Back to Journals » Journal of Inflammation Research » Volume 14
Mechanosensitive Piezo1 Channel Evoked-Mechanical Signals in Atherosclerosis
Authors Shinge SAU, Zhang D, Achu Muluh T , Nie Y, Yu F
Received 11 May 2021
Accepted for publication 3 July 2021
Published 27 July 2021 Volume 2021:14 Pages 3621—3636
DOI https://doi.org/10.2147/JIR.S319789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shafiu A Umar Shinge, 1 Daifang Zhang, 1, 2 Tobias Achu Muluh, 3 Yongmei Nie, 1, 4 Fengxu Yu 1, 4
1Cardiovascular Surgery Department, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 2Clinical Research Center, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 3Oncology Department, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 4Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
Correspondence: Yongmei Nie; Fengxu Yu Tel +8619938597025
; +8613980259707 Email [email protected]; [email protected]
Abstract: Recently, more and more works have focused and used extensive resources on atherosclerosis research, which is one of the major causes of death globally. Alongside traditional risk factors, such as hyperlipidemia, smoking, hypertension, obesity, and diabetes, mechanical forces, including shear stress, pressure and stretches exerted on endothelial cells by flow, is proved to be crucial in atherosclerosis development. Studies have recognized the mechanosensitive Piezo1 channel as a special sensor and transducer of various mechanical forces into biochemical signals, and recent studies report its role in atherosclerosis through different mechanical forces in pressure, stretching and turbulent shear stress. Based on our expertise in this field and considering the recent advancement of atherosclerosis research, we will be focusing on the function of Piezo1 and its involvement in various cellular mechanisms and consequent involvement in the development of atherosclerosis in this review. Also, we will discuss various functions of Piezo1 involvement in atherosclerosis and come up with new mechanistic insight for future research. Based on the recent findings, we suggest Piezo1 as a valid candidate for novel therapeutic innovations, in which deep exploration and translating its findings into the clinic will be a new therapeutic strategy for cardiovascular diseases, particularly atherosclerosis.
Keywords: atherogenesis, Piezo1 channel, endothelial cell, inflammation, shear stress, mechanotransduction
Graphical Abstract:
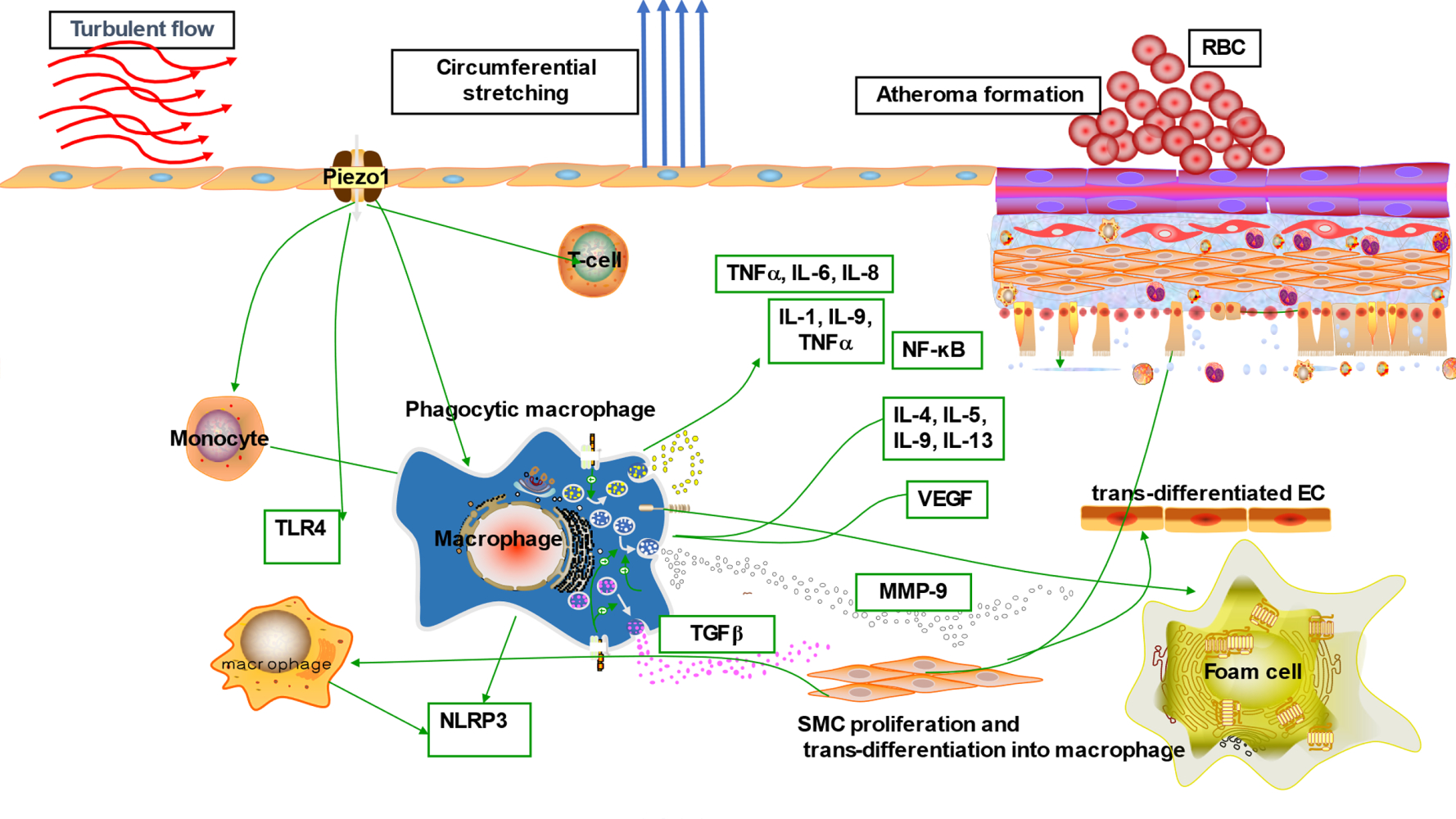
Introduction
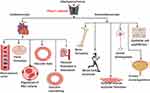
Atherosclerosis (AS) is a major global killer, and is the basis of cardiovascular diseases (CVDs) comprising myocardial infarction (MI), ischemic heart disease (IHD) and stroke. AS is a multifactorial inflammatory disorder,1–4 and is the root of cardiovascular disease.5 AS remains a leading cause of death globally, claiming millions of lives every year, as estimated in the 2015 global burden of disease study (17.9 million) about 30% of whole death globally.6 There is an increased in its prevalence due to the global rise in obesity and diabetes, with more than half of the world population becoming obese, leading to a steep rise in cardiovascular diseases (CVDs) burden; it is estimated that the global death from CVDs may go beyond 23.6 million by the year 2030. At the onset of AS, endothelial cell (EC) defense is triggered by a number of factors, including mechanical forces such as shear stress and stretching, which leads to EC adaptive responses thereby developing a distinct phenotype to interact with other traditional risk factors,7 and facilitate the initiation of AS. The significance of mechanical forces in cardiovascular physiology and pathology have been reported for decades. Shear stress mediated by Piezo1 is among the significant factors and the initiator of inflammation and vascular endothelial cell (VEC) dysfunction.8 Cells detect and liberate mechanical forces using various biochemical and molecular mechanisms. In the cardiovascular system, frictional force and pressure are generated in the vessels, endothelial Piezo1 senses and transduces such forces which are the determinant of the physiological competence of the system throughout life. Piezo1 serves a crucial function in angiogenesis, embryonic development and is crucial for endothelial shear stress-senses, blood pressure regulation, and physical activities during exercise etc.9 As our knowledge of this field increases, various functions of Piezo1 are emerging. Piezo1 is a significant player in cardiovascular physiology (Figure 1), renal and hematopoietic systems.10 Its function is known in embryonic vessel development,11 RBC stability in both human and mouse,9 control of blood pressure, physical activities, hypertension-dependent arterial remodeling, urinary osmoregulation, epithelial equilibrium and axonal development.11 It also serves the function of adjusting erythrocyte volume,12–14 and in prostate cancer.15 Piezo1 global knockout in mice leads to embryonic fatality,10,12,16–19 indicating its significance during embryogenesis.20 Additionally, severe defects in vessel maturation and remodeling, and disability in NO production and vessel dilation responding to flow were both observed following Piezo1 global deletion or EC-specific disruption.21 Also, Piezo1 in partnership with Piezo2 serves the function of baroreceptor reflex as their dual disruption obliterates baroreceptor responses to sodium nitroprusside or phenylephrine.22 Piezo1 appears to function in a wide area of cell biology, as illustrated in Figure 1, and its pathologic importance in humans was also indicated.11,23–25 The broad concept of Piezo1 functions was also reviewed in recent articles.9,26–28 Collectively, Piezo1 remains a significant player in a broad area of cell physiology, considering its expression and functions in diverse tissues, not limited to the cardiovascular system (Figure 1). This indicates that defects or mutations in this channel may impair various functions in ECs and other cell types. Therefore, pharmacological manipulation of either activation or inhibition of this channel may come up with a new trend in clinical practice. Different abnormalities or up/downregulation of cellular activities were observed in several studies following the silencing/blocking, deletion, cell specific or global knockout and deficiency of Piezo1.9,28–33 Since the recognition of Piezo1 &2 in 2010, they have been regarded as one of the most significant classes of mechanosensory proteins. It is now feasible to investigate the mechanism of Piezo1 engagement in physiological and pathophysiological processes as a result of recent developments in understanding their topology, the identification of the agonists Yoda1, Jedi1&2 as well as the antagonist GsMTx4, Ruthenium Red. Recently, cardiovascular aspects of Piezo1 channel have been reviewed elsewhere, but here we review the current understanding of Piezo1 channel and specifically focus on its immune/inflammatory mechanisms of atherosclerosis involving different mechanotransduction processes as summarized in the graphic abstract, which have not been reviewed to date. We also raise awareness of the realization of its pharmacology and suggested new directions for future research toward the development of advanced therapeutic strategies for prevention and management of atherosclerosis.
