients with ALK or ROS1 rearrangement whose disease failed to respond to a previous tyrosine kinase inhibitor. Clin Lung Cancer. 2019;20(3):215–221. doi:10.1016/j.cllc.2018.12.020
23. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®) for non-small cell lung cancer (version 2.2019). Available from: https://www.nccn.org/professionals/physician_gls/recently_updated.aspx.
24. Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non–small-cell lung cancer. J Clin Oncol. 2017;2017:
25. Patil T, Smith DE, Bunn PA, et al. The incidence of brain metastases in stage IV ROS1-rearranged non–small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol. 2018;13(11):1717–1726. doi:10.1016/j.jtho.2018.07.001
26. Coleman N, Yousaf N, Arkenau HT, Welsh L, Popat S. Lorlatinib salvages central nervous system–only relapse on entrectinib in ROS1-positive NSCLC. J Thorac Oncol. 2020;15(8):e142–e144. doi:10.1016/j.jtho.2019.12.115
27. Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK Inhibitors in preclinical models. Cancer Cell. 2015;28(1):70–81. doi:10.1016/j.ccell.2015.05.010
28. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi:10.1016/S1470-2045(17)30680-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.