Back to Journals » Clinical Epidemiology » Volume 11
Medication Use During Pregnancy in Mainland China: A Cross-Sectional Analysis of a National Health Insurance Database
Authors Zhang J , Ung COL , Wagner AK, Guan X , Shi L
Received 11 September 2019
Accepted for publication 20 November 2019
Published 10 December 2019 Volume 2019:11 Pages 1057—1065
DOI https://doi.org/10.2147/CLEP.S230589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Jingyuan Zhang,1 Carolina Oi Lam Ung,2 Anita Katharina Wagner,3 Xiaodong Guan,1,3,4 Luwen Shi1,4
1Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University, Beijing, People’s Republic of China; 2State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, People’s Republic of China; 3Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; 4International Research Center for Medicinal Administration, Peking University, Beijing, People’s Republic of China
Correspondence: Luwen Shi; Xiaodong Guan
Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University, Beijing, People’s Republic of China
Tel/Fax +86-010-82805019; +86-13911232199
Email [email protected]; [email protected]
Purpose: This study aims to illustrate the prevalence and patterns of medication use among pregnant women in mainland China.
Patients and methods: Hospital and drugstore service data for a nationally representative sample of basic medical insurance (BMI) beneficiaries in 2015 were obtained from the China Health Insurance Association (CHIRA) database. A total of 7946 women who had singleton deliveries in 2015, aged between 12 and 54, and whose records in the CHIRA database covered at least one trimester were included in this study. We conducted descriptive analyses of sample characteristics, medication use prevalence, and number and types of medications used.
Results: We found that 11.7% of women used at least one medication during the course of pregnancy (median number of medications used = 6.7). Medication use was more common among those who were older, residing in Eastern China, or employed. Most commonly used medication groups by the Anatomical Therapeutic Chemical Classification System were B (Blood and blood forming organs, 49.3%), A (Alimentary tract and metabolism, 48.1%), G (Genito urinary system and sex hormones, 38.1%) and J (Antiinfectives for systemic use, 31.6%). Intravenous solutions, vitamins and minerals, progestogens, and beta-lactam antibacterials were the most frequently used medications from each of these four ATC groups, respectively. Moreover, 7.1% used at least one medication contraindicated in pregnancy.
Conclusion: This study showed that around one in 10 women used medication during pregnancy in mainland China and found possible cases of inappropriate or unsafe medication use.
Keywords: medication use, pregnancy, China, cross-sectional, health insurance database
Introduction
Medication use during pregnancy has drawn global attention since the thalidomide disaster in 1960’s.1 Considering the potential risks of maternal medication exposure to the fetus, clinicians and women must carefully weigh the risks and benefits of medication use during pregnancy. On one hand, most medications and their metabolites can pass the placental barrier. Except for medications indicated for pregnant women, most medications should thus be avoided during pregnancy.2 On the other hand, withholding necessary medication treatments may place the life of the mother and the fetus in danger.3,4
There is a scarcity of information on medication use during pregnancy. In 1987, the World Health Organization (WHO) Regional Office for Europe conducted a collaborative study on drug use in pregnancy to assess maternal medication use internationally.5–7 Since then, as an effective approach exploring potential risks related to medication use during pregnancy, various drug utilization studies have been conducted to assess the prevalence and patterns of medication use during pregnancy in different countries and regions.8–10 Furthermore, researchers have identified factors associated with maternal medication use such as age under 2511 or above 35 years,12 lower education level,13,14 less household income,14 presence of smoking habits,11 and poorer health status.11,13
The number of live births was 17.6 million in 2017 in China.15 Previous studies found a wide variation in the prevalence of medication use by pregnant women across different regions ranging from 12.4% to 75.9%.16–20 Dietary supplements (38.7–65.2%),20 traditional Chinese medicines (TCM, 7.8–47.5%),17,18 antibiotics (1.7–34.3%),16–18 and hormones (3.7–7.2%)17,18 were reportedly the most commonly used medications during pregnancy in the country. In these studies, patient-reported data were collected through survey studies at local hospitals that involved small sample sizes. Due to the size, location and design of the studies, the findings could easily be subject to selection and recall biases which might contribute to variations in the prevalence estimation. So far, no national data about the prevalence of medication use during pregnancy in China is available. To fill this important knowledge gap and to better inform clinical practice, we aimed to estimate the prevalence of medication use among pregnant women across China based on a national health insurance database. We further assessed the prevalence and patterns of medication use stratified by the stage of pregnancy, and identified the most commonly used medications in each trimester.