Structure of Piezo1 Channel
Human mechanically activated (MA) channel molecular identity was mysterious for a long time until the revolutionary discovery of Piezo1 & 2, which may increase our understanding of cellular signaling and mechanotransduction. Piezo1 proteins are collectively arranged to form a three blade-like propeller inserted within the lipid bilayer which makes the central ion pore that senses mechanical forces.9,34–36 Its extracellular propeller domain serves as a detector of flow related-shear and other mechanical stress.16,37 In respect to structural components, Piezo1 can be categorized as follows.
Exceptional 38‑TM Topography
With advancement of Piezo1 research, high-resolution structures of mice Piezo1 (mPiezo1) were shortly disclosed, showing that each subdomain has a special 38-TM topography. The inner helixes (IH) and outer helix (OH) of the center of the pore module are the 2 TM subdomains (TM37 and TM38) nearest to the protein’s core. The remaining 36 TM regions (TM1-36) are organized into a blade resembling a curved structure with 9 repeating folds comprising four TM regions each, known as transmembrane helical units (THUs).38–40
Exceptionally Curving Blades
Each subdomain’s 9 peripheral THUs produce bladelike architectures, each twisted clockwise. As seen from a line parallel to the plasma membranous planes, next to the TM25–TM36 and periphery of the TM13-24 these are based at a 100° and a 140° angle, respectively. The L-shaped helical structures produced by TM13, TM17, TM21, TM25, and TM29 are another essential feature of the blades. Both structural characteristics tend to be suitable for inducing regional membrane curvature as well as mechano-sensing. Surprisingly, the peripheral TM13-24 tends to be located inside a strongly bent membrane plane, implying that the Piezo1 channel has the ability to bend the membrane in which it sits. Previous research suggested that cell membrane deflection and tension may control Piezo1.26,27,38,39,41
Central Cap
Using topographical predicting simulation, a study by Kamajaya’s team,42 discovered that Piezo1 residues 2210 to 2457 produced an extracellular loop next to the last TM region from the C-terminus, known as C-terminal extracellular domain (CED). The central cap was not expressed when residues 2218 to 2453 were removed from the Piezo1 protein, implying that this area trimerized to produce the central cap. The central cap comprises the CED in the shape of trimeric complexes that encapsulated the extracellular-vestibule (EV) with apertures, disclosed by another study.27,38,39,43
The Ion Conducting Pore
Piezo proteins compose a trimeric ion conducting channel comprising residues 2189 to 2547, that composes the last two TMs. An EV in the cap region, a transmembrane-vestibule (MV) within the membrane, and the intracellular-vestibule (IV) beneath the membrane make up the continuous central channel. The MVs are located on top of and beneath the membrane, and both the EV and IV have an opening that binds them. DEEED (2393–2397), a patch of negative charge residues located in the opening of the extracellular “cap” structure made up of the CED, is necessary for effective ion conducting and determining the preference for cations than anions. Furthermore, divalent calcium ion selectivity, unitary conductance, and aperture blocking can be caused by two essential acidic residues, E2495 and E2496, situated at the CTD-constituting IV.26,39,40,44
The Intracellular Beam
Piezo1 possess 3 beam-like structures from intracellular aspect, each measuring 90 nm in length and arranged at a 30° angle to the membranous plane. The beam composition is made up of residues H1300-S1362. The long intracellular THU7-8 loop has about 390 residues, which could supply the beam with a structural foundation for force transmitting. The 3 longer intracellular beams serve as a functional barrier between the distal THUs and the central ion conducting orifice, as well as supporting the entire TM framework. The mutated protein was missing after residues 1280 to 1360 were removed, indicating the beam’s structural significance.26,27,39
The Anchor
The OH-IH pair is connected into the C-terminal domains (CTD) planes by a hairpin structure called the anchor, which pushes the OH-CED-IH-composing area of 1 subdomain to the adjacent subdomain in a dextral aspect. Three helices (α1, α2, and α3) make up the anchor. The inverted V-shaped structure formed by helices α1 and α2 was discovered to keep the ion-conducting pore’s stability. The longer α3 helical unit interacts with the polar residue-rich α2–3 turning in the anchor and the glutamate-rich portion of the CTD through a lysine-rich anchor-OH linker which runs parallel onto the membranous plane. A few mutations in Piezo1 have been identified to account for serious disorders at regions such as KKKK (2182-K2185), T2143, T2142 (T2127 in human Piezo1), R2514, E2523, and E2522, which are situated at α3 in the anchor. SERCA2, a Piezo-interactor protein, also inhibits Piezo1 by interacting with the anchor-OH linker. These results affirm the anchor portion’s structural and functional significance.26,38,40
Piezo1 Functions
The Role of Piezo1 in Helical Flow
The circulatory system generates a variety of flow patterns within the vascular beds, including laminar, disturbed or turbulent, and oscillatory or helical flow, depending on the nature of vessel.8 Piezo1 channel was recently recognized as a specialized mechanosensor that senses blood flow and integrates the signal into a genealogical program for vascular formation and maturation.45–47 Due to the effects of flow pattern on vascular health, helical flow patterns have received considerable attention in modern days. The helical pattern of flow is symbolized by increased velocity48 with a high shear stress that is known to be sensed by Piezo1,9,27 and is now considered as a physiologic type of flow.48,49 It is now thought to be an atheroprotective flow,50 considering that it could minimize blood cell adherence to the vessel wall, prohibit LDL buildup, and improve perfusion and oxygen delivery. Furthermore, hemodynamic efficacy of a vascular device is enhanced by helical flow,48,51 as summarized in Table 1. Piezo1 may sense helical flow and act as a key regulator of vascular health through various mechanisms, as it senses and transduces different flow patterns into biochemical signals. Therefore, through Piezo1 mechanotransductions, the roles of helical flow might minimize the burdens on vasculature as well as protecting it from atherosclerotic pathologies, intimal hyperplasia and thromboembolism, through the suppression of proinflammatory signaling resulting from low and turbulent shear stress.52 Altogether, these data demonstrate the physiological importance of Piezo1 mediated helical flow, its mechanisms in cardiovascular biology signaling and its therapeutic potentials for targeting atherosclerosis and other CVDs.
 |
Table 1 Summarizing the Suggested Clinical Significance of Helical Flow |
Piezo1 in VSMCs Shear Stress-Induced Plaque Formation
Piezo1 is the specialized sensor and transducer of mechanical forces including shear stress. It enables EC,9,53 and vascular smooth muscle cells (VSMCs),20 to detect and respond to changes in their environment. Turbulent shear senses via Piezo1,8 increases EC protein and gene expressions of VSMC mitogens including platelet-derived growth factors (PDGF), ET-1, and vascular endothelial growth factor (VEGF). EC expression of VSMCs migration inhibitor (plasminogen activator inhibitor (PAI)-1) and effective suppressors of cellular growth and migration, such as NO and TGFβ, are all downregulated by turbulent shear. The EC downregulation of these growth inhibitors by disturbed shear encourages VSMCs to migrate into the intima via an interrupted internal elastic lamina (IEL). VSMCs develop synthetic phenotype in the intima, replicate and generate collagen, including other (ECM) proteins. With time, VSMCs54 together with fibroblast that is known to be regulated by Piezo1,55 form the fibrous cap along with lipid core, building up what is known as atherosclerosis plaque.
Piezo1 Channel as Shear Sensors
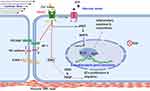
Shear sensing is vital in cardiovascular physiology, and shear stress is among the factors guiding the developing vessel during embryogenesis.17 Piezo1 is a specialized and authentic shear stress sensor, as proved by much evidence. ECs detect shear stress and convey the signaling inside the cells, which induces cell responses to changes in its surroundings; abnormality in these responses could result in various disorders, including CVDs.56 In response to shear stress, Piezo1 activates cascades of downstream mechanisms, which alter the cell behavior based on the type of stimuli.17,57 It senses physiological shear to enhance angiogenesis and vessel maturation.34 Earlier discoveries about Piezo1 unveiled its importance for shear stress detection.18 With Piezo1, EC oriented and aligned in the flow direction, while in its absence, EC failed to do so.12,34 Collectively, these qualify Piezo1 as an authentic shear stress sensor; and tuning Piezo1 through pharmacological approaches may change the story of clinical practice. Shear stress triggers EC Piezo1, which causes Ca2+ inflow and phosphorylating of AKT and eNOS, resulting in enhanced NO generation and VSMC-dependent vessel dilatation. Comparatively, VSMC Piezo1 is triggered by stretch and is active in vascular remodeling mechanisms under pathological conditions, resulting in a reduction in vessel diameter. Both Piezo1-dependent processes successfully sustain basal BP control.58 In human embryonic kidney cells, transfecting Piezo1 caused prompt shear stress–evoked Ca2+ influx or ion current, shear stress quickly stimulates endogenous Piezo1 in membrane patches extracted from native endothelium, and Piezo1 knockout disrupts embryonic vascular maturation, which is thought to be caused by shear stress.9
Piezo1 Correlation with Inflammation and Atherosclerosis
Increasing evidence has reported Piezo1 involvement in various mechanisms of cell biology, including health and disease. Nearly all cells, tissues and organs are exposed to various degrees of mechanical forces, and Piezo1 is the principal mechano-sensor in various cells and tissues, thus linking Piezo1 to multiple mechanisms of inflammation and various disease pathogenesis particularly atherosclerosis. Mechanical forces, including shear stress, are the primary stimuli for cell physiology, since they supply signaling for cellular homeostasis. Piezo1 is known to mediate wide varieties of such forces, therefore dysregulation of Piezo1 signal may alter homeostasis and result in disease processes such as atherosclerosis. ECs respond to these stimuli such as laminar or turbulent shear mediated by Piezo1, and this triggers pro- or anti-inflammatory signals leading to inflammation and atherosclerosis depending on the flow pattern. Piezo1’s significance in cardiovascular physiology and pathology is becoming more widely recognized, hence the next section will discuss in detail the Piezo1 mechanisms in inflammation and atherosclerosis. Furthermore, evidence shows that unidirectional and turbulent flow senses by Piezo1, causes distinct signaling in ECs, leading to an anti- or pro-atherogenic phenotype.8 Each of the unidirectional and turbulent flow triggers initiate signal thoroughfare involving Piezo1.8,59 The trigger of NF-κB upregulates proinflammatory as well as pro-atherogenic genes,60,61 leading to growth of AS, while KLF triggering sustains vessel architecture (Figures 2 and 3). Furthermore, various in vivo and ex vivo studies on pre-flowed ECs culture, demonstrate that unidirectional and turbulent flow-induced athero-protective and atherogenic signals both require Piezo1.
Piezo1 Mediated Mechanisms in the Initiation and Progression of Inflammation and Atherosclerosis
The majority of prior studies on atherosclerosis pointed to elevated LDL levels as the primary culprit.62 Only recent works recognized that hypercholesterolemia and atherosclerosis development are linked via inflammatory processes. Therefore, immunological response and inflammation have been regarded as key contributors in the initiation and amplification of atherosclerosis.63,64 Mechanosensitive Piezo1 channel was recently reported to play a crucial role in immune cells and inflammatory processes.65–68 Over time, research has shed more light on inflammatory responses in atherosclerosis, providing strong evidence that inflammation is a major factor in all stages of atherogenesis.69 Furthermore, inflammatory signaling increases thrombosis, which is responsible for ischemic heart disease and the majority of myocardial infarctions and cerebrovascular diseases.
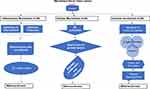
Stages of Atheroma Formation
(A) Initially EC undergo inflammatory triggers in response to noxious stimuli,70 including atherogenic shear from flow mediated by Piezo1,71 as discussed in Piezo1 Mediated Shear-Induced Inflammation and Atherosclerosis; (B) monocytes recruitment to atheroma region discussed in 5.3; (C) cytokines and chemoattractive molecules engaged in promoting the recruiting of additional immune/inflammatory cells to the intima discussed in 5.5; (D) later monocyte differentiates into macrophages and engulfs lipoprotein particles to transform into lipid engorged foam cells; (E) then foam cells release proinflammatory molecules, and growth factors including reactive leading to the proliferation, migration, discussed in 5.5, and phenotypic switching or transdifferentiation of VSMCs into SMC derived macrophages in the intima.72,73 (F) Apoptosis of macrophages leading to the development of “necrotic” core of the mature plaque; (G) macrophages and SMC augment the process,74 through secretion of matrix metalloproteinases (MMPs) particularly MMP-9,75 which leads to the degradation of extracellular matrix, resulting in the plaque’s fibrous cap thinning; (H) plaque rupture due to weakness of the fibrous cap leading to thrombogenic coagulation and finally thrombosis and obstruction of vessel lumen,76 which limits the tissue perfusion and results in ischemic heart disease.69,70 Piezo1 signaling is known to play crucial roles in other immune/inflammatory mechanisms of atherosclerosis as discussed in 5.4.
Piezo1 Mediated Shear-Induced Inflammation and Atherosclerosis
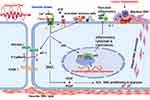
Beside the established risk factors of atherosclerosis, it is convincing that turbulent shear mediated by Piezo1 is crucial for EC activation, leading to initiating of inflammation and atherosclerosis.8,77,78 Disturbed flow sensed via Piezo1 produces turbulent shear, which is a considerable risk for atherosclerosis, as studies reported its effect on EC.78,79 Turbulent shear80 triggers pro-inflammatory EC phenotype; disorders such as AS appeared to originate from such EC phenotypes.8 Numerous studies suggested Piezo1 associates with different inflammatory pathways and signaling mechanisms in the development of AS (Figure 3). Piezo1 was recently found to be expressed and function in inflammatory cells including monocytes, macrophages65 and T-cells.81 Studies reported that activation of monocytes and macrophage by Piezo1 triggers proinflammatory signals leading to expression of various cytokines and chemokines,65–67 a crucial event during atherogenesis. Cytokines such as TNF-α, IL-1, 2, 3, 6, 10, 12, 15, 18, CXCL8, IFN-γ, M-CSF, TGF-β1, 2, and 3 are critical components of inflammation and play a significant role in AS pathogenesis. The most interesting fact is that in the lack of Piezo1, inflammatory cytokines and chemokines, as well as the transcriptional factor, hypoxia-inducible factor 1 (HIF-1), were not expressed by macrophages and monocytes subjected to pressure cycles. Additionally, PECAM-1, VCAM-1 and ICAM-181 are also found in disturbed flow regions of ECs, and reduced shear upregulates the P-selectin82 and monocyte chemotactic protein-1 (MCP-1) mRNA expression in correlation with an increased number of monocytes bounded to EC. Interestingly, following the loss of Piezo1, the rise in Vcam-1 expression and the number of CD68-positive cells was markedly decreased, and turbulent flow-evoked inflammation signals were also blocked. Comparatively, subjecting human umbilical artery endothelial cells (HUA ECs) to turbulent flow leads to NF-κB triggering, as evidenced by p65 phosphorylating at serine 536. Furthermore, in athero-susceptible sites, there were less integrin activities, inflammatory signals and atherosclerosis, following the selective depletion of endothelial Piezo1 or Gq/G11 in mouse. Both in vivo and ex vivo EC Piezo1 and Gq/G11 mediate inflammatory EC signal in response to turbulent flow, leading to EC dysfunction and AS, also equal signaling pathway detects laminar flow and triggers eNOS.21,81,83 Hence, turbulent and laminar flow tend to stimulate Piezo1- and Gq/G11-mediating signals, which mediates both athero-protective and proatherogenic signals, described in the figure legends8,84–86 (Figures 2 and 3). In AS, inflammation involves several mechanisms and cross-talk between various pathways, therefore targeting inflammation through Piezo1 approach could be wise. Altogether, these findings support the idea that Piezo1 might not just mediate unidirectional flow-evoked anti-atherogenic signals but is also necessary for turbulent flow-mediated endothelial inflammation and atherogenesis.
