Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Medication management patterns among Medicare beneficiaries with chronic obstructive pulmonary disease who initiate nebulized arformoterol treatment
Authors Celli BR, Navaie M, Xu Z, Cho-Reyes S, Dembek C, Gilmer TP
Received 22 December 2018
Accepted for publication 25 April 2019
Published 15 May 2019 Volume 2019:14 Pages 1019—1031
DOI https://doi.org/10.2147/COPD.S199251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Bartolome R Celli,1 Maryam Navaie,2,3 Zhun Xu,4 Soojin Cho-Reyes,2 Carole Dembek,5 Todd P Gilmer4
1Chronic Obstructive Pulmonary Disease Center, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA; 2Global Strategy, Advance Health Solutions, LLC, New York, NY, USA; 3School of Professional Studies, Columbia University, New York, NY, USA; 4Department of Family Medicine and Public Health, University of California San Diego, La Jolla, CA, USA; 5Global Health Economics and Outcomes Research, Sunovion Pharmaceuticals Inc, Marlborough, MA, USA
Purpose: Global evidence-based treatment strategies for chronic obstructive pulmonary disease (COPD) recommend using long-acting bronchodilators (LABDs) as maintenance therapy. However, COPD patients are often undertreated. We examined COPD treatment patterns among Medicare beneficiaries who initiated arformoterol tartrate, a nebulized long-acting beta2 agonist (LABA), and identified the predictors of initiation.
Methods: Using a 100% sample of Medicare administrative data, we identified beneficiaries with a COPD diagnosis (ICD-9 490–492.xx, 494.xx, 496.xx) between 2010 and 2014 who had ≥1 year of continuous enrollment in Parts A, B, and D, and ≥2 COPD-related outpatient visits within 30 days or ≥1 hospitalization(s). After applying inclusion/exclusion criteria, three cohorts were identified: (1) study group beneficiaries who received nebulized arformoterol (n=11,886), (2) a subset of the study group with no LABD use 90 days prior to initiating arformoterol (n=5,542), and (3) control group beneficiaries with no nebulized LABA use (n=220,429). Logistic regression was used to evaluate predictors of arformoterol initiation. Odds ratios (ORs), 95% confidence intervals (CIs), and p values were computed.
Results: Among arformoterol users, 47% (n=5,542) had received no LABDs 90 days prior to initiating arformoterol. These beneficiaries were being treated with a nebulized (50%) or inhaled (37%) short-acting bronchodilator or a systemic corticosteroid (46%), and many received antibiotics (37%). Compared to controls, beneficiaries who initiated arformoterol were significantly more likely to have had an exacerbation, a COPD-related hospitalization, and a pulmonologist or respiratory therapist visit prior to initiation (all p<0.05). Beneficiaries with moderate/severe psychiatric comorbidity or dual-eligible status were significantly less likely to initiate arformoterol, as compared to controls (all p<0.05).
Conclusion: Medicare beneficiaries who initiated nebulized arformoterol therapy had more exacerbations and hospitalizations than controls 90 days prior to initiation. Findings revealed inadequate use of maintenance medications, suggesting a lack of compliance with evidence-based treatment guidelines.
Keywords: long-acting beta2-agonists, arformoterol, nebulized therapy, COPD, Medicare, treatment patterns
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States (US),1,2 affecting 16 million people,1,3 and accounting for more than $32.1 billion in medical costs.4 By 2020, the economic burden of COPD is projected to increase to $49 billion.4 To better manage the clinical spectrum of COPD, US and international guidelines recommend the use of maintenance therapy with long-acting bronchodilators (LABDs).5–7 Despite these guidelines, adequate management of COPD remains a challenge, as does curtailing its rising economic toll.8,9
Past studies on COPD treatment patterns have revealed that health care providers do not consistently prescribe medications in accordance with evidence-based strategies.10–16 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) therapeutic strategy sheds light on this issue by highlighting that two out of three COPD patients receive pharmacotherapy that is inconsistent with evidence-based guidelines.5 A closer examination of these inconsistencies reveals that patients are often undertreated with LABDs, despite exacerbations and multiple hospitalizations.15,17–20 A recent study conducted on commercially insured patients found that 55% of the members diagnosed with COPD did not receive guideline-recommended maintenance medications.18 In that study, only 64% of COPD patients with a history of exacerbations received a prescription for maintenance medication. Similar findings of undertreatment of COPD have been documented in managed Medicare populations where research has shown 40% to 71% receive no maintenance treatment, including almost 69% with moderate to severe COPD.13 Additional studies on medication adherence patterns among Medicare beneficiaries with COPD have documented that 40% to 50% fail to use any maintenance medications.21,22 Although maintenance medication use by Medicare beneficiaries has improved over time,23 suboptimal disease management continues to be a persistent clinical challenge.23,24
Given that 12% of all Medicare beneficiaries and 17% of dual-eligible beneficiaries (ie, Medicare and Medicaid recipients) suffer from COPD,25 it is imperative to better understand specific medication treatment patterns to improve care management. The primary objective of our study was to examine COPD medication management patterns among Medicare beneficiaries who initiated nebulized arformoterol tartrate (neb arformoterol), a long-acting beta2 agonist (LABA), for maintenance therapy. Our secondary objective was to identify the predictors associated with initiating neb arformoterol.
Methods
Data source
Our study used a 100% sample of Medicare administrative data from the Chronic Condition Warehouse at the Centers for Medicare and Medicaid Services (CMS). All data were obtained from Master Beneficiary Summary Files. Information specific to hospitalizations (including skilled nursing facility) was extracted from MedPar files. Data regarding outpatient services were extracted from Carrier and Outpatient (OP) files. Home health care utilization was obtained from Home Health (HH) files. Medication use was extracted from Part D and Durable Medical Equipment (DME) files. The data were analyzed on the secure Virtual Research Data Center (VRDC) provided by CMS. All individual Medicare beneficiaries were provided a unique identifier for this study. No identifying information such as name, social security number, and Medicare number were used. The Human Research Protections Program at the University of California San Diego approved our study which was subject to a data use agreement with CMS.
Sample and cohort selection
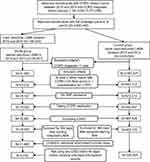
Our primary study population was 10,371,035 Medicare beneficiaries who had a COPD-related claim (ie, International Classification of Disease, 9th Revision, Clinical Modification codes 490.xx, 492.xx, 494.xx, 496.xx) between 2010 and 2014 and a COPD diagnosis before January 1, 2013 (Figure 1). Our study sample was further refined to 3,642,497 beneficiaries who met the inclusion criteria of having continuous coverage under Medicare Parts A, B, and D. Among these beneficiaries, 106,442 used nebulized LABA therapy during 2010 and 2014 and 3,536,055 beneficiaries did not use nebulized LABA therapy during this time period.
To support the analyses described below, a start date was randomly assigned to control group beneficiaries consistent with the distribution of initiation dates for nebulized LABA therapy in the study group. Both samples (study group and controls) were limited to beneficiaries: (a) with a diagnosis of COPD for at least one year, (b) with at least 2 office claims with COPD 30 days apart or one hospitalization for COPD in the prior year, (c) without an admission to a skilled nursing facility, (d) who used COPD medication(s), and (e) without end-stage renal disease who survived for at least 364 days following initiation of nebulized LABA therapy or their assigned start date. We further limited the sample of users of nebulized LABA therapy to those using arformoterol only. As a final restriction, we limited both samples to beneficiaries who did not use any LABD medication in the 90 days prior to initiation of nebulized LABA therapy or their assigned start date. After applying these criteria, the following three cohorts of Medicare beneficiaries were identified: (1) a study group of beneficiaries who were treated with neb arformoterol (n=11,886), (2) a subset of the study group who had no use of LABD in the 90 days prior to initiating neb arformoterol (n=5,542), and (3) a control group of beneficiaries who had no claims for a nebulized LABA between 2010 and 2014 and no use of LABD in the 90 days prior to their assigned start date (n=220,429).
Measures
The following measures were extracted from Medicare files: (a) demographic characteristics such as age, gender, and race; (b) dual-eligibility status; (c) comorbidities as measured by the Chronic Illness and Disability Payment System diagnostic classification;26 (d) COPD medications based on drug refill rates specific to short-acting bronchodilators (SABDs) which included short-acting muscarinic antagonists (SAMAs) and short-acting beta-agonists (SABAs), LABAs, long-acting muscarinic antagonists (LAMAs), inhaled corticosteroids (ICS), systemic corticosteroids (CS), methylxanthines, phosphodiesterase (PDE4) inhibitors, non-specific PDE inhibitors, and mucolytics; (e) antibiotic use; (f) oxygen use; (g) frequency of exacerbations; and (h) health resource utilization (HRU) including medical equipment use, hospitalizations (all cause and COPD-specific), intensive care unit stays, pulmonary specialist visits (inpatient and outpatient), emergency department (ED) visits, use of skilled nursing facilities, and home health care visits.
Data analysis
Univariate and bivariate analyses were conducted to examine beneficiary characteristics and treatment patterns including computing comparative means, frequencies, and proportions. Cell sizes below 11 were suppressed per CMS’ requirements. Logistic regression analysis was used to evaluate the predictors associated with initiation of neb arformoterol. Odds ratios (ORs), 95% confidence intervals (CIs), and p-values were computed. p<0.05 denoted statistical significance. All analyses were performed using SAS Enterprise Guide 7.1.
Results
Sociodemographic characteristics
The majority of Medicare beneficiaries were ≥70 years old, female, non-Hispanic white, and from either the southern or Midwest region of the US. In addition, more than one-third of the beneficiaries were dual-eligible (Table 1).
 | Table 1 Sociodemographic characteristics of study cohorts |
Clinical characteristics and medication management patterns
Among beneficiaries who initiated neb arformoterol (n=11,886), the most frequent comorbidities were cardiovascular, musculoskeletal, gastrointestinal, and psychiatric disease (Table 2). Medication treatment patterns revealed that 47% (n=5,542) of beneficiaries had received no LABDs 90 days prior to initiating neb arformoterol, and 55% had received no SABDs (Figure 2).
 | Table 2 Comorbidities and types of COPD medications filled by Medicare beneficiaries 90 days before initiating nebulized arformoterol by (N=11,886) |
The most common COPD treatments received by beneficiaries before initiating neb arformoterol were inhaled (49%) or nebulized (45%) SABDs, systemic CS (48%), followed by LABA/ICS combination therapy (37%), and monotherapy LAMAs (33%) (Figure 3). Many beneficiaries (38%) were also receiving antibiotics. Most of the beneficiaries who had received no LABDs prior to initiating neb arformoterol were being treated with a nebulized (50%) or inhaled (37%) SABD, or a systemic CS (46%) (Figure 4).
The following changes in medication patterns were observed 90 days after initiation of neb arformoterol: (a) reduced use of inhaled SABDs (45% vs 38%), systemic CS (48% vs 37%), LABA/ICS (37% vs 19%), LAMAs (33% vs 28%), and antibiotics (38% vs 30%), and (b) increased use of nebulized SABD (49% vs 53%) and nebulized CS (13% vs 63%) (Figure 3).
The most noticeable change was an increase in concomitant neb arformoterol + nebulized CS (63%), a substitution for the observed decrease in inhaled LABA + ICS dual therapy. Other common concomitant therapies included neb arformoterol + nebulized SABD (53%) or inhaled SABD (38%), neb arformoterol + systemic CS (37%), and neb arformoterol + inhaled LAMA (28%).
Predictors associated with initiating arformoterol treatment among beneficiaries who received no LABDs 90 days prior
A comparison of the treatment characteristics between the subsample of beneficiaries from the study group who had received no LABDs 90 days prior to initiating neb arformoterol (n=5542) and controls revealed that a significantly higher proportion of study group beneficiaries received nebulized SABDs (22.7 vs 10.8%, respectively; p<0.05), antibiotics (36.5 vs 22.8%, respectively; p<0.05), and systemic (46.5 vs 24.1%, respectively; p<0.05) or nebulized CS (16.7 vs 3.5%, respectively; p<0.05) (Table 3). In addition, more study group beneficiaries received spirometry tests than control beneficiaries (9.7 vs 4.2%, respectively; p<0.05).
 | Table 3 Treatment characteristics of Medicare beneficiaries who received no long-acting bronchodilators 90 days before initiating nebulized arformoterol compared with controls |
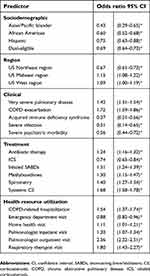
After adjusting for potential confounders (eg, age, gender), the results revealed that the strongest predictors of neb arformoterol initiation were receiving outpatient care from a pulmonologist (OR 2.36, 95% CI 2.22, 2.51) or a respiratory therapist (OR 1.80, 95% CI 1.43, 2.27), having had an exacerbation (OR 1.72, 95% CI 1.59, 1.86), use of a systemic CS (OR 1.68, 95% CI 1.58, 1.78), and having had a COPD-related hospitalization (OR 1.54, 95% CI 1.37, 1.74) (Table 4).
By contrast, predictors that were strongly associated with a reduced likelihood of initiating neb arformoterol included presence of a severe infection (OR 0.31, 95% CI 0.14, 0.65), having acquired immunodeficiency syndrome (OR 0.37, 95% CI 0.21, 0.66) or a severe psychiatric comorbidity (OR 0.56, 95% CI 0.44, 0.72), being dual-eligible (OR 0.69, 95% CI 0.64, 0.73), and using an ICS (OR 0.74, 95% CI 0.65, 0.84). Racial/ethnic minorities (ie, Asian/Pacific Islanders, African Americans, and Hispanics) were also significantly less likely to receive neb arformoterol as compared with Caucasian beneficiaries (Table 4).
Discussion
To our knowledge, this is the first study in the literature to examine COPD treatment patterns among Medicare beneficiaries who initiated neb arformoterol and to identify the key predictors of initiation. We found several emerging themes with important implications for clinical practice. First, nearly 1 in 2 Medicare beneficiaries had received no LABDs for maintenance treatment 90 days prior to initiating neb arformoterol. Instead, these beneficiaries were primarily being treated with short-acting agents (ie, a nebulized or inhaled SABD). Our findings are consistent with previous reports that 40% to 50% of Medicare beneficiaries with COPD receive no maintenance medications.21,22 Patients with mild COPD are generally not candidates for maintenance therapy. Our findings that 50% of the Medicare beneficiaries in our sample had experienced an exacerbation, 23% had an ED visit, and 10% had a COPD-related hospitalization in the 90 days prior to initiating neb arformoterol suggest that these beneficiaries had more advanced COPD.5,27 It is concerning that maintenance therapy was not initiated among apparently sicker beneficiaries earlier in their treatment regimen, suggesting undertreatment of COPD consistent with previous studies.27–30 These results also indicate a lack of compliance with GOLD therapeutic strategies, indicating the need for continued education to increase physician awareness about the importance of following recommended guidelines to lower the risk of exacerbations and hospitalizations.5,31,32
A second emerging theme from our study was that concomitant therapy with neb arformoterol was common including dual therapy with a CS, SABD, or LAMA. Once arformoterol was initiated, treatment with a nebulized CS and SABD increased, but the use of all other concomitant medications decreased. The GOLD therapeutic strategy recommends combination therapy for COPD patients with moderate to severe airflow limitation.5,33 Whether the use of neb arformoterol was administered as a primary or augmented therapy was unclear since 19% of the beneficiaries were using a handheld inhaler for LABA + ICS inhaler in conjunction with neb arformoterol 90 days after initiation. One potential explanation could be that these beneficiaries may have had difficulty using a handheld device, leading to a prescription for a nebulizer as a strategy to improve disease management.27,33 Another possibility could be that beneficiaries wanted to have the flexibility of a portable handheld inhaler in addition to a nebulizer to use at home.
An unexpected but illuminating finding was that moderate to severe psychiatric comorbidity was negatively associated with neb arformoterol initiation among beneficiaries who had received no LABDs 90 days prior to starting arformoterol. This result was counterintuitive. In Medicare populations, concurrent COPD and psychiatric conditions, such as depression and anxiety, have been associated with increased exacerbations, HRU, and costs.34–36 Furthermore, among these beneficiaries, low adherence to COPD maintenance medications is common.21,22,36,37 Given the relative ease of using a nebulizer (eg, requires fewer steps to administer medications than most handheld inhalers), increasing reliance on nebulization may improve adherence to prescribed regimens by lowering the procedural burden associated with inhaler use.21,38,39
Another key finding of our study was that dual-eligible beneficiaries who had not received a LABD for maintenance treatment were less likely to initiate neb arformoterol when compared to controls. This result is concerning because dual-eligible beneficiaries with COPD tend to be sicker, have higher rates of exacerbations and hospitalizations, more comorbidities, higher HRU, and a greater cost burden to treat.40–46 Our finding is also somewhat perplexing. Nebulized medications are covered under Medicare Part B as a durable medical equipment benefit, whereas other drugs are covered under Medicare Part D.47 For full-benefit dual-eligible individuals who represent the vast majority of beneficiaries (>70%), Part D covers the costs of nebulized therapy and does not require beneficiary cost-sharing.48,49 Since we found that dual eligibility was a negative predictor of neb arformoterol initiation and most of these beneficiaries would have had no out-of-pocket cost, our results suggest a potential lack of access to nebulized therapy in this patient population. Given that dual-eligible beneficiaries tend to be more medically needy,40,42–44 addressing the potential lack of adequate maintenance therapy in this population should become a priority.32,50
The rationale for using neb arformoterol has been well described in the literature.28 In two 12-week, double-blind randomized controlled trials that compared neb arformoterol to placebo, patients treated with arformoterol had improved and sustained lung function and lower exacerbation rates.51,52 In another 52-week trial, neb arformoterol treated patients were found to have greater improvements in health status and health-related quality-of-life than patients who received placebo.53,54 Research comparing nebulized LABA therapies has shown that patients treated with arformoterol have fewer exacerbations, lower inpatient costs, and lower COPD-related costs (primarily related to hospitalizations) when compared with patients treated with formoterol.55,56 In Medicare populations, LABA therapy has been shown to significantly lower the risk of hospitalizations, resulting in fewer inpatient days and lower total health care utilization when compared with SABA therapy.57–59 Research specific to medication adherence has found significantly lower risk of hospitalizations and decreased health care costs among Medicare beneficiaries who adhere to COPD maintenance medication regimens as compared to beneficiaries who discontinue maintenance therapy.36,60,61 Because 69% of the COPD patients who are hospitalized (mainly due to exacerbations) are primarily insured through Medicare,62 preventing acute exacerbations and subsequent hospitalizations through timely LABD maintenance therapy would be consistent with Medicare’s Hospital Readmission Reduction Program in the era of value-based health care.63–67
The findings of the current study must be viewed in light of limitations inherent to retrospective observational studies that rely on administrative claims data. There was no information available on certain factors that may have influenced medication management such as cognitive impairment, limited hand-breath coordination abilities, poor dexterity, or patient preferences.27,68–72 In addition, no direct assessment of COPD severity, such as measures of lung function, was available. However, our inclusion criteria required a COPD diagnosis of ≥1 year, and studies have shown that by the time a COPD diagnosis is given, most patients are beyond mild disease.1,2,73–75 Moreover, COPD patients typically receive a handheld inhaler as the first line of treatment and switching to or receiving augmented therapy with a nebulizer is often due to poor symptom control.31,76 Furthermore, the use of ICD-9 codes to classify beneficiaries with psychiatric disorders may have underrepresented the true prevalence of these conditions given that clinicians often fail to recognize mental illness.77,78
Conclusion
In this study, we found that 47% of the Medicare beneficiaries with COPD who initiated neb arformoterol had received no LABDs 90 days prior, despite multiple comorbidities, exacerbations, and hospitalizations. Furthermore, beneficiaries with moderate to severe psychiatric comorbidity and those who were dual-eligible were less likely to receive neb arformoterol when compared with controls, implying the need to increase access to nebulized maintenance therapy for these more vulnerable populations. Taken together, our findings suggest that clinical practice is not yet well aligned with current GOLD therapeutic strategies for COPD care management.
Acknowledgments
We thank Vaidy Ganapathy, PhD, formerly an employee of Sunovion Pharmaceuticals Inc., and Cynthia Ingrao, RN, BSN, DNP, from Advance Health Solutions, for their support with various stages of study implementation. We also extend our appreciation to Gulshan Sharma, MD, MPH, for his insightful support with analytic plan development and results interpretation. An earlier version of this paper with interim findings was presented at the CHEST 2017 Annual Meeting as a podium presentation. The presentation’s abstract was published in “Poster Abstracts” in CHEST: https://journal.chestnet.org/article/S0012-3692(17)32324-3/abstract. DOI: https://doi.org/10.1016/j.chest.2017.08.806.
Author contributions
BRC, MN, ZX, SCR, CD, and TPG all made substantial contributions to the conception, design, and/or interpretation of data. ZX and TPG were responsible for data acquisition and data analysis. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
BRC received consultation remuneration as a member of the Medical Advisory Board at Advance Health Solutions, LLC. He has also been an expert pulmonologist consultant for Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. He reports personal fees from Boehringer INgelheim, Glaxo Smith Kline, Astra Zeneca, Novartis, and Chiesi, outside the submitted work. MN and SCR are employed by Advance Health Solutions, LLC which received funding from Sunovion Pharmaceuticals Inc. to oversee this study. ZX and TPG are employed by the University of California San Diego which received a grant from Advance Health Solutions, LLC to conduct this study. CD is employed by Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Murphy SL, Xu JQ, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. In: Sudol. J, editor. National Vital Statistics Reports. Vol. 66. no. 6. Hyattsville, MD: National Center for Health Statistics. 2017. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06.pdf.
2. Han MK, Hanania NA, Martinez FJ. Confronting the challenge of COPD: what is new in the approaches to diagnosis, treatment, and patient outcomes. Chest. 2018;154(4):984–985. doi:10.1016/j.chest.2018.08.1024
3.
4. Ford ES, Murphy LB, Khaviou O, Giles WH, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi:10.1378/chest.14-0972
5.
6. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi:10.7326/0003-4819-155-3-201108020-00008
7. Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625–637. doi:10.1183/13993003.01170-2015
8. Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002 to 2010. Copd. 2017;14(1):72–79. doi:10.1080/15412555.2016.1199669
9. Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi:10.1378/chest.14-2434
10. Yawn BP, Make B. Treatment of chronic obstructive pulmonary disease in the primary care setting: how can we achieve more for our patients? Am J Med. 2018;131(9S):7–14. doi:10.1016/j.amjmed.2018.05.004
11. Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83. doi:10.2147/COPD.S122013
12. Diette GB, Orr P, McCormack MC, Gandy W, Hamar B. Is pharmacologic care of chronic obstructive pulmonary disease consistent with the guidelines? Popul Health Manag. 2010;12(1):21–26. doi:10.1089/pop.2008.0048
13. Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic pulmonary respiratory disease (COPD) among patient treated with combination of long-acting bronchodilators or inhaled chorticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi:10.2147/COPD.S27032
14. Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of COPD: a retrospective analysis of us managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:201–209. doi:10.2147/COPD.S25805
15. Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi:10.1016/j.amjmed.2008.09.042
16. Ford ES, Mannino DM, Giles WH, Wheaton AG, Liu Y, Croft JB. Prescription practices for chronic obstructive pulmonary disease: findings from the national ambulatory medical care survey 1999–2010. COPD. 2014;11(3):247–255.
17. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Resp Med. 2016;4(6):473–526. doi:10.1016/S2213-2600(16)00094-1
18. Diette GB, Dalal AA, D‘Souza AO, Lunacsek OE, Nagar SP. Treatment patterns of chronic obstructive pulmonary disease in employed adults in the United States. Int J Chron Obstruct Pulmon Dis. 2015;10:415–422. doi:10.2147/COPD.S75034
19. Baker CL, Zou KH, Su J. Long-acting bronchodilator use after hospitalization for COPD: an observational study of health insurance claims data. Int J Chron Obstruct Pulmon Dis. 2014;9:431–439. doi:10.2147/COPD.S59322
20. Amin AN, Bollu V, Stensland MD, Netzer L, Ganapathy V. Treatment patterns for patients hospitalized with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2018;75(6):359–366. doi:10.2146/ajhp160979
21. Qian J, Simoni-Wastila L, Rattinger GB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2014;29(1):49–57. doi:10.1002/gps.3968
22. Stuart BC, Simoni-Wastilla L, Zuckerman IH, et al. Impact of maintenance therapy on hospitalization and expenditures for Medicare beneficiaries with chronic obstructive pulmonary disease. Am J Geriatr Pharmacother. 2010;8(5):441–453. doi:10.1016/j.amjopharm.2010.10.002
23. Nishi SPE, Maslonka M, Zhang W, Kuo YF, Sharma G. Pattern and adherence to maintenance medication use in Medicare beneficiaries with chronic obstructive pulmonary disease: 2008–2013. Chronic Obstr Pulm Dis. 2018;5(1):16–26. doi:10.15326/jcopdf.5.1.2017.0153
24. Dhamane AD, Schwab P, Hopson S, et al. Association between adherence to medications for COPD and medications for other chronic conditions in COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;12:115–122. doi:10.2147/COPD.S114802
25.
26. Kronick R, Gilmer TP, Dreyfus T, Ganiats TG CDPS-Medicare: the chronic illness and disability payment system modified to predict expenditures for Medicare beneficiaries. Final Report to CMS. 2002. Available from: http://cdps.ucsd.edu/CDPS_Medicare.pdf.
27. Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrer JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9(1):58–72. doi:10.3109/15412555.2011.630047
28. Terasaki J, Nishi SPE, Ameredes BT, Sharma G. Arformoterol: rationale for use in chronic obstructive pulmonary disease. Clin Invest. 2014;4(5):429–439. doi:10.4155/cli.14.32
29. Lindenauer PK, Shieh M-S, Pekow PS, Stefan MS. Use and outcomes associated with long-acting bronchodilators among patients hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1186–1194. doi:10.1513/AnnalsATS.201407-311OC
30. Bishwakarma R, Zhang W, Kuo YF, Sharma G. Long-acting bronchodilators with or without inhaled corticosteroids and 30-day readmission in patients hospitalized for COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:477–486. doi:10.2147/COPD.S122354
31. Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585–2596. doi:10.2147/COPD.S114034
32. Mannino DM, Yu TC, Zhou H, Higuchi K. Effects of GOLD-adherent prescribing on COPD symptom burden, exacerbations, and health care utilization in a real-world setting. Chronic Obstr Pulm Dis. 2015;2(3):223–235. doi:10.15326/jcopdf.2.3.2014.0151
33. Dhand R, Mahler DA, Carlin BW, et al. Results of a patient survey regarding COPD knowledge, treatment experiences, and practices with inhalation devices. Respir Care. 2018;63(7):833–839. doi:10.4187/respcare.05715
34. Singh G, Zhang W, Kuo YF, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905–915. doi:10.1378/chest.15-0449
35. Albrecht JS, Huang TY, Park Y, et al. New episodes of depression among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2016;31(5):441–449. doi:10.1002/gps.4348
36. Albrecht JS, Park Y, Hur P, et al. Adherence to maintenance medications among older adults with chronic obstructive pulmonary disease. The role of depression. Ann Am Thorac Soc. 2016;13(9):1497–1504. doi:10.1513/AnnalsATS.201602-136OC
37. Wei YJ, Simoni-Wastila L, Albrecht JS, et al. The association of antidepressant treatment with COPD maintenance medication use and adherence in a comorbid Medicare population: a longitudinal cohort study. Int J Geriatr Psychiatry. 2018;33(2): e212-e220. doi:10.1002/gps.4713
38. Foster JA, Yawn BP, Maziar A, Jenkins T, Rennard SI. Enhancing COPD management in primary care settings. MedGenMed. 2007;9(3):24.
39. van Geffen WH, Douma WR, Slebos DJ, Kerstjens HA. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;(8):CD011826. doi:10.1002/14651858.CD011826.pub2.
40. D’Souza AO, Shah M, Dhamane AM, Dalal AA. Clinical and economic burden of COPD in a Medicaid population. COPD. 2014;11(2):212–220. doi:10.3109/15412555.2013.836168
41. Jiang HJ, Wier LM, Potter DEB, Burgess J Potentially preventable hospitalizations among Medicare-Medicaid dual eligibles, 2008. Statistical Brief #96. Rockville, MD: Agency for Healthcare Quality and Research; 2010. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb96.pdf.
42.
43. Figueroa JF, Lyon Z, Zhou X, Grabowski DC, Jha AK. Persistence and drivers of high-cost status among dual-eligible Medicare and Medicaid beneficiaries: an observational study. Ann Intern Med. 2018;169(8):528–534. doi:10.7326/M18-0085
44. Bynum JPW, Austin A, Carmichael D, Meara E. High-cost dual eligibles’ service use demonstrates the need for supportive and palliative models of care. Health Aff. 2017;36(7):1309–1317. doi:10.1377/hlthaff.2017.0157
45. Westney G, Foreman MG, Xu J, Henriques King M, Flenaugh E, Rust G. Impact of comorbidities among Medicaid enrollees with chronic obstructive pulmonary disease, United States, 2009. Prev Chronic Dis. 2017;14:E31. doi:10.5888/pcd14.160333
46. Chapel JM, Ritchey MD, Zhang D, Wang G. Prevalence and medical costs of chronic diseases among adult Medicaid beneficiaries. Am J Prev Med. 2017;53(6S2):S143–S154. doi:10.1016/j.amepre.2017.07.019
47.
48.
49.
50. Walsh EG, Wiener JM, Haber S, Bragg A, Freiman M, Ouslander JG. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home- and community-based services waiver programs. Jags. 2012;60:821–829. doi:10.1111/j.1532-5415.2012.03920.x
51. Baumgartner RA, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Hanrahan JP. Nebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29(2):261–278. doi:10.1016/j.clinthera.2007.02.009
52. Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5(1):25–34. doi:10.1080/15412550701816187
53. Donohue JF, Ganapathy V, Bollu V, Stensland MD, Nelson LM. Health status of patients with moderate to severe COPD after treatment with nebulized arformoterol tartrate or placebo for 1 year. Clin Ther. 2017;39(1):66–74. doi:10.1016/j.clinthera.2016.11.021
54. Donohue JF, Bollu VK, Stull DE, et al. Long-term health-related quality-of-life and symptom response profiles with arformoterol in COPD: results from a 52-week trial. Int J Chron Obstruct Pulmon Dis. 2018;13:499–508. doi:10.2147/COPD.S141729
55. Chen YJ, Makin C, Bollu VK, Navaie M, Celli BR. Exacerbations, health services utilization, and costs in commercially-insured COPD patients treated with nebulized long-acting β2-agonists. J Med Econ. 2016;19(1):11–20. doi:10.3111/13696998.2015.1079530
56. Ganapathy V, Stensland MD. Health resource utilization for inpatients with COPD treated with nebulized arformoterol or nebulized formoterol. Int J Chron Obstruct Pulmon Dis. 2017(12):1793–1801.
57. Ejzykowicz F, Bolly VK, Rajagopalan K, Hay JW. Health care use and costs among Medicare patients with chronic obstructive pulmonary disease treated with short-acting beta agonists or long-acting beta agonists. J Clin Pathways. 2016;2(3):31–38.
58. Bollu V, Ejzykowicz F, Rajagopalan K, Karafilidis J, Hay JW. Risk of all-cause hospitalization in COPD patients initiating long-acting or short-acting beta agonist therapy. J Med Econ. 2013;16(8):1082–1088. doi:10.3111/13696998.2013.815625
59. Bollu V, Guérin A, Gauthier G, Hiscock R, Wu EQ. Readmission risk in chronic obstructive pulmonary disease patients: comparative study of nebulized β2-agonists. Drugs Real World Outcomes. 2017;4(1):33–41. doi:10.1007/s40801-016-0097-y
60. Simoni-Wastila L, Wei Y-J, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all-cause hospitalization and spending in a Medicare population. Am J Geriatr Pharmacother. 2012;10(3):201–210. doi:10.1016/j.amjopharm.2012.04.002
61. Albrecht JS, Khokhar B, Huang TY, et al. Adherence and healthcare utilization among older adults with COPD and depression. Respir Med. 2017;129:53–58. doi:10.1016/j.rmed.2017.06.002
62. Wier LM, Elixhauser A, Pfuntner A, Au DH Overview of hospitalizations among patients with COPD, 2008. Statistical Brief #106. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb106.jsp.
63. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benefits. 2014;7(2):98–106.
64. Shah T, Press VG, Huisingh-Scheetz M, Sr W. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. doi:10.1016/j.chest.2016.05.002
65. Derdak S. Prevention of COPD readmissions: work in progress. Respir Care. 2017;62(1):133–134. doi:10.4187/respcare.05350
66. Press VG, Konetzka RT, White SR. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr Opin Pulm Med. 2018;24(2):138–146. doi:10.1097/MCP.0000000000000454
67. Barrecheguren M, González C, Miravitlles M. What have we learned from observational studies and clinical trials of mild to moderate COPD? Respir Res. 2018;19(1):177. doi:10.1186/s12931-018-0882-0
68. Sharafkhaneh A, Wolf RA, Goodnight S, Hananua NA, Make BJ, Tashkin DP. Perceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectives. COPD. 2013;10(4):482–492. doi:10.3109/15412555.2013.773302
69. Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm. 2011;68(13):1221–1232. doi:10.2146/ajhp100452
70. Baird C, Lovell J, Johnson M, Shiell K, Ibrahim JE. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–139. doi:10.1016/j.rmed.2017.06.006
71. Ding B, Small M, Scheffel G, Holmgren U. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936. doi:10.2147/COPD.S154525
72. Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228. doi:10.1089/jamp.2014.1142
73. Martinez FJ, Han MK, Allinson JP, et al. At the Root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540–1551. doi:10.1164/rccm.201710-2028PP
74. Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. 2017;12:2593–2610. doi:10.2147/COPD.S132236
75. Agustí A, Celli B. Natural history of COPD: gaps and opportunities. ERJ Open Res. 2017;3(4):00117–2017. doi:10.1183/23120541.00117-2017
76. Wise RA, Acevedo RA, Anzueto AR, et al. Guiding principles for the use of nebulized long-acting beta2-agonists in patients with COPD: an expert panel consensus. Chronic Obstr Pul Dis. 2017;4(1):7–20.
77. Grossberg GT, Beck D, Zaidi SNY. Rapid depression assessment in geriatric patients. Clin Geriatr Med. 2017;33(3):383–391. doi:10.1016/j.cger.2017.03.007
78.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.