Back to Journals » Cancer Management and Research » Volume 11
Mechanisms Underlying Therapeutic Effects Of Traditional Chinese Medicine On Gastric Cancer
Authors Yang L, Zhao L, Zeng T , Chen H, Shao J, Yang S, Tao Z, Yang J, Chen T, Lin X , Chen X, Tang M
Received 4 June 2019
Accepted for publication 27 August 2019
Published 16 September 2019 Volume 2019:11 Pages 8407—8418
DOI https://doi.org/10.2147/CMAR.S218214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Linjun Yang,1,* Liqian Zhao,1,* Tianni Zeng,1 Hong Chen,1 Jingjing Shao,1 Song Yang,1 Zheying Tao,1 Jingqin Yang,1 Tongke Chen,1 Xiaokun Lin,2 Xiwen Chen,1 Mosheng Tang3
1Department of Laboratory Animal Centre, Laboratory Animal Centre, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Pediatric Surgery, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 3Department of Radiotherapy and Chemotherapy, Lishui City People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mosheng Tang
Department of Radiotherapy and Chemotherapy, Lishui City People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang, People’s Republic of China
Tel +86 0577 86689908
Email [email protected]
Xiwen Chen
Laboratory Animal Centre, Wenzhou Medical University, Lucheng district, Wenzhou, Zhejiang, People’s Republic of China
Tel +86 0577 86689907
Email [email protected]
Abstract: Gastric cancer (GC) is one of the most common malignant tumors in the world. It is the fourth most common cancer and has the second highest mortality rate globally. Metastasis is an important feature of gastric cancer and is the most common cause of death. Exploring the mechanism underlying the metastasis of gastric cancer and searching for new drug targets has become the focus of several studies. Traditional Chinese medicine may show promise for treatment of gastric cancer. In this review, we report the recent progress in research on the anti-metastasis activity of Chinese medicine, to facilitate clinical development of treatments for gastric cancer.
Keywords: Chinese medicine, metastasis, gastric cancer, cell cycle, angiogenesis
Introduction
Gastric cancer is the most common malignant tumor of the digestive system. China accounts for 42.6% of gastric cancer cases globally,1 and one of the main causes of death is metastasis of malignant tumors.2 With the continuous study of traditional Chinese medicine, numerous studies have illuminated that in cancer treatment it can be used to enhance the efficacy and diminish the side effects. Chen et al.3 Found that Huaier make the effective of HCC after curative liver resection which can extend RFS and reduce extrahepatic recurrence. In addition, Von Hagens et al.4 illustrated that in a phase I study, patients who have metastatic breast cancer with Long-term oral artesunate can consolidate the original curative effect and safety. Deeken et al.5 indicated that intravenous artesunate is 18 mg/kg which the treatment of solid tumor was well tolerated and diminishing the side effect. Compared to chemotherapy and radiotherapy in Western medicine, traditional Chinese medicine places considerable emphasis on overall regulation, and it has been proved to be an effective therapy for reducing side effects, improving the survival rate, and prolonging the survival period in cancer model systems. There are some anti-radiation traditional Chinese medicine in radiotherapy.6 Traditional Chinese medicine is proved to be efficacious in increasing the sensitivity to chemo- and radio-therapies.7 And evidence from the meta-analysis suggested that traditional Chinese medicine combined with chemotherapy has a positive effect on gastric cancer treatment.8 It can be seen that traditional Chinese medicine is of great significant for assitant-therapy to decline the side effect, safe and tolerable. The purpose of this review is to clarify the mechanism by which traditional Chinese medicine helps in the treatment of gastric cancer and prevention of metastasis as shown in Table 1.
 |  |  |  |
Table 1 Traditional Chinese Medicine On Gastric Cancer |
Inhibition Of Proliferation Of Gastric Cancer Cells
The proliferation of tumor cells is the foundation of tumor invasion and metastasis. Excessive proliferation of tumor cells at the primary focal center increases pressure and promotes invasion and metastasis in the direction of low pressure, resulting in metastasis to neighboring tissue and distant organs. Traditional Chinese medicine and its components can suppress the proliferation of gastric cancer by arresting the cell cycle, inhibiting telomerase activity, inducing apoptosis via the mitochondrial pathway, and regulating inflammation-related factors.