Materials and Methods
Data Source
By the end of 2015, over 1.33 billion people in China (97% of the population) were enrolled in one of the three public health insurance schemes: (1) the Urban Employee Basic Medical Insurance (UEBMI) which was mandatory for urban employees; (2) the Urban Resident Basic Medical Insurance (URBMI) which covered mainly the unemployed, children, students, and the disabled in urban areas; and (3) the New Rural Cooperative Medical Scheme (NRCMS) for rural residents.21 Medical service utilization information of every UEBMI and URBMI (hereafter jointly referred to as BMI) beneficiary in hospitals and BMI designated drugstores is routinely collected in BMI databases at city level and includes: (1) BMI beneficiaries’ demographic information, eg, unique personal identifiers, birth date, and gender; (2) inpatient and outpatient medical encounter information, eg, principal diagnosis for each encounter coded with International Classification of Diseases 10th Revision (ICD-10) codes, admission and discharge dates, and healthcare facility information; and (3) all medical service utilization data at each encounter, eg the name, date, price, and quantity of medications dispensed and procedures performed whether they are covered by the BMI or not.
The China Health Insurance Association (CHIRA) employs a two-stage systematic sampling design to obtain a national representative sample of BMI beneficiaries and extracts cross-sectional medical service utilization data annually from the city-level BMI databases. The extract forms the unique national health insurance database known as the CHIRA database. Using sampling proportional to size, the percentage of sampled BMI beneficiaries is 2% in the centrally administered municipalities and provincial capitals, 5% in the prefecture-level cities, and 10% in the counties.22
The data in our study were obtained from the CHIRA database in 2015, which at the time contained inpatient and outpatient diagnosis and medical service utilization data of 37.3 million BMI beneficiaries sampled from 82 cities nationwide, representing about 2% of the total population in Mainland China. Since the CHIRA data was de-identified, this study was exempt from Institutional Review Board review by the Ethics Committee of Peking University Health Science Center, Beijing, China (No. IRB00001052-17022).
Study Population
Women aged 12–54 with a singleton delivery between January 1, 2015 and December 31, 2015 were identified in the CHIRA database from one of the two beneficiary groups predefined according to ICD-10: (1) codes O80-O84 and (2) codes O60-O75. As direct measures of gestational age such as the last menstrual period (LMP) were lacking in the database, we used a delivery date-based algorithm validated by Li et al (2013) to assign pregnancy trimesters. We assumed a gestational duration of 270 days (245 days for preterm delivery), with three 90-day trimesters during pregnancy (65 days of the third trimester for preterm delivery) based on admission date for delivery.23 Only women who had a singleton delivery in 2015, were between 12 and 54 years old at delivery, and whose records in the CHIRA database covered at least one trimester were included in this study.
Medication Information
In the CHIRA database, medications were categorized as Western medicines or TCM according to the registration status as shown distinctively in the registration approval number given by the National Medicinal Product Administration (NMPA). Western medicines were further classified according to the WHO Anatomical Therapeutic Chemical (ATC) classification system. Consistent with previous studies, we classified vitamins (A11A-A11J), minerals (A12A-A12C), iron preparations (B03A), and folic acids (B03B) as medications indicated in pregnancy.9,24,25 We also identified the US Food and Drug Administration (FDA) Pregnancy Risk Category X (contraindicated during pregnancy) medications as listed in the MicroMedex and the Monthly Index of Medical Specialties (MIMS) databases.26–28 Medication dispensing records with missing information (name of medication, dispensing date) were excluded from the analysis.
Outcome Measures and Statistical Analysis
To measure the extent to which medications were used in each trimester and throughout the entire pregnancy, we estimated the prevalence of overall medication use as the proportion of women with any medication dispensed. The prevalence of specific medication use was defined as the percentage of women with a specific medication dispensed of all women with at least one medication dispensed in the time frame of interest. Among women with at least one medication dispensed in each trimester and during the entire pregnancy, we calculated the median number of different medications dispensed based on their generic names.
We conducted descriptive analyses of sample characteristics, medication use prevalence, number and types of medications used. Numerical variables were summarized with medians and categorical variables with proportions. To test differences between groups, we used Chi-square tests for categorical variables and Wilcoxon rank-sum tests for numerical variables.
Results
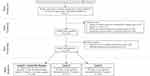
A total of 11,373 women aged 12–54 with a singleton delivery in 2015 were identified in the national database, of which 7946 women had records in the CHIRA database that covered at least one trimester. According to our estimates of each gestational period, among these 7946 women, there were 2896, 5377, and 7946 women included in the analysis of medication use during the first trimester/entire pregnancy, second, and third trimester, respectively (Figure 1, Supplementary material 1).
Demographic Information of the Study Sample and the Users
The majority of the pregnant women included in this study were between 25 and 29 years old, lived in Eastern China, were covered by URBMI and underwent spontaneous delivery. We found that 11.7% of 2896 women (from the entire pregnancy group) used at least one medication during the entire course of pregnancy. The prevalence of medication use during the entire pregnancy and in each trimester was further stratified based on the demographic characteristics of the women included in their corresponding groups as shown in Table 1. Medication use during pregnancy was more common among older women with the prevalence among women aged 35 or above three times higher than that among women under 25 years old. Women in Eastern areas or those with a job (enrolled in the UEBMI) had a considerably higher prevalence of medication use, ranging from five to ten times higher than their counterparts in Middle and Western regions and those enrolled in the URBMI. The same associations were also observed in each of the three-trimester groups.
 |
Table 1 Demographic Information of the Study Sample and Medication Users |
The Types and Number of Medications Used During Pregnancy and in Each Trimester
As shown in Table 2, the prevalence of medication use including and excluding vitamins and minerals during pregnancy were similar (11.7% and 11.5%). Overall, only 2.0% of pregnant women used iron preparations or folic acid during pregnancy with an increasing prevalence from the first to the third trimester (0.4–1.7%). The proportion of women using Western medicines during pregnancy (11.1%) was more than twice that of women using TCM (5.2%). The median number of medications used during pregnancy per women was 6.7. Women who used four or more medications accounted for more than half (52.5%) of women who used at least one medication during pregnancy.
 |
Table 2 The Types and Number of Medications Used During the Course of Pregnancy and in Each Trimester |
As shown in Supplementary material 2, among the women who used at least one medication during the course of pregnancy, 65.5% used medications in only one trimester and 12.4% used medications throughout all three trimesters. One in two women (50.1%) used medication during the first trimester.
Most Commonly Used Western Medicines During Pregnancy
Table 3 shows the prevalence of Western medicine use by ATC Class (first code level) with the highest prevalence for class B (blood and blood forming organs, 49.3%), followed by class A (alimentary tract and metabolism, 48.1%), class G (genito urinary system and sex hormones, 38.1%) and class J (antiinfectives for systemic use, 31.6%). Further information about the most commonly used Western medicines by ATC third-level codes and by generic names is provided in Supplementary material 3 and Supplementary material 4. The most commonly used ATC code B medicines included solutions for injection or infusion (B05X, B05B) such as glucose (28.9%) and sodium chloride (23.9%), iron preparations (B03A) and vitamin B12 and folic acid (B03B). In ATC group A, multivitamins (A11A), vitamin C (A11G), other vitamin preparations (A11H), such as potassium chloride (8.3%) and magnesium sulfate (6.2%) as well as omeprazole (3.0%) were the most frequently used medicines. The use of progestogens (G03D) was significantly more prevalent during the first trimester (53.5% in the group T1) than in the other two trimesters (10.0% in group T2, 3.0% in group T3). The prevalence of antiinfective medicine use (ATC code J) remained relatively stable throughout the three trimesters (21.2% in group T1, 23.3% in group T2, 21.9% in group T3) and amoxicillin (beta-lactam antibacterial agent, J01C) was the most commonly used antibiotic.
 |
Table 3 The Prevalence of Use of Western Medicines by ATC 1st Level Code Among Medication Users in Each Group (%) |
As shown in Table 4, 7.1% of women (from the entire pregnancy group) were exposed to potentially harmful medicines (FDA Category X) during pregnancy. The prevalence of use in group T1, group T2 and group T3 were 5.3%, 1.9% and 5.9%, respectively. The most frequently used Category X medicines were hormonal preparations such as oxytocin (3.0%) and misoprostol (0.9%). The use of the category X antiviral drug ribavirin during pregnancy (1.5%) was also prominent.
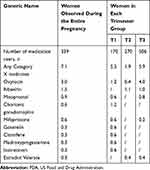 |
Table 4 The Prevalence of FDA Category X Medicines Among Medication Users in Each Group (%) |
Discussion
In this study, we found that 11.7% of women in mainland China used at least one medication during the course of pregnancy. The prevalence of overall medication use was much lower than those reported in other countries: 46.2–57.6% in Nordic countries,9,14,29,30 63.5–68.8% in the North America and Japan,28,31,32 and over 90% in Germany and France.33,34 However, the median number of 6.7 medications among medication users found in this study was higher than the average of 1–4 medications per user reported in most developed countries.24 Given diverse study methods (including differences in recruitment strategies, data sources, criteria for medications included, and analysis methods) and health system contexts (health insurance system and drug policies), interpretation of differences in medication use in pregnancy across studies is limited. However, the relatively low prevalence of medication use among pregnant women and the higher number of medication used per user observed in this study suggest that international variations do exist.
In this study, the prevalence of overall medication use varied by trimester and sample characteristics. More intense medication use during the first trimester (50.1%) is consistent with previous studies,28 which may be due to a potentially higher demand for medication treatment in early pregnancy, or a result of unidentified or unplanned pregnancy.35,36 Similar to previous researchers,37–39 we found that older women, those enrolled in the UEBMI, or those living in Eastern areas had significantly higher medication use compared to their respective counterparts. Women enrolled in UEBMI or living in Eastern areas tended to have higher income levels and better access to medical services which might lead to more intense medication use.
A few medications accounted for a large proportion of total antenatal medication use. Exposure to intravenous solutions, vitamins and minerals, progesterone, and antibiotics like amoxicillin, were prevalent during pregnancy, which is in accordance with findings by others.33,40,41 However, the use of iron preparations or folates was uncommon (2.0%). That may be because our study did not capture information about free distribution of folates and iron supplements in communities. These products are recommended for pregnant women by WHO (2016) to prevent neural tube defects,42 a preventable cause of morbidity. In China, a nationwide program was launched in 2009 by the National Health Commission that aimed to prevent neural tube defects. All women of child-bearing age who planned to become pregnant were given access to folates and iron supplements free of charge from their community health service centers.43
The common use of intravenous solutions accompanied with other injections during pregnancy found in this study may be mainly due to the typical prescribing pattern involving potential overuse of intravenous medications in mainland China.44 Given that the oral route should be used whenever possible and intravenous fluids can usually be avoided in patients who are capable of eating and drinking,45 the appropriateness and safety of the prevalent use of intravenous infusion among pregnant women in China remain questionable. It is also noteworthy that 53.5% of women with medication exposure during the first trimester used progestogens (G03D), possibly in an attempt to prevent early pregnancy loss. However, the American College of Obstetrician and Gynecologists (ACOG) Guideline has stated that conclusive evidence supporting progestogen use to avoid early pregnancy loss is lacking.46,47 Physicians should balance benefits with risks based on the patient’s condition and prescribe with caution only when clearly indicated.
The use of contraindicated medications during pregnancy warrants special attention. The prevalence of potentially harmful (FDA Category X) medicine use (7.1%) was higher than those reported in the United States (2.9%),27 Canada (2.5–3.9),28,48 the United Kingdom (1.0%),49 France (1.6%),34 Serbia (0%),50 and North Ethiopia (0%).51 The most commonly used Category X medicines was oxytocin (2.95%), especially in the third trimester which may be used in the treatment of delay in labour.52 The use of ribavirin was also prominent (1.5%) despite the availability of more effective and safer alternatives (eg, Category B medicines such as sofosbuvir or Category C medicines such as oseltamivir) for pregnant women in China.53,54 Efforts are needed to understand the reasons for use of Category X products and, where appropriate, to facilitate interventions to avoid unnecessary medication exposure during pregnancy.
To our best knowledge, this is the first study illustrating the prevalence and patterns of medication use during pregnancy in mainland China based on a unique and representative national health insurance database. Compared to traditional health insurance data which only cover reimbursable items, the CHIRA database can be considered an “extensive health insurance database” that contains information on all medications dispensed to BMI beneficiaries, both prescription medicines and over-the-counter products (OTC), whether or not they are covered by the local BMI schemes. The CHIRA database enabled a more accurate and reliable assessment of medication use than other data sources and may be considered an important database to advance Chinese population-based research for integration of real-world evidence into clinical practice and local policymaking.
However, our results may be subject to several limitations. First, because the CHIRA database mainly contains information on BMI beneficiaries and rural residents mostly enroll in the NRCMS, our findings may not be generalizable to pregnant women in rural areas. Second, we employed an algorithm to estimate gestational stages, which may lead to misclassification of medication exposure periods. However, given that the validated algorithm has been proved to perform well in classifying prenatal medication exposure status,23 its impact on the results should be limited. Third, we only included pregnancies resulting in a singleton live birth. These women may have had better health status than those who had miscarriages, terminations of pregnancy, and stillbirths. This may result in some bias to our estimates of prevalence of medication use during pregnancy. Fourth, we examined high-risk (category X) medication use according to the FDA pregnancy risk category, which has been replaced by a narrative labelling system in 2015 because of its oversimplification and incompetence to guide clinical practice. Our reference to the former FDA pregnancy risk category can only provide a rough description of medication use risk during pregnancy on a population basis. The appropriateness and safety of medication use require further evaluation on a case-by-case basis. Finally, our analysis was based on medication dispensing records from pharmacies and did not take into account other potential pathways of medication supply; we also had no data on the treatment adherence of pregnant women, which may lead to some overestimation of medication exposure in pregnancy. In addition, since there were only a limited number of variables captured in the CHIRA database, we were unable to fully explore all the possible factors associated with medication use during pregnancy, such as parity, complication of chronic diseases and so on.
Conclusion
This study showed that in 2015 around one in 10 women used at least one medication during pregnancy in mainland China; there may be cases of inappropriate or unsafe medication use. More efforts should be made to understand the reasons for possible over- and underuse of medications in pregnancy and to design, implement, and test interventions to optimize safe and appropriate use of medications during pregnancy.
Acknowledgments
We are grateful for staff of the China Health Insurance Association (CHIRA) database for their assisting in collecting the data.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. XDG and JYZ conceived the study idea and contributed to the study design. JYZ implemented literature review, cleaned and checked the data, wrote the code, did the analysis for the study. JYZ and COLU wrote the initial draft, and amended the final manuscript. XDG, AKW supervised and checked the analysis, gave suggestions to the initial draft, and reviewed the final manuscript. LWS contributed to data access, supervised the study design and data interpretation, and reviewed the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 71774005). The funders were not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang TJ, Yang TS, Liang HM. Thalidomide and congenital abnormalities. Lancet. 1963;1(7280):552–553. doi:10.1016/S0140-6736(63)91347-3
2. De Jong-van den Berg LTW, Vandenberg PB, Haaijer-Ruskamp FM, Dukes MNG, Wesseling H. Investigating drug-use in pregnancy - methodological problems and perspectives. Pharm Weekblad-Sci Ed. 1991;13(1):32–38. doi:10.1007/BF01963881
3. Rubin PC. Prescribing in pregnancy. General principles. Br Med J (Clin Res Ed). 1986;293(6559):1415–1417. doi:10.1136/bmj.293.6559.1415
4. Sørensen HT, Nielsen GL, Andersen AMN, et al. Drug use in pregnancy. Principal problems and a review of newer utilization studies. Clin Res Regul Aff. 1996;13(3–4):181–197. doi:10.3109/10601339609035951
5. Sabo A, Stanulovic M, Jakovljevic V, Grujic Z. Collaborative study on drug use in pregnancy: the results of the follow-up 10 years after (Novi Sad Centre). Pharmacoepidemiol Drug Saf. 2001;10(3):229–235. doi:10.1002/pds.585
6. Hoffmann A, Jager O, Peiker G, Reimann I. Drug use in pregnancy - East German data of an international collaborative study. Int J Clin Pharmacol Ther. 1992;30(11):462–464.
7. Reimann IR, Karpinsky C, Hoffmann A. Epidemiological data on drug use during pregnancy in Thuringia, East Germany, 1993. Int J Clin Pharmacol Ther. 1996;34(2):80–83.
8. Dejongvandenberg L. Drug utilization studies in pregnancy: what can they contribute to safety assessment? Pharm World Sci. 1993;15(4):171–172. doi:10.1007/BF01880561
9. Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K. Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106 000 pregnancies in Norway 2004–2006. Br J Clin Pharmacol. 2008;65(5):653–660. doi:10.1111/j.1365-2125.2008.03102.x
10. Grzeskowiak LE, Gilbert AL, Morrison JL. Investigating outcomes associated with medication use during pregnancy: a review of methodological challenges and observational study designs. Reprod Toxicol. 2012;33(3):280–289. doi:10.1016/j.reprotox.2012.01.006
11. Smolina K, Hanley GE, Mintzes B, Oberlander TF, Morgan S. Trends and determinants of prescription drug use during pregnancy and postpartum in British Columbia, 2002–2011: a population-based cohort study. PLoS One. 2015;10(5):16. doi:10.1371/journal.pone.0128312
12. Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):8. doi:10.1016/j.ajog.2011.02.029
13. Riley EH, Fuentes-Afflick E, Jackson RA, et al. Correlates of prescription drug use during pregnancy. J Womens Health. 2005;14(5):401–409. doi:10.1089/jwh.2005.14.401
14. Olesen C, Thrane N, Henriksen TB, Ehrenstein V, Olsen J. Associations between socio-economic factors and the use of prescription medication during pregnancy. Eur J Clin Pharmacol. 2006;62(7):547–553. doi:10.1007/s00228-006-0119-x
15. National Health Commission of the People’s Republic of China. Statistical Bulletin on Medical and Health Development in 2017. National Health Commission of the People’s Republic of China; 2018. Available from: http://www.moh.gov.cn/guihuaxxs/s10743/201806/44e3cdfe11fa4c7f928c879d435b6a18.shtml.
16. Huang Y. Study on perception of drug use safety and statu quo of drug use among pregnant women. World Latest Med Inf. 2016;88:150–151.
17. Han C, Feng X, Tang H, Zhong L. A drug utilization investigation on 550 pregnant women. Chin J Pharmacovigil. 2012;09:558–560.
18. Yi J, Cen N. A survey of drug use and safety of pregnant women in a hospital of China. China Pharm. 2009;20(29):2314–2317.
19. Feng Y, Wu G, Yan W, Chen L. Drug utilization study on pregnant women in Guangxi Zhuang Autonomous Region of China. China Pharm. 2011;28:2605–2607.
20. Zhu X, Qi X, Hao J, et al. Pattern of drug use during the first trimester among Chinese women: data from a population-based cohort study. Eur J Clin Pharmacol. 2010;66(5):511–518. doi:10.1007/s00228-009-0781-x
21. The State Council Information Office of the People’s Republic of China. The Right to Development: China’s Philosophy, Practice and Contribution.
22. Xia L, Li J-H, Zhao K, Wu H-Y. Incidence and in-hospital mortality of acute aortic dissection in China: analysis of China Health Insurance Research (CHIRA) Data 2011. J Geriatric Cardiol. 2015;12(5):502–506. doi:10.11909/j.issn.1671-5411.2015.05.021
23. Li Q, Andrade SE, Cooper WO, et al. Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiol Drug Saf. 2013;22(5):524–532. doi:10.1002/pds.v22.5
24. Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf. 2011;20(9):895–902. doi:10.1002/pds.2184
25. Bakker MK, Jentink J, Vroom F, Van den Berg PB, de Walle HEK, de Jong-van Den Berg LTW. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. Bjog. 2006;113(5):559–568. doi:10.1111/j.1471-0528.2006.00927.x
26. Gerald G, Briggs RKF, Towers CV, Forinash AB. Drugs in Pregnancy and Lactation.
27. Lee E, Maneno MK, Smith L, et al. National patterns of medication use during pregnancy. Pharmacoepidemiol Drug Saf. 2006;15(8):537–545. doi:10.1002/(ISSN)1099-1557
28. Daw JR, Mintzes B, Law MR, Hanley GE, Morgan SG. Prescription drug use in pregnancy: a retrospective, population-based study in British Columbia, Canada (2001–2006). Clin Ther. 2012;34(1):239–249. doi:10.1016/j.clinthera.2011.11.025
29. Malm H, Martikainen J, Klaukka T, Neuvonen PJ. Prescription drugs during pregnancy and lactation - a Finnish register-based study. Eur J Clin Pharmacol. 2003;59(2):127–133. doi:10.1007/s00228-003-0584-4
30. Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden - assessed by the prescribed drug register and the medical birth register. Clin Epidemiol. 2011;3:43–50. doi:10.2147/CLEP.S16305
31. Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191(2):398–407. doi:10.1016/j.ajog.2004.04.025
32. Nishigori H, Obara T, Nishigori T, et al. Drug use before and during pregnancy in Japan: the Japan environment and children’s study. Pharmacy. 2017;5(2). doi:10.3390/pharmacy5020021
33. Egen-Lappe V, Hasford J. Drug prescription in pregnancy: analysis of a large statutory sickness fund population. Eur J Clin Pharmacol. 2004;60(9):659–666. doi:10.1007/s00228-004-0817-1
34. Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc J. Prescription of drugs during pregnancy in France. Lancet. 2000;356(9243):1735–1736. doi:10.1016/S0140-6736(00)03209-8
35. Floyd RL, Decoufle P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;17(2):101–107. doi:10.1016/S0749-3797(99)00059-8
36. Webster WS, Freeman JA. Prescription drugs and pregnancy. Expert Opin Pharmacother. 2003;4(6):949–961. doi:10.1517/14656566.4.6.949
37. Donati S, Baglio G, Spinelli A, Grandolfo ME. Drug use in pregnancy among Italian women. Eur J Clin Pharmacol. 2000;56(4):323–328. doi:10.1007/s002280000149
38. Refuerzo JS, Blackwell SC, Sokol RJ, et al. Use of over-the-counter medications and herbal remedies in pregnancy. Am J Perinatol. 2005;22(6):321–324. doi:10.1055/s-2005-873235
39. Odalovic M, Kovacevic SV, Nordeng H, Ilic K, Sabo A, Tasic L. Predictors of the use of medications before and during pregnancy. Int J Clin Phar. 2013;35(3):408–416. doi:10.1007/s11096-013-9750-7
40. Beyens MN, Guy C, Ratrema M, Ollagnier M. Prescription of drugs to pregnant women in France: the HIMAGE study. Therapie. 2003;58(6):505–511. doi:10.2515/therapie:2003082
41. Gagne JJ, Maio V, Berghella V, Louis DZ, Gonnella JS. Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol. 2008;64(11):1125–1132. doi:10.1007/s00228-008-0546-y
42. World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: World Health Organization; 2016. Available from:: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/.
43. Zhang X, Liu J, Jin Y, et al. Folate of pregnant women after a nationwide folic acid supplementation in China. Matern Child Nutr. e12828.
44. Yuan S. China should reduce the overuse of intravenous infusion. Br Med J. 2014;348:g1262. doi:10.1136/bmj.g1262
45. National Institute for Health and Care Excellence (NICE). Intravenous fluid therapy in adults in hospital: clinical guideline [CG174]. 2017. Available from: https://www.nice.org.uk/guidance/cg174.
46. The American College of Obstetrician and Gynecologists (ACOG). Early pregnancy loss: practice bulletin [Number 200]. 2015. Available from: https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Early-Pregnancy-Loss.
47. World Health Organization; Stuart MC, Kouimtzi M, Hill S. WHO Model Formulary 2008. Geneva: World Health Organization; 2009. Available from: http://www.who.int/iris/handle/10665/44053.
48. Yang T, Walker MC, Krewski D, et al. Maternal characteristics associated with pregnancy exposure to FDA category C, D, and X drugs in a Canadian population. Pharmacoepidemiol Drug Saf. 2008;17(3):270–277. doi:10.1002/(ISSN)1099-1557
49. Hardy JR, Leaderer BP, Holford TR, Hall GC, Bracken MB. Safety of medications prescribed before and during early pregnancy in a cohort of 81,975 mothers from the UK general practice research database. Pharmacoepidemiol Drug Saf. 2006;15(8):555–564. doi:10.1002/(ISSN)1099-1557
50. Odalovic M, Kovacevic SV, Ilic K, Sabo A, Tasic L. Drug use before and during pregnancy in Serbia. Int J Clin Pharm. 2012;34(5):719–727. doi:10.1007/s11096-012-9665-8
51. Molla F, Assen A, Abrha S, et al. Prescription drug use during pregnancy in Southern Tigray region, North Ethiopia. BMC Pregnancy Childbirth. 2017;17:6. doi:10.1186/s12884-017-1359-8
52. The WHO Reproductive Health Library. WHO Recommendation on the Use of Oxytocin Alone for Treatment of Delay in Labour. Geneva: World Health Organization;2014
53. Ghulmiyyah LM, Alame MM, Mirza FG, Zaraket H, Nassar AH. Influenza and its treatment during pregnancy: a review. J Neonatal Perinatal Med. 2015;8(4):297–306. doi:10.3233/NPM-15814124
54. Spera AM, Eldin TK, Tosone G, Orlando R. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8(12):557–565. doi:10.4254/wjh.v8.i12.557
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.