Piezo1 Mediated Immune/ Inflammatory Response in Atherosclerosis
Mechanical stress exerted on EC in the form of disturbed flow is among the primary activators of the endothelial immune defense system. Activation of EC is always followed by the recruitment of immune cells such as monocytes, which later differentiate into macrophages and transform to lipid-engorged foam cells, the hallmark of AS. Numerous studies reported the dominance of immune cells on the site of an earlier plaque. Triggered T cells, including macrophages, have been revealed to reside on early and part of matured AS plaques, and their transcription components speed up the plaque advancement, indicating the significant function served by innate and adaptive immunity in AS cytopathology.64,87,88 Turbulent flow is thought to activate the endothelial immune system that initiates the process of AS. Traditionally ECs oppose adherence of inflammatory cells, but EC exposure to turbulent shear mediated by Piezo1 activates the endothelial expressions of adhesive molecules that promote the attaching of immune cells (Figure 3). Studies reported the significant role of mechanical force on immune cell functioning, and Piezo1 appears to be crucial in T-cell activation and recruitment.89,90 Additionally, findings by Solis et al. revealed the physiologic function of Piezo1, including mechano-sensing in immunity.65 Adaptive immunity has a significant effect on atherogenesis, with pro- and anti-atherogenic impacts exerted by T cells sub-classes.91 As reported by Solis, Piezo1 detects oscillatory pressure in myeloid cells, and triggers pro-inflammatory responses. Mechanical activation of macrophages, including monocytes, stimulates expressions of pro-inflammatory and chemo-attractive mediators, which all rely on Piezo1.65 Intriguingly, in lack of Piezo1, macrophages, including monocytes subjected to cyclical pressure, could not express inflammatory cytokines and chemokines.67 Altogether these findings demonstrated that Piezo1 is crucial for immune response during atherogenesis (Figure 3).
Piezo1 Activation of NLRP3 Inflammasome and TLR4 Signaling in Regulation of Inflammation and Atherosclerosist
Atherosclerosis is a chronic progressive inflammatory disorder that is believed to be linked to the triggering of NLRP3 inflammasome and TLRs signaling. According to a growing body of data, NLRP3 inflammasome and TLR4 appears to have a causal role in the onset and advance of atherosclerosis.92,93 Given the importance of mechanical forces in regulating cellular and tissue growth, as well as in disease pathogenesis,94 including atherosclerosis, mechanosensitive Piezo1 channel could be considered as a crucial player in various mechanisms of CVDs particularly atherosclerosis.28 Piezo1 channel was reported to be expressed and function in vascular cells including ECs,95 VSMCs,20 as well as immune cells (myeloid cells) such as monocyte, macrophages,65 B and T cells.68,90 On the other hand, NLRP3 and TLRs,96,97 are also expressed by both immune cells and endothelium. NLRP3 inflammasomes and TLRs are well-known multi-protein complex immunological sensors that augment inflammation in response to a variety of danger-signaling ligands inclusive of pathogen-associated molecular patterns (PAMPs) from invading microbes (e.g., LPS) or mislocated commensal pathogens, as well as danger-associated molecular patterns (DAMPs) or alarmins like endogenous factors,98 inclusive of RNA and DNA, HMGB1, amyloid-β, cholesterol crystals, mitochondrial damage, or ROS, as well as noxious stimuli like disturbed shear mediated by Piezo1, which results in accumulation and triggering of caspase-1. Innate immune responses mainly rely on the detection of PAMPs and DAMPs via the pattern-recognition receptors (PPR), including Toll-like receptors and NLRs, particularly TRL4 and NLRP3. A recent study shows that application of Yoda1 in BV2 cell upregulates the expressions of TLR4, moreover, significant elevation of TLR4 and Piezo1 expressions were seen following the application of Yoda1 and LPS; this finding suggest that Piezo1 is crucial for TRL4 signaling.99 NLRP3 activators are thought to cause one or more downstream cellular processes or diseases rather than directly interacting with NLRP3. The NLRP3 inflammasome is triggered by two distinct signals: toll-like receptor 4 (TLR4) ligands lipopolysaccharide (LPS) binding to its receptor, which causes NLRP3 and pro-IL-1 transcriptional upregulating via NF-B (1st signaling). TLR4 can also deliver 1st signaling independently of new protein synthesis through its adaptors myeloid differentiation factor 88 (MyD88), interleukin 1 receptor-associated kinase 1 (IRAK1), and IRAK4. NLRP3 activation requires a posttranscriptional alteration; NLRP3 deubiquitination mediates by BRCA1/BRCA2-containing complex subunit 3 (BRCC3) (2nd signaling). The NLRP3 inflammasome is assembled and activated by the second signal through NLRP3-activating substances (e.g., ATP, ROS, oxidized mitochondrial DNA (mtDNA), and other noxious stimuli including mechanical stretch and disturbed or oscillatory shear mediated by Piezo1, followed by proinflammatory caspase-mediated pyroptosis.97,100 Recent findings92,100–102 suggested that in ECs, various insults like disturbed flow, initiate the NLRP3 inflammasome triggering, and Piezo1 is a newly discovered mechanosensitive channel that senses and transduces different flow patterns into biochemical signals.103,104 In ECs simulation of turbulent flow and oscillating shear extensively intensifies the generation of active caspase-1 and IL-1β.105 Furthermore, Sun et al. reported that Piezo1 triggering enhances the assembly of NLRP3 inflammasome, as indicated by caspase 1 upregulating and generation of IL-1β which was reversed after Piezo1 siRNA transfecting. Interestingly, the abrogation of Piezo1 dependent NLRP3 inflammasome trigger was observed following the Ca2+/NF-κB pathway suppression. The author further revealed that mechanical stretching enhanced expression of Piezo1 and intracellular Ca2+ accumulation that upregulates NLRP3 through the NF-B pathway triggering. It also acts as a direct secondary stimulus, promoting NLRP3 assembly, caspase-1 triggering, and the generation of IL-1β.106 In tissue-resident mice alveolar macrophages, Wu et al. found that cyclical stretching known to be mediated by Piezo1, triggers the NLRP3 inflammasome through mitochondrial ROS generation, suggesting that this mechanism might be connected to lung inflammation caused by mechanical ventilation. Mechanical stress appears to be a risk factor for NLRP3 inflammasome activation, according to this study.107 Whereas another study found that application of prolonged compressive stress to epidermal tissue increased NLRP3 and caspase-1 protein expressions while reduced IL-1β expression.108 Contrarily, another study revealed that in macrophages, cyclical stretching inhibits the NLRP3 inflammasome.109 This implies that further studies are necessary to explain these contradictory findings. The mechanosensitive Piezo1 channel appeared as principal transducer of mechanical cues into Ca2+ dependent signaling, and Ca2+ signaling was critical in various cellular physiology and pathologies. Piezo1 triggering is necessary for various Ca2+ dependent pathways. Many downstream pathways including that of TLR4, NF-B, mTOR, and JNK1,110 which are controlled by intracellular Ca 2+ signals, have been demonstrated to be the major molecular processes involved in immune processes including inflammation and atherosclerosis.
Piezo1 Mechanical Trigger of Proliferation, Migration and Apoptosis in Atherogenesis
Owing to the pulsative aspect of blood supply, vascular cells (VECs & VSMCs) are exposed to hemodynamic forces in the form of shear stress, pressure, circumferential/ distention or stretches. Mechanosensitive Piezo1 senses such forces in various intensities, allowing their translation into biological signals within the cell, leading to triggering of downstream pathways.9,111,112 Depending on whether the cells are subjected to physiologic or sub-physiologic/supraphysiologic mechanical forces, this trigger can differ. Significant physiological mechanical force is crucial in maintaining vascular homeostasis.72 While sub-physiologic or supraphysiologic forces may induce alterations in gene expression that encourage inflammation, cell proliferation, migration, apoptosis and vascular remodeling, that are pivotal process in atherosclerosis development.113,114 Multiple studies demonstrated the effects of Piezo1-mediated mechanical signals on vascular and other cells (immune/inflammatory cells) involving proliferation, migration, apoptosis and remodeling during disease pathogenesis,65,66,68,90,105 particularly atherosclerosis (Figure 4). Pathological inflammation, cell proliferation, migration and apoptosis of EC, VSMCs and macrophages is the hallmark of atherosclerosis pathogenesis, including several CVDs.115 Piezo1 is necessary for sensing and transduction of various mechanical forces including shear stress, pressure, and cyclic stretch. VSMCs proliferation was reported to be increased by cyclic stretch in vitro.72,73 Piezo1 is a well-recognized mechanically triggered channel that enables Ca2+ influx,47,116 and Ca2+ is a highly recognized modulator of cell proliferation, migration and apoptosis, thus, it serves a crucial function in atherogenesis.8,9 Additionally, ERK and Akt/mTOR pathways are crucial for cell survival, differentiation, proliferation, migrating and apoptosis,117–119 and intracellular Ca2+ signaling can control these pathways.120 Stretching was shown to enhance mice and rabbit VSMCs proliferation by activating the extracellular signal-regulated-kinase (ERK) pathway in a synergistic manner with oxidized LDL and norepinephrine. Furthermore, 15% stretching of mouse aortic SMCs exhibited higher ERK and Akt triggering, as well as an increase in insulin-induced cellular proliferation.72 In pathological stretching, however, unregulated proliferation of ECs was seen due to upregulating of oncogene c-Myc expression in human umbilical vein endothelial cells (HUVEC). HUVECs depleted in Piezo1 display decreased expression of VEGF, cell proliferation, and migration in stationary states, demonstrating the significance of Piezo1 in this mechanism.12 When ECs were stretched, they transdifferentiated into SMCs as elevated expression of specific SMC marker genes (SM22, -SMA, caldesmon-1, SM MHC, and calponin) was observed, while endothelial markers were reduced. The appearance of SMC markers on EC means that during mechanical stretching, EC plasticity towards the SMC phenotype occurs, which may lead to the plaque progression.73 Comparatively, Piezo1 overexpression or dysregulation can lead to increase in Ca2+ overload. The elevated level of intracellular Ca2+ can activate the Akt/mTOR pathways through calmodulin (CaM) or CaM-dependent protein kinase II (CaMKII) and, as a result, accelerate cellular proliferation and migration, important processes in plaque formation and cancer. Piezo1 plays a crucial role in cell migration, as MCF-7 cells can migrate better when Piezo1 is overexpressed. While application of Piezo1 agonist GsMTx-4 impaired the migrating of MCF-7 breast cancer cells.28 Collectively, these results demonstrate that Piezo1 is needed for inflammation cell proliferation, migration, apoptosis and, that these are critical stages in the development of AS.
Piezo1 Pharmacology
The accumulated reports and preceding experiments collectively demonstrate the mechanosensitive value of Piezo1 in both physiology and pathology, particularly atherosclerosis. They may be used as diagnostic biomarkers, as well as pharmacologic and genetic targets for innovative and advanced therapeutic approaches. Despite the infancy of Piezo1 research and its pharmacology, the rapid discovery of its pharmacologic agents presents an outstanding therapeutic opportunity. Recent studies reported the recognition of a few molecules including Yoda1,121 and Jedi1/2,122 as potent activators of Piezo1, and GsMTx-4,123–126 Ruthenium Red (RR)47,127 as its non-specific inhibitors, inclusive of Yoda1 analog called Dooku1,11 and the most recent molecule Tubeimoside1 (TBMS1),128 that have reversible antagonizing capacity of Yoda1-evoked Piezo1 trigger, as well as the binding site including activating mechanisms.112 Altogether, the agonistic and antagonistic capability of the molecules on Piezo1 demonstrated the medicinal and pathophysiological significance of Piezo1 in atherosclerosis and including cancer and other disorders. Nevertheless, Piezo1 drug targeting for therapeutic approaches remains challenging and a difficult task that requires further investigations.
Future Research
Piezo1 channel is a newly identified ion channel, which touches many areas in cell biology. It is expressed in different cell types, and its function may differ in different species, tissues, and local cellular environments. Despite the rapid advancement in Piezo1 research, more roles are yet to be explored. Piezo1 will be a novel candidate for global researchers to further explore its physiological and pathological functions as well as thorough understanding of its pharmacology. Additionally, future investigation on Piezo1 regulation of accompanying mechanisms related to AS will shine a light toward the future prospective and promising therapeutic strategies that may help to reduce the burden of disease and cost of treatment in various conditions, particularly AS in CVDs.
Conclusion
Atherosclerosis is the basis of CVDs, and the critical subject in cardiovascular research and the long-term major issue of global health care. Great efforts and advancement in biomedical as well as clinical research have brought significant achievements in the management of CVDs burden, particularly AS. Despite these remarkable achievements, residual risks yet remain, as many individuals suffer the disability of heart functions leading to the increased CVDs epidemic. AS is a multifactorial disorder; that makes it a complex disease which requires multiple approaches beyond lipid-lowering and lifestyle improvement. Besides these primary and other risks, considering mechanical forces including shear stress in form of disturbed flow should also be crucial. Consistent with the accumulating evidence that specific plaque location is in the curved and branching regions of the arteries where the flow is low and disturbed,129 and immune/inflammatory mechanisms involving Piezo1 signaling play critical role during the process, this shows that even newborn babies are counted in this risk, due to the branching and curved nature of blood vessels. This indicates the importance of turbulent shear, mediated by Piezo1 in the development and progression of atherosclerosis. There have been several experiments that have elucidated the mechanisms of Piezo1 signaling and its regulatory roles in the trigger of various inflammatory signals that initiate downstream events including activation of NLRP3 inflammasome and TLR4. However, we need to be clear that the initiation of signaling necessary inflammatory mechanisms and their downstream effects in NLRP3 inflammasome triggering play critical roles at different stages of triggering and the impacts they generate are miscellaneous. Piezo1 signaling has various impacts on inflammatory cells and their function including the initiation of inflammatory mechanisms and downstream triggering of the NLRP3 inflammasome, TLR4 and related Ca2+ dependent signaling which play crucial roles in the initiation and progression of atherosclerosis. This paper provides a new perspective where not just Piezo1 inflammatory signals and the downstream activation of TLR4 and NLRP3 inflammasome but also the signaling hubs between them are promising strategies to target against atherosclerosis that are worthwhile for future research. In this regard the scientific community should come up with new strategies using interdisciplinary approaches to resolve the challenges of disparity between in vivo physiological systems that have heterogeneity in cell behavior across vascular beds compared with our current homogenic in vitro experimental system. To achieve this, the following arguments should be considered.
Endothelial Model
HUVEC is the most commonly used model in research; the disparity of endothelial heterogeneity across vascular beds is rarely taken into account, in which endothelium from other origins could give different outcomes to the same approach. Indeed for clarity, a single study approach should be tested on endothelial models of multiple origin, as the cell behavior is spatially different across vascular beds.
Individual Mechano-Sensing Protein, Its Correlation with Other Proteins and Signaling Pathways
Several studies reported Piezo1 as an independent mechano-sensor and transducer of itself, independent of other ancillary proteins, while other works reported Piezo1 functions in correlation with other mechanosensitive proteins, such as TRPV4. This could be due to the cross-talk between different proteins in different cellular mechanisms and correlation with signaling pathways in a particular cell activity. Therefore, new approaches and strategies are required to resolve this argument. Further study targeting several mechanisms involving various proteins and their correlation in regulating multiple cellular signaling pathways are necessary, to elucidate more on particular function of each protein individually, and in correlation with others in different mechanism of cellular physiology.
Physiological Model of Flow in Different Vascular Beds and Rhythmic Changes
With physiological flow in vivo, shear pattern is transiently dynamic due to physiological variations in vascular bed, cardiac output or other metabolic needs, compared with ex vivo experimental systems that are commonly employed, the laminar or oscillatory shear pattern applied is always steady with no variation in intensity, amplitude or pattern (seen with in vivo) throughout the experiment. This creates a considerable gap among ex vivo and in vivo systems. Therefore, the shear sensing of Piezo1, the downstream impact, endothelial response and consequent gene expression may vary between ex vivo and in vivo. The transient variations of shear intensity and amplitude which commonly happen with in vivo should be considered when using in vitro experimental systems, since the common experimental systems used only apply steady, laminar, oscillatory or low turbulent shear which is not enough to precisely symbolize in vivo physiological shear experienced by EC. Here arises the requirement for advancing the current ex vivo experimental system to precisely meet the conditions similar to that of the in vivo physiological flow system.
In summary, Piezo1 is a promising candidate in cardiovascular research, due to the evidence already available on its physiological and pathological relevance in various angle of cardiovascular, regardless of its novelty. Its broad expression makes it likely to serve vital functions in regulating undiscovered aspects of cardiovascular physiology and pathology, and produce multiple functional outcomes depending on cell type and mechanisms involved. Therefore, innovative strategies through Piezo1 pharmacological approach will be advantageous for multiple therapies in cardiovascular disease, especially atherosclerosis.
Lastly, an intriguing fact in the Piezo1 pharmacology is the recent discovery of its activator (Yoda1), and inhibitor Grammostola spatulata mechano-toxin 4 (GsMTx4). It shows that with deep understanding of both Piezo1 and its pharmacology, their translation into the clinic may unlock the door and could be the promising therapeutic target for AS burden and other CVDs.
Abbreviations
AS, atherosclerosis; CVDs, cardiovascular disease; MI, myocardial infarction; IHD, ischemic heart disease; ECs, endothelial cells; VEC, vascular endothelial cell; RBCs, red blood cells; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; IFN-γ, interferon gamma; IL, interleukin; MCP-1, monocyte chemotactic protein-1; PECAM-1, platelet endothelial cell adhesion molecule; ICAM-1, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; KLF, Kruppel like factor; NF-κB, nuclear factor kappa B; eNOS, endothelial nitric oxide synthase; GM-CSF, granulocyte-macrophage colony-stimulating factor; ROX, reactive oxygen species; HUVECs, human umbilical vein endothelial cells.
Acknowledgments
Great appreciation to National Natural Science Foundation of China, Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province, and Luzhou Municipal People’s Government-Southwestern Medical University Science and Technology Strategic Coopration Project for supporting this work, and special regards to all the teams and staff of the Department of cardiovascular surgery and Clinical Research Center of Southwest Medical University for raising the discussions and exciting research environment for this paper.
Author Contributions
All authors made a significant contribution to the work reported, right from conception, study design, execution, acquisition of data, analysis and interpretation, and took part in drafting, revising and critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from National Natural Science Foundation of China [No.:31860261], and [No.: 11462022], together with “Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province” in 2019 [No.: xtcx2019-03 and No.:xtcx2019-04], and Luzhou Municipal People’s Government - Southwest Medical University Science and Technology Strategic Cooperation Project (No.: 2018LZXNYD-ZK27 and No.: 2018LZXNYD-ZK40) 2018.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi:10.1161/ATVBAHA.108.179705
2. Pourcet B, Staels B. Alternative macrophages in atherosclerosis: not always protective! J Clin Invest. 2018;128(3):910–912. doi:10.1172/JCI120123
3. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi:10.1056/NEJM199901143400207
4. Ruscica M, Corsini A, Ferri N, Banach M, Sirtori CR. Clinical approach to the inflammatory etiology of cardiovascular diseases. Pharmacol Res. 2020;159:104916.
5. O’Morain VL, Ramji DP. The potential of probiotics in the prevention and treatment of atherosclerosis. Mol Nutr Food Res. 2020;64(4):e1900797. doi:10.1002/mnfr.201900797
6. Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Global Health. 2020;8(5):e721–e729. doi:10.1016/S2214-109X(20)30117-0
7. Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34(10):2191–2198. doi:10.1161/ATVBAHA.114.303422
8. Albarran-Juarez J, Iring A, Wang S, et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215(10):2655–2672. doi:10.1084/jem.20180483
9. Beech DJ, Kalli AC. Force sensing by piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol. 2019;39(11):2228–2239. doi:10.1161/ATVBAHA.119.313348
10. Lukacs V, Mathur J, Mao R, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun. 2015;6:8329. doi:10.1038/ncomms9329
11. Evans EL, Cuthbertson K, Endesh N, et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. 2018;175(10):1744–1759. doi:10.1111/bph.14188
12. Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem. 2014;289(46):31673–31681. doi:10.1074/jbc.R114.612697
13. Coste B, Murthy SE, Mathur J, et al. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun. 2015;6:7223. doi:10.1038/ncomms8223
14. Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015;4:e12088. doi:10.7554/eLife.12088
15. Han Y, Liu C, Zhang D, et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int J Oncol. 2019;55(3):629–644. doi:10.3892/ijo.2019.4839
16. Geng J, Zhao Q, Zhang T, Xiao B. In touch with the mechanosensitive piezo channels: structure, ion permeation, and mechanotransduction. Curr Top Membr. 2017;79:159–195.
17. Hyman AJ, Tumova S, Beech DJ. Piezo1 channels in vascular development and the sensing of shear stress. Curr Top Membr. 2017;79:37–57.
18. Li J, Hou B, Beech DJ. Endothelial Piezo1: life depends on it. Channels (Austin). 2015;9(1):1–2. doi:10.4161/19336950.2014.986623
19. Ridone P, Vassalli M, Martinac B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev. 2019;11(5):795–805. doi:10.1007/s12551-019-00584-5
20. Retailleau K, Duprat F, Arhatte M, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13(6):1161–1171. doi:10.1016/j.celrep.2015.09.072
21. Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126(12):4527–4536. doi:10.1172/JCI87343
22. Burke SD, Jordan J, Harrison DG, Karumanchi SA. Solving baroreceptor mystery: role of PIEZO ion channels. J Am Soc Nephrol. 2019;30(6):911–913. doi:10.1681/ASN.2019020160
23. Alper SL. Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr Topics Membranes. 2017;79:97–134. doi:10.1016/bs.ctm.2017.01.001
24. Martin-Almedina S, Mansour S, Ostergaard P. Human phenotypes caused by PIEZO1 mutations; one gene, two overlapping phenotypes? J Physiol. 2018;596(6):985–992. doi:10.1113/JP275718
25. Zarychanski R, Schulz VP, Houston BL, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–1915. doi:10.1182/blood-2012-04-422253
26. Xiao B. Levering mechanically activated piezo channels for potential pharmacological intervention. Annu Rev Pharmacol Toxicol. 2020;60:195–218. doi:10.1146/annurev-pharmtox-010919-023703
27. Fang XZ, Zhou T, Xu JQ, et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021;11(1):13. doi:10.1186/s13578-020-00522-z
28. De Felice D, Alaimo A. Mechanosensitive piezo channels in cancer: focus on altered calcium signaling in cancer cells and in tumor progression. Cancers (Basel). 2020;12(7):1780. doi:10.3390/cancers12071780
29. Chang JE, Buechler MB, Gressier E, Turley SJ, Carroll MC. Mechanosensing by Peyer’s patch stroma regulates lymphocyte migration and mucosal antibody responses. Nat Immunol. 2019;20(11):1506–1516. doi:10.1038/s41590-019-0505-z
30. Song J, Liu L, Lv L, et al. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int. 2020;44(7):1491–1502. doi:10.1002/cbin.11344
31. Sun Y, Li M, Liu G, et al. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J Cancer Res Clin Oncol. 2020;146(5):1139–1152. doi:10.1007/s00432-020-03179-w
32. Velasco-Estevez M, Gadalla KKE, Linan-Barba N, Cobb S, Dev KK, Sheridan GK. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia. 2020;68(2):356–375. doi:10.1002/glia.23722
33. Zhong M, Komarova Y, Rehman J, Malik AB. Mechanosensing Piezo channels in tissue homeostasis including their role in lungs. Pulm Circ. 2018;8(2):2045894018767393. doi:10.1177/2045894018767393
34. Kang H, Hong Z, Zhong M, et al. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 2019;316(1):C92–C103. doi:10.1152/ajpcell.00346.2018
35. Rode B, Shi J, Endesh N, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8(1):1–11 doi:10.1038/s41467-017-00429-3.
36. Lim CG, Jang J, Kim C. Cellular machinery for sensing mechanical force. BMB Rep. 2018;51(12):623–629. doi:10.5483/BMBRep.2018.51.12.237
37. Douguet D, Patel A, Xu A, Vanhoutte PM, Honore E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci. 2019;40(12):956–970. doi:10.1016/j.tips.2019.10.002
38. Zhao Q, Zhou H, Chi S, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–492. doi:10.1038/nature25743
39. Jiang Y, Yang X, Jiang J, Xiao B. Structural designs and mechanogating mechanisms of the mechanosensitive piezo channels. Trends Biochem Sci. 2021;46(6):472–488. doi:10.1016/j.tibs.2021.01.008
40. Zheng W, Gracheva EO, Bagriantsev SN. A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels. Elife. 2019;8:e44003. doi:10.7554/eLife.44003
41. Liang X, Howard J. Structural biology: piezo senses tension through curvature. Curr Biol. 2018;28(8):R357–R359. doi:10.1016/j.cub.2018.02.078
42. Kamajaya A, Kaiser JT, Lee J, Reid M, Rees DC. The structure of a conserved piezo channel domain reveals a topologically distinct beta sandwich fold. Structure. 2014;22(10):1520–1527. doi:10.1016/j.str.2014.08.009
43. Wu J, Lewis AH, Grandl J. Touch, tension, and transduction - the function and regulation of piezo ion channels. Trends Biochem Sci. 2017;42(1):57–71. doi:10.1016/j.tibs.2016.09.004
44. Gottlieb PA. A tour de force: the discovery, properties, and function of piezo channels. Curr Top Membr. 2017;79:1–36.
45. Li J, Hou B, Tumova S, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–282. doi:10.1038/nature13701
46. Ranade SS, Qiu Z, Woo SH, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111(28):10347–10352. doi:10.1073/pnas.1409233111
47. Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi:10.1126/science.1193270
48. Liu X, Sun A, Fan Y, Deng X. Physiological significance of helical flow in the arterial system and its potential clinical applications. Ann Biomed Eng. 2015;43(1):3–15. doi:10.1007/s10439-014-1097-2
49. Baratchi S, Chen YC, Peter K. Helical flow: a means to identify unstable plaques and a new direction for the design of vascular grafts and stents. Atherosclerosis. 2020;300:34–36. doi:10.1016/j.atherosclerosis.2020.03.002
50. De Nisco G, Hoogendoorn A, Chiastra C, et al. The impact of helical flow on coronary atherosclerotic plaque development. Atherosclerosis. 2020;300:39–46. doi:10.1016/j.atherosclerosis.2020.01.027
51. Zhang L, Yu J, Wei W. Advance in targeted immunotherapy for graft-versus-host disease. Front Immunol. 2018;9:1087. doi:10.3389/fimmu.2018.01087
52. Chen Z, Fan Y, Deng X, Xu Z. Swirling flow can suppress flow disturbances in endovascular stents: a numerical study. ASAIO J. 2009;55(6):543–549. doi:10.1097/MAT.0b013e3181b78e46
53. Beech DJ. Endothelial Piezo1 channels as sensors of exercise. J Physiol. 2018;596(6):979–984. doi:10.1113/JP274396
54. Chatzizisis YS, Giannoglou GD, Parcharidis GE, Louridas GE. Is left coronary system more susceptible to atherosclerosis than right? A pathophysiological insight. Int J Cardiol. 2007;116(1):7–13. doi:10.1016/j.ijcard.2006.03.029
55. Blythe NM, Muraki K, Ludlow MJ, et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J Biol Chem. 2019;294(46):17395–17408. doi:10.1074/jbc.RA119.009167
56. Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res. 2013;99(2):260–268. doi:10.1093/cvr/cvt084
57. Beech DJ, Xiao B. Piezo channel mechanisms in health and disease. J Physiol. 2018;596(6):965–967. doi:10.1113/JP274395
58. Fels B, Kusche-Vihrog K. It takes more than two to tango: mechanosignaling of the endothelial surface. Pflugers Arch. 2020;472(4):419–433. doi:10.1007/s00424-020-02369-2
59. Brown BM, Nguyen HM, Wulff H. Recent advances in our understanding of the structure and function of more unusual cation channels. F1000Res. 2019;8:123. doi:10.12688/f1000research.17163.1
60. Nagel T, Resnick N, Dewey CF, Gimbrone MA. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol. 1999;19(8):1825–1834. doi:10.1161/01.ATV.19.8.1825
61. Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal. 2011;15(5):1405–1414. doi:10.1089/ars.2010.3679
62. Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91(4):281–291. doi:10.1161/01.RES.0000029784.15893.10
63. Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116(2):307–311. doi:10.1161/CIRCRESAHA.116.301313
64. Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72(17):2071–2081. doi:10.1016/j.jacc.2018.08.1043
65. Solis AG, Bielecki P, Steach HR, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573(7772):69–74. doi:10.1038/s41586-019-1485-8
66. Walmsley SR. Pressure regulate immune cell function. Nature. 2019;573(7772):41–42. doi:10.1038/d41586-019-02339-4
67. Williams ER. PIEZO1 promotes inflammation. Sci Signal. 2019;12(598):eaaz4154.
68. Liu CSC, Raychaudhuri D, Paul B, et al. Cutting edge: piezo1 mechanosensors optimize human T cell activation. J Immunol. 2018;200(4):1255–1260. doi:10.4049/jimmunol.1701118
69. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. doi:10.1161/CIRCRESAHA.114.302721
70. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi:10.1161/hc0902.104353
71. Chatterjee S. Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory pathways. Front Physiol. 2018;9:524. doi:10.3389/fphys.2018.00524
72. Mantella LE, Quan A, Verma S. Variability in vascular smooth muscle cell stretch-induced responses in 2D culture. Vasc Cell. 2015;7:7. doi:10.1186/s13221-015-0032-0
73. Jufri NF, Mohamedali A, Avolio A, Baker MS. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc Cell. 2015;7:8. doi:10.1186/s13221-015-0033-z
74. Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–744. doi:10.1038/s41569-019-0227-9
75. Chen Y, Waqar AB, Nishijima K, et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J Cell Mol Med. 2020;24(7):4261–4274. doi:10.1111/jcmm.15087
76. Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123(1):27–36. doi:10.1172/JCI63108
77. Heo KS, Berk BC, Abe J. Disturbed flow-induced endothelial proatherogenic signaling via regulating post-translational modifications and epigenetic events. Antioxid Redox Signal. 2016;25(7):435–450. doi:10.1089/ars.2015.6556
78. Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiologica. 2017;219(2):382–408. doi:10.1111/apha.12725
79. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi:10.1152/physrev.00047.2009
80. Tovar-Lopez F, Thurgood P, Gilliam C, et al. A microfluidic system for studying the effects of disturbed flow on endothelial cells. Front Bioeng Biotechnol. 2019;7:81. Doi:10.3389/fbioe.2019.00081
81. Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends Mol Med. 2017;23(9):850–868. doi:10.1016/j.molmed.2017.07.007
82. Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci. 2019;26(1):56. doi:10.1186/s12929-019-0551-8
83. Wang S, Iring A, Strilic B, et al. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J Clin Invest. 2015;125(8):3077–3086. doi:10.1172/JCI81067
84. Tanaka K, Joshi D, Timalsina S, Schwartz MA. Early events in endothelial flow sensing. Cytoskeleton. 2021. doi:10.1002/cm.21652
85. Nakayama A, Albarran-Juarez J, Liang G, et al. Disturbed flow-induced Gs-mediated signaling protects against endothelial inflammation and atherosclerosis. JCI Insight. 2020;5(23). doi:10.1172/jci.insight.140485.
86. Givens C, Tzima E. Endothelial mechanosignaling: does one sensor fit all? Antioxid Redox Signal. 2016;25(7):373–388. doi:10.1089/ars.2015.6493
87. Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108(2):251–259. doi:10.1172/JCI200111380
88. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi:10.1056/NEJMra043430
89. Pageon SV, Govendir MA, Kempe D, Biro M. Mechanoimmunology: molecular-scale forces govern immune cell functions. Mol Biol Cell. 2018;29(16):1919–1926. doi:10.1091/mbc.E18-02-0120
90. Liu CSC, Ganguly D. Mechanical cues for T cell activation: role of piezo1 mechanosensors. Crit Rev Immunol. 2019;39(1):15–38. doi:10.1615/CritRevImmunol.2019029595
91. Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5(196):196ra100. doi:10.1126/scitranslmed.3006133
92. Jin Y, Fu J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J Am Heart Assoc. 2019;8(12):e012219. doi:10.1161/JAHA.119.012219
93. Chen X, Guo X, Ge Q, Zhao Y, Mu H, Zhang J. ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis. Oxid Med Cell Longev. 2019;2019:1–18.
94. Ingber DE. From mechanobiology to developmentally inspired engineering. Philos Trans R Soc Lond B Biol Sci. 2018;373(1759):20170323. doi:10.1098/rstb.2017.0323
95. Faucherre A, Moha Ou Maati H, Nasr N, et al. Piezo1 is required for outflow tract and aortic valve development. J Mol Cell Cardiol. 2020;143:51–62. doi:10.1016/j.yjmcc.2020.03.013
96. Asmussen A, Fink K, Busch H-J, et al. Inflammasome and toll-like receptor signaling in human monocytes after successful cardiopulmonary resuscitation. Critical Care. 2016;20(1):1–5. doi:10.1186/s13054-016-1340-3.
97. Arbore G, Kemper C. A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol. 2016;46(7):1563–1573. doi:10.1002/eji.201546131
98. Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233(3):2116–2132. doi:10.1002/jcp.25930
99. Luo H, Liu H, Bian W, Chen B, Yang D, Yang M. Yoda1 activates piezo1 in vitro to simulate the upregulation of piezo1 in the infected brain: piezo1 participates in the immune activation of microglia. 2021.
100. Koushki K, Shahbaz SK, Mashayekhi K, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. 2021;60(2):175–199.
101. Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73.
102. Liu Y, Dai Y, Li Q, et al. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci Lett. 2020;736:135279. doi:10.1016/j.neulet.2020.135279
103. Wu J, Goyal R, Grandl J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat Commun. 2016;7:12939. doi:10.1038/ncomms12939
104. Chen X, Wanggou S, Bodalia A, et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron. 2018;100(4):799–815 e797. doi:10.1016/j.neuron.2018.09.046
105. Maruyama K, Nemoto E, Yamada S. Mechanical regulation of macrophage function - cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1beta secretion in murine macrophages. Inflamm Regen. 2019;39:3. doi:10.1186/s41232-019-0092-2
106. Sun Y, Leng P, Song M, et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca(2+)/NF-kappaB pathway. Int Immunopharmacol. 2020;85:106681. doi:10.1016/j.intimp.2020.106681
107. Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol. 2013;190(7):3590–3599. doi:10.4049/jimmunol.1200860
108. Stojadinovic O, Minkiewicz J, Sawaya A, et al. Deep tissue injury in development of pressure ulcers: a decrease of inflammasome activation and changes in human skin morphology in response to aging and mechanical load. PLoS One. 2013;8(8):e69223. doi:10.1371/journal.pone.0069223
109. Maruyama K, Sakisaka Y, Suto M, et al. Cyclic stretch negatively regulates IL-1beta secretion through the inhibition of NLRP3 inflammasome activation by attenuating the AMP kinase pathway. Front Physiol. 2018;9:802. doi:10.3389/fphys.2018.00802
110. Hu N, Zhang Y. TLR4 knockout attenuated high fat diet-induced cardiac dysfunction via NF-kappaB/JNK-dependent activation of autophagy. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2001–2011. doi:10.1016/j.bbadis.2017.01.010
111. Lacroix JJ, Botello-Smith WM, Luo Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat Commun. 2018;9(1):2029. doi:10.1038/s41467-018-04405-3
112. Botello-Smith WM, Jiang W, Zhang H, et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun. 2019;10(1):4503. doi:10.1038/s41467-019-12501-1
113. Goldblatt ZE, Cirka HA, Billiar KL. Mechanical regulation of apoptosis in the cardiovascular system. Ann Biomed Eng. 2021;49(1):75–97. doi:10.1007/s10439-020-02659-x
114. Chan DD, Van Dyke WS, Bahls M, et al. Mechanostasis in apoptosis and medicine. Prog Biophys Mol Biol. 2011;106(3):517–524. doi:10.1016/j.pbiomolbio.2011.08.002
115. Muluh TA, Chen Z, Li Y, et al. Enhancing cancer immunotherapy treatment goals by using nanoparticle delivery system. Int J Nanomedicine. 2021;16:2389–2404. doi:10.2147/IJN.S295300
116. Gnanasambandam R, Bae C, Gottlieb PA, Sachs F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One. 2015;10(5):e0125503. doi:10.1371/journal.pone.0125503
117. Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. doi:10.1016/j.tibs.2011.03.006
118. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi:10.1016/j.cell.2017.02.004
119. Aminzadeh A, Salarinejad A. Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells. Toxicol Res (Camb). 2021;10(1):84–90. doi:10.1093/toxres/tfaa104
120. Zhang R, Zhang N, Zhang H, et al. Celastrol prevents cadmium-induced neuronal cell death by blocking reactive oxygen species-mediated mammalian target of rapamycin pathway. Br J Pharmacol. 2017;174(1):82–100. doi:10.1111/bph.13655
121. Syeda R, Xu J, Dubin AE, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015;4:e07369. Doi:10.7554/eLife.07369
122. Wang Y, Chi S, Guo H, et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9(1):1300. doi:10.1038/s41467-018-03570-9
123. Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295–6300. doi:10.1021/bi200770q
124. Beqja D, Haidar S, Nikolaev M, Shen Y, Denholm B. Transgenic tarantula toxin: a novel tool to study mechanosensitive ion channels in Drosophila. J Insect Physiol. 2020;127:104116. doi:10.1016/j.jinsphys.2020.104116
125. Gnanasambandam R, Ghatak C, Yasmann A, et al. GsMTx4: mechanism of inhibiting mechanosensitive ion channels. Biophys J. 2017;112(1):31–45. doi:10.1016/j.bpj.2016.11.013
126. Suchyna TM. Piezo channels and GsMTx4: two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog Biophys Mol Biol. 2017;130(Pt B):244–253. doi:10.1016/j.pbiomolbio.2017.07.011
127. Coste B, Xiao B, Santos JS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi:10.1038/nature10812
128. Liu S, Pan X, Cheng W, et al. Tubeimoside I antagonizes yoda1-evoked piezo1 channel activation. Front Pharmacol. 2020;11:768. doi:10.3389/fphar.2020.00768
129. Zhao Y, Ren P, Li Q, et al. Low shear stress upregulates CX3CR1 expression by inducing VCAM-1 via the NF-kappaB pathway in vascular endothelial cells. Cell Biochem Biophys. 2020;78(3):383–389. doi:10.1007/s12013-020-00931-4
130. Fan Y, Xu Z, Jiang W, Deng X, Wang K, Sun A. An S-type bypass can improve the hemodynamics in the bypassed arteries and suppress intimal hyperplasia along the host artery floor. J Biomech. 2008;41(11):2498–2505. doi:10.1016/j.jbiomech.2008.05.008
131. Caro CG, Seneviratne A, Heraty KB, et al. Intimal hyperplasia following implantation of helical-centreline and straight-centreline stents in common carotid arteries in healthy pigs: influence of intraluminal flow. J R Soc Interface. 2013;10(89):20130578. doi:10.1098/rsif.2013.0578
132. Zhan F, Fan Y, Deng X. Swirling flow created in a glass tube suppressed platelet adhesion to the surface of the tube: its implication in the design of small-caliber arterial grafts. Thromb Res. 2010;125(5):413–418. doi:10.1016/j.thromres.2009.02.011
133. Doty DB, Flores JH, Doty JR, Millar RC. Mitral valve replacement with homograft. Semin Thorac Cardiovasc Surg. 1999;11(4 Suppl 1):191–193.
134. Chen Z, Zhan F, Fan Y, Deng X. A novel way to reduce thrombus build-up in vena cava filters. Catheter Cardiovasc Interv. 2011;78(5):792–798. doi:10.1002/ccd.23107
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.