Cell Cycle Arrest
Uncontrolled cell proliferation is one of the fundamental biological characteristics of tumors, based on dysregulation of the cell cycle. Various tumor components operate through cell cycle arrest. Cell cycle-dependent kinase (CDK2 and CDK4) plays a key role in cell cycle progression and is closely related to the entry of cells into S and G1 phase.9 Cell cycle arrest of tumor cells by traditional Chinese medicine is related to the loss of cell cycle-dependent protein kinase inhibitory factor activity, thereby inhibiting the CDK suppression signal and leading to cell cycle regulation disorder. Several studies have reported that10,11 matrines (including sophocarpine, oxysophocarpine, and sophoridine), astragalus polysaccharides (including ascorbic acid, glucose, and fructose), and cinobufotalin (an anti-cancer drug) can induce G1 cycle arrest. Sun et al.12 found that Coleusin factor induced cell cycle arrest at the G0/G1 stage in SGC-7901 cells. Other studies13,14 reported that berberine and ailanthone induced G2/M cell cycle arrest and inhibited the proliferation of gastric cancer cells.
Inhibition Of Telomerase Activity
Recent studies have discovered that telomerase is expressed in more than 85% of cancers, making telomerase an attractive and viable target for the development of anti-cancer therapeutics.15,16 Telomerase may play an important role in the process of tumor proliferation. Moreover, inhibiting tumor cell telomerase activity through traditional Chinese medicine can effectively inhibit tumor cell proliferation and interfere with metastasis of tumor cells.
Çalışkan et al.17 found that telomerase activity regulates the expression of human telomerase reverse transcriptase (hTERT) in gastric cancer cells, and suggested that inhibiting telomerase activity may be a potential anti-cancer therapeutic mechanism. Another study18 utilized the PCR-TRAP method to evaluate telomerase activity in BGC-823 human gastric carcinoma cells, and found that gambogic acid (GA) can inhibit telomerase activity by inhibiting the phosphorylation of Akt and reducing the expression of hTERT. Duan et al.19 demonstrated that piperlongumine (PL) acts as an anti-cancer agent by inhibiting telomerase activity and cell proliferation in vitro and in vivo.
Mitochondrial Apoptosis Pathway
Wang et al.20 found that the ethyl acetate extract of Celastrus orbiculatus (C. orbiculatus extract, COE) could change the mitochondrial membrane potential of the cell, downregulate the apoptosis-related proteins Bax and caspase, and increase the expression of bcl-2 and PI3K/Akt. These data suggest that the COE may inhibit the proliferation and metastasis of gastric cancer cells by inducing apoptosis. Pseudolaric acid (PAB) has been shown to promote the apoptosis of various cells. Wang et al.21 evaluated the molecular mechanism of the antitumor activity of PAB in human leukemia U937 cells. U937 cells were observed to be activated by the Bcl-2-mediated mitochondrial pathway, and activation of the caspase-dependent pathway was regulated by PAB. In addition, the activity of caspase-3 increased after PAB treatment. This study thus showed that PAB can enhance U937 cell apoptosis, at least in part through the activation of the mitochondrial apoptotic pathway. Mao et al.22 found that crocodile bile accelerated cell apoptosis via the mitochondrial apoptosis pathway. Simultaneously, it reduced the mitochondrial membrane potential, and increased the production of reactive oxygen species, the Bax/Bcl-2 ratio, the levels of activated caspase-3, and the release of cytochrome C. These data indicate that crocodile choline is a potent inhibitor of gastric cancer cells via the mitochondrial apoptosis pathway.
Other Aspects
The literature shows that traditional Chinese medicine also exhibits anti-gastric cancer effects through autophagy.23 Curcumin24 may inhibit proliferation and induce the autophagy and apoptosis in GC cells through MTT assay and transmission electron microscopy (TEM). Lei25 find that mulberry anthocyanins intervented SGC-7901 cells induced autophagy byTEM observation results.
Some studies have shown that traditional Chinese medicine can inhibit the effect of tumor cells through ROS. Kim DH et al26 reported for the first time that ISL induces apoptosis of renal cell carcinoma Caki cells by producing ROS, thus inducing p53 and inhibiting Stat3 signaling pathway. Silybin27 can induce caspase-dependent cell death in vitro and inhibit the growth of glioma in vivo through Ca2+/ROS/MAPK-mediated pathway.
There are other natural products, bitter melon extract (BME), suppressing the cancer proliferation via different mechanisms. Bhattacharya et al.28 shown that BME enhances NK cell-mediated HNSCC killing activity and reveals the potential immunomodulatory effects of BME. Muhammad et al.29 found that BME attains anti-tumor activity by induction of autophagy and AMPK/mTOR pathway. Subsequently, they demonstrated that BME oral feeding effectively inhibited cancer cell growth in isogenic and xenograft mouse models. In addition, Bhattacharya et al.30 shown that BME inhibited cell proliferation in BME orally-fed mouse tumors via lowering expression of proliferating cell nuclear antigen (PCNA) and c-Myc. Both of their studies indicates BME high potential clinical application.
Interference With Angiogenesis
By co-culturing endothelial cells with human or animal macrophages or supernatants, it was found that the angiogenic extracts caused chemotaxis of the peritoneal macrophages and human mononuclear cells in guinea pigs. These results imply that tumor extracts act indirectly to induce angiogenesis in vivo via their effect on host macrophages.31 Angiogenesis can be controlled by inhibiting the proliferation of vascular endothelial cells (VECs) and by regulating vascular growth factors.
Inhibition Of The Proliferation Of VECs
Angiogenesis is an essential pathological process in the metastasis of malignant tumors, and endothelial cell proliferation is the basis of angiogenesis. Therefore, the discovery and screening of drugs that inhibit the proliferation of VECs has become a focus of tumor clinical research. Zang et al.32 reported that Luteolin has an inhibitory effect on gastric cancer angiogenesis and Vasculogenic mimicry (VM) formation induced by inhibiting VEGF secretion, which is dependent on Notch1 expression. Huang et al.33 observed that curcumin could inhibit GC-MSC driven angiogenesis, which inhibits the proliferation of vascular endothelial cells, producing an anti-tumor effect. Lynne M Howells et al supported that in the treatment of patients with metastatic colorectal cancer. Curcumin is well safety and tolerated combining with FOLFOX chemotherapy. Bing et al.34 reported that Xiaotansanjie decoction attenuates microvessel density by inhibiting the proliferation of vascular endothelial cells in gastric cancer.
Angiogenic Factors
Under conditions of local hypoxia, several molecular responses are induced during tumor growth which activate hypoxia-inducible factors. The transcription factor HIF accumulates in endothelial cells under hypoxic conditions and binds to the VEGF promoter to induce VEGF expression, resulting in angiogenesis.35 Dai et al.36 reported that Ginsenoside Rg3 inhibits tumor growth and reduces lymph node metastasis by inhibiting the expression of VEGF-C, VEGF-D, and VEGFR-3 in an orthotopic mouse model of human gastric cancer. Pang et al.37 reported that xiaotan sanjie can inhibit proliferation of human gastric adenocarcinoma cells MKN-45 by attenuating tumor angiogenesis, which may be related to downregulation of the mRNA and protein expressions of VEGF-C and VEGFR-3.
Inhibition Of Gastric Cancer Cell Movement
Local invasion and distant metastasis are the most important biological features of malignant carcinoma. Tumors with invasive growth can not only continue to grow at the original site, but also spread directly to the surrounding tissue, and can also spread to other parts of the body through a variety of ways. Therefore, inhibiting the metastasis of gastric cancer cells is particularly important in the treatment of tumor, including inhibition of the migration and invasion of gastric cancer cells and the adhesion of gastric cancer cells.
Migration And Invasion
The migration and invasion capabilities of gastric cancer cells are associated with metastasis.38 Once proliferating gastric cancer cells reach a certain density, they will invade the adjacent basement membrane and extracellular matrix, penetrate blood vessels, and enter the circulatory system. Via migration through blood vessels, a small metastasis focus will be formed on a target organ.39 Other studies have also indicated that MMP-2 and MMP-9 facilitate cell migration and invasion. MMP-2 is involved in the metabolism of type IV collagen in the basement membrane, which is often the initial step in cancer invasion and metastasis.40,41 In addition, MMP-9, which is the main enzyme for degrading extracellular matrix (ECM), is an important mediator of migration and invasion for gastric cancer cells.42 Lu et al.43 reported that Silibinin acts by inhibiting the invasion and metastasis of human gastric cancer SGC-7901 cells, and by downregulating MMP-9 and MMP-2. Yan et al.44 found that Baicalein could inhibit the proliferative, adhesive, invasive, and migratory abilities of SGC-7901 cells. The underlying mechanism was the inhibition of the expression of MMP-9 and MMP-2 by suppressing the activity of the p38 signaling pathway. Zhang et al.45 demonstrated that alisol B can regulate phosphorylation of vasodilator-stimulated phosphoprotein (VASP) to inhibit gastric cancer cell adhesion and migration.
Adhesion
An increase in cell adhesion molecule expression and degradation of the extracellular matrix can control cancer metastasis and reduce migration of cancer cells.
Regulating The Expression Of Adhesion Molecules
Adhesion molecules regulate cell-cell adhesion, as well as adhesion of cells to the extracellular matrix. Reducing adhesion between malignant tumor cells and enhancing adhesion of tumor cells to host cells and the extracellular matrix are two of the basic mechanisms of malignant tumor metastasis. Adhesion is regulated by focal adhesion molecules including CD44 and E-Cad. One of the functions of CD44 is to regulate the adhesion and migration of tumor cells. In the active state, the cytoplasmic domain of CD44 interacts with the actin cytoskeleton, which promotes migration of tumor cells.46 Sun et al.47 found that HepG2 cells cultured with the serum of rats fed with Biejiajian Pills showed lower expressions of β-catenin protein both in the cytoplasm and the nuclei along with the inhibition of GSK-3β phosphorylation and reduced expression of CD44v6 and VEGF. Biejiajian Pills suppress the proliferation and invasiveness of hepatocellular carcinoma. Xu et al.48 found that Huaier could partly reverse the epithelial-mesenchymal transition (EMT) by decreasing the expression of the mesenchymal markers N-cadherin and vimentin and increasing the expression of the epithelial marker E-cadherin. Huaier extract significantly inhibited both the migratory and invasive abilities of gastric cancer cells. Ma et al Indicated that by Comprehensive clinical analysis, Huaier might enhance clinical therapeutic effects and decline the side ones with Gastrointestinal cancers.
Inhibiting Extracellular Matrix Degradation
MMPs are the major zymogen family proteins that degrade tumor extracellular matrix (ECM). MMPs can promote tumor cell invasion of the ECM by degrading it, which permits tumor cells to migrate to other sites. Wu et al.49 found that the combination of Apatinib and AsPs could inhibit the expression of phosphorylated AKT (p-AKT) and MMP-9 expression. Apatinib, in combination with AsPs, showed enhanced inhibitory effects on cell proliferation, migration, and invasion compared with Apatinib monotherapy. Jiao et al.50 found that the migration and proliferation of the SGC-7901 cells were decreased after incubation with different concentrations of Tanshinone IIA (TSN) in a dose-dependent manner. Moreover, the expression levels of Ki-67, PCAN, MMP-2, MMP-9, and FOXM1 were decreased, and P21 was increased in the TSN-treated SGC-7901 cells.
Modulation Of Metastasis-Related Genes To Inhibit Metastasis
Inhibition of gastric cancer cell metastasis can be achieved by regulating metastasis-related gene expression in gastric cancer. In 2003, Ishikawa et al.51 reported that VEGF-C and -D genes may be associated with lymphatic metastasis of early gastric cancer, which suggested that VEGF-C and -D genes inhibit tumor metastasis. Dai et al.36 reported that ginsenosides can target vegf-c, vegf-d, and vegfr-3 genes in mouse models of human gastric cancer cells and inhibit the progression and metastasis of malignant tumors. Bao et al.52 reported that naringenin may inhibit the migration and invasion of human gastric cancer SGC-7901 cells by reducing the expression of metastasis related genes MMP-2 and MMP-9. Liu et al.53 reported that Dehydroeffusol can significantly inhibit the migration and invasion of gastric cancer cells in vivo and in vitro. Dehydroeffusol can inhibit gene promoter activity, influence the expression of the vasculogenic mimicry master gene VE-cadherin, and significantly reduce the expression of metastasis associated genes in gastric cancer cells (MMP2), thus effectively inhibiting gastric cancer metastasis. The proliferation of gastric cancer cells not only increases the pressure between gastric cancer cells, but also enhances metastasis. Most traditional Chinese medicines exhibit their anti-metastasis effect by regulating the relevant oncogenes and anti-oncogenes to further inhibit cellular proliferation and induce apoptosis. Recent studies have demonstrated that metastasis-associated genes include Bcl-2, Bax, maspin, and p53. Mu et al.54 showed that baicalein has anti-apoptotic activity by downregulating Bcl-2 and upregulating Bax in SGC-7901 cells. Qian et al.55 indicated that COE could induce apoptosis of human gastric cancer MGC-803 cells overexpressing the maspin gene.
Regulation Of Inflammation-Related Factors
Cancer-related inflammation can promote tumorigenesis, tumor growth, and tumor metastasis in many types of cancers. Therefore, inhibiting cancer-related inflammation significantly improves the effects of cancer therapy. It is known that neoplastic cells often over-express proinflammatory mediators including proteases, eicosanoids, cytokines, and chemokines.56 Moreover, 40 years ago, the phenomenon of TNF-mediated cell death in vitro and its physiological and pathophysiological effects were first described.57,58 Blocking TNF causes TNFR1 to no longer activate MAPK and NF-κB signaling, resulting in downregulation of proinflammatory genes.59 Curcumin plays an anti-cancer role by inhibiting the occurrence, progression, and metastasis of various cancers, and inhibits cancer by two main processes that affect angiogenesis and tumor growth. Curcumin inhibits lymph node metastasis in gastric cancer, and its pleiotropic activity is mediated by modulating proinflammatory cytokines, and other signaling molecules, Human protein STAT3, NF-κB, COX-2, 5-LOX, C- reactive protein, prostaglandin E (2), prostatic specific antigen, adhesion molecule, phosphorylase kinase, triglyceride, transforming growth factor beta, ET-1 HO-1, creatinine, ALT, and AST.60–63 Patchouli alcohol (PA), the main active ingredient of Pogostemonis herba, has anti-Helicobacter pylori and gastroprotective functions. In addition, PA significantly reduced the levels of pro-inflammatory cytokines such as interleukin −4 (IL-4), IL-2, and tumor necrosis factor (TNF-alpha), and increased the level of the anti-inflammatory cytokine IL-13 in GES-1 cells. To a certain extent, reducing the inflammatory reaction of the gastric mucosa can help block the precancerous lesions of gastric cancer.64
Conclusion
This article summarizes how Chinese medicine can intervene at multiple levels and affect multiple targets to influence the metastasis of gastric cancer as shown in Figure 1. However, it is difficult to confirm the active ingredients of traditional Chinese medicines due to their multimodal anti-tumor effects and complex composition, thus greatly limiting their potential. Additionally, clinical research for traditional Chinese medicine still lacks in-depth study and evaluation criteria, and as a result its authenticity is largely unrecognized. These findings suggest that the multi-effects of traditional Chinese medicine should be combined with those of Western medicine, allowing integrative medicine to be used to control tumors. It is expected that further rigorous research will demonstrate that traditional Chinese medicine has clinical significance for treatment of the metastasis of gastric cancer and will lead to improvement of preventive measures.
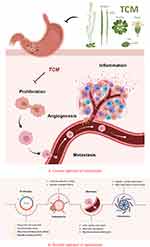 |
Figure 1 Mechanisms underlying therapeutic effects of traditional Chinese medicine on gastric cancer. (A) General approach of mechanisms. (B) Detailed approach of mechanisms. |
With the continuous discovery and deeper exploration of the mechanism of gastric cancer and identification of the new molecular targets, the target of Chinese medicine in gastric cancer has been found, and the effects of traditional Chinese medicine have been determined at the molecular level, which provides the basis for clinical applications of traditional Chinese medicine.
Acknowledgments
We thank the grants provided from Natural Science Foundation of China Grant (81673733), Zhejiang Provincial Natural Science Foundation of China Grant (LQ17H120009), College Student Innovation and Entrepreneurship Training Program of China (201710343015).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Tsai SC, Huang SF, Chiang JH, et al. The differential regulation of microRNAs is associated with oral cancer. Oncol Rep. 2017;38(3):1613. doi:10.3892/or.2017.5851
3. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018. doi:10.1136/gutjnl-2018-315983
4. Von Hagens C, Walter-Sack I, Goeckenjan M, et al. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2). Phytomedicine. 2018;54:140–148.
5. Deeken JF, Wang H, Hartley M, et al. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81:587–596. doi:10.1007/s00280-018-3533-8
6. Bo-Shu L, Dong LI, Jin-Ping L, et al. Anti-radiation traditional Chinese medicine and natural products: researchadvances. J Int Pharm Res. 2015;4:453–462.
7. Mao X, Zhang Y, Lin N. Application and perspectives of traditional Chinese medicine in the treatment of liver cancer. Cancer Transl Med. 2015;1(3):101. doi:10.4103/2395-3977.159538
8. Yunbo C, Guijuan Z, Xiaoping C, et al. Jianpi Bushen, a traditional Chinese medicine therapy, combined with chemotherapy for gastric cancer treatment: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:1–14.
9. Fisher RP. Getting to S: CDK functions and targets on the path to cell-cycle commitment. F1000research. 2016;5:2374. doi:10.12688/f1000research
10. Zhang J, Li Y, Chen X, et al. Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells.. Oncol Rep. 2011;26(1):115. doi:10.3892/or.2011.1266
11. Afroze SH, Sloan J, Osuji GC, et al. Cinobufotalin impedes Sw.71 cytotrophoblast cell line function via cell cycle arrest and apoptotic signaling. Mol Cell Biochem. 2016;422(1–2):189. doi:10.1007/s11010-016-2820-0
12. Sun B, Geng S, Huang X, et al. Coleusin factor exerts cytotoxic activity by inducing G0/G1 cell cycle arrest and apoptosis in human gastric cancer BGC-823 cells. Cancer Lett. 2011;301(1):95–105. doi:10.1016/j.canlet.2010.10.010
13. Shi HL, Wu XJ, Liu Y, et al. Berberine counteracts enhancedIL −8 expression of AGS cells induced by evodiamine. Life Sci. 2013;93(22):830–839. doi:10.1016/j.lfs.2013.09.010
14. Chen Y, Zhu L, Yang X, et al. Ailanthone induces G2/M cell cycle arrest and apoptosis of SGC-7901 human gastric cancer cells. Mol Med Rep. 2017;16(5):6821–6827. doi:10.3892/mmr.2017.7491
15. Kosciolek BA, Kalantidis K, Tabler M, et al. Inhibition of telomerase activity in human cancer cells by RNA interference. Mol Cancer Ther. 2003;2(3):209.
16. Ivancich M, Schrank Z, Wojdyla L, et al. Treating cancer by targeting telomeres and telomerase. Antioxidants. 2017;6(1). doi:10.3390/antiox6010015.
17. Çalışkan EC, Atalay MC, Miser ES, et al. Normal and tumour tissue mRNA expressions of telomerase complex genes in several types of cancer. Balkan Med J. 2017;34(3):269–274. doi:10.4274/balkanmedj.2015.1616
18. Zhao Q, Yang Y, Yu J, et al. Posttranscriptional regulation of the telomerase hTERT by gambogic acid in human gastric carcinoma 823 cells. Cancer Lett. 2008;262(2):223–231. doi:10.1016/j.canlet.2007.12.002
19. Duan C, Zhang B, Deng C, et al. Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumour Biol. 2016;37(8):1–12.
20. Wang H, Tao L, Ni T, et al. Anticancer efficacy of the ethyl acetate extract from the traditional Chinese medicine herb Celastrus orbiculatus against human gastric cancer.. J Ethnopharmacol. 2017;205:147–157. doi:10.1016/j.jep.2017.04.030
21. Wang D, Xin Y, Tian Y, et al. Pseudolaric Acid B inhibits gastric cancer cell metastasis in vitro and in haematogenous dissemination model through PI3K/AKT, ERK1/2 and mitochondria-mediated apoptosis pathways. Exp Cell Res. 2017;352:34–44.
22. Mao XM, Fu QR, Li HL, et al. Crocodile cholin from Crocodylussiamensis induces apoptosis of human gastric cancer. Tumour Biol. 2017;39(3):1010428317694320. doi:10.1177/1010428317694320
23. Li Z, Oncology DO. Observation of the preventive effect of compound Kushen injection on chemotherapy adverse reactions in postoperative gastric cancer patients and its effects on immune function. Hebei J Tradit Chin Med. 2016;38:42–44.
24. Fu H, Wang C, Yang D, et al. Curcumin regulates proliferation, autophagy and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J Cell Physiol. 2017;233:4634–4642.
25. Lei Z, Jie Z, Jun L, et al. Study on mulberry anthocyanins induced autophagy and apoptosis of human gastric cancer SGC-7901 cell autophagy. J Chinese Med Mater. 2016;39:1134–8.
26. Kim DH, Park J, Chae I, et al. Isoliquiritigenin inhibits the proliferation of human renal carcinoma Caki cells through the ROS-mediated regulation of the Jak2/STAT3 pathway. Oncol Rep. 2017;38:575–583. doi:10.3892/or.2017.5677
27. Kim KW, Choi CH, Kim TH, et al. Silibinin inhibits glioma cell proliferation via Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res. 2009;34(8):1479–1490. doi:10.1007/s11064-009-9935-6
28. Bhattacharya S, Muhammd N, Steele R, et al. Bitter melon enhances natural killer mediated toxicity against head and neck cancer cells. Cancer Prev Res. 2017;10(6):337–344. doi:10.1158/1940-6207.CAPR-17-0046
29. Muhammad N, Steele R, Isbell ST, et al. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget. 2017;8:39. doi:10.18632/oncotarget.19887
30. Bhattacharya S, Muhammad N, Steele R, et al. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget. 2016;7(22):33202–33209. doi:10.18632/oncotarget.8898
31. Mostafa LK, Jones DB, Wright DH. Mechanism of the induction of angiogenesis by human neoplastic lymphoid tissue: studies employing bovine aortic endothelial cells in vitro. J Pathol. 1980;132(3):207–216. doi:10.1002/path.1711320303
32. Zang M, Hu L, Zhang B, et al. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem Biophys Res Commun. 2017;490(3):913. doi:10.1016/j.bbrc.2017.06.140
33. Huang F, Yao Y, Wu J, et al. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κB/VEGF signaling. Am J Transl Res. 2017;9(12):5538–5547.
34. Bing Y, Long L, Ying Z, et al. XiaotanSanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World J Gastroenterol. 2014;20(36):13105. doi:10.3748/wjg.v20.i36.13105
35. Ahluwalia A, Jones MK, Tarnawski AS. Key role of endothelial importin-α in VEGFexpression and gastric angiogenesis: novel insight into aging gastropathy. Am J Physiol Gastrointest Liver Physiol. 2014;306(4):338–345. doi:10.1152/ajpgi.00382.2013
36. Dai X, Liu D, Liu M, et al. Anti-metastatic efficacy of traditional Chinese medicine (TCM) ginsenoside conjugated to a VEFGR-3 antibody on human gastric cancer in an orthotopic mouse model. Anticancer Res. 2017;37(3):979. doi:10.21873/anticanres.11407
37. Pang B, Wei PK, Li YJ. Effect of xiaotan sanjie recipe on expressions of VEGF-C and VEGFR-3 in nude mice with transplanted human gastric adenocarcinoma cell MKN-45. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(2):204–208.
38. Li Y, Tan BB, Zhao Q, et al. ZNF139 promotes tumor metastasis by increasing migration and invasion in human gastric cancer cells. Neoplasma. 2014;61(3):291. doi:10.4149/neo_2014_050
39. Wu -T-T, Lu J, Zheng PQ, et al. Yiqi Huayu Jiedu Decoction inhibit the invasion and metastasis of gastric cancer cells through TGF-β/Smad pathway. Evid Based Complement Alternat Med. 2017;2017(19):1–8.
40. Łukaszewicz-Zając M, Mroczko B, Guzińska-Ustymowicz K, et al. Matrix metalloproteinase 2 (MMP-2) and their tissue inhibitor 2 (TIMP-2) in gastric cancer patients. Adv Med Sci. 2013;58(2):235–243. doi:10.2478/ams-2013-0018
41. Chen SX, Yin JF, Lin BC, et al. Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumour Biol. 2016;37(5):6801–6812. doi:10.1007/s13277-015-4404-0
42. Arcone R, Palma M, Pagliara V, et al. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced matrix metalloproteinase-9/2. Biochim Open. 2016;3(C):56–63. doi:10.1016/j.biopen.2016.10.002
43. Lu S, Zhang Z, Chen M, et al. Silibinin inhibits the migration and invasion of human gastric cancer SGC7901 cells by downregulating MMP-2 and MMP-9 expression via the p38MAPK signaling pathway. Oncol Lett. 2017;14(6):7577.
44. Yan X, Rui X, Zhang K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep. 2015;33(2):737–743. doi:10.3892/or.2014.3669
45. Zhang J, Su K, Shi W, et al. Matrine inhibits the adhesion and migration of BCG823 gastric cancer cells by affecting the structure and function of the vasodilator-stimulated phosphoprotein (VASP). Acta Pharmacol Sin. 2013;34(8):1084–1092. doi:10.1038/aps.2013.15
46. Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185 HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272(44):27913–27918. doi:10.1074/jbc.272.44.27913
47. Sun H, He S, Wen B, et al. [Effect of Biejiajian Pills on Wnt signal pathway molecules β-catenin and GSK-3β and the target genes CD44v6 and VEGF in hepatocellular carcinoma cells]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(10):1454–1458.
48. Xu Z, Zheng G, Wang Y, et al. Aqueous Huaier extract suppresses gastric cancer metastasis and epithelial to mesenchymal transition by targeting twist.. J Cancer. 2017;8(18):3876–3886. doi:10.7150/jca.20380
49. Wu J, Yu J, Wang J, et al. Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed Pharmacother. 2018;100:176–183. doi:10.1016/j.biopha.2018.01.140
50. Jiao Y, Wang X, Li Y, et al. Tanshinone IIA suppresses gastric cancer cell proliferation and migration by downregulation of FOXM1. Oncol Rep. 2017;37(3):1394. doi:10.3892/or.2017.5408
51. Ishikawa M, Kitayama J, Kazama S, et al. Expression of vascular endothelial growth factor C and D (VEGF-C and-D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol. 2003;33(1):21–27. doi:10.1093/jjco/hyg008
52. Bao L, Liu F, Guo HB, et al. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol. 2016;37(8):11365–11374. doi:10.1007/s13277-016-5013-2
53. Liu W, Meng M, Zhang B, et al. Dehydroeffusol effectively inhibits human gastric cancer cell-mediated vasculogenic mimicry with low toxicity. Toxicol Appl Pharmacol. 2015;287(2):98. doi:10.1016/j.taap.2015.05.003
54. Mu J, Liu T, Jiang L, et al. The traditional Chinese medicine baicalein potently in hibits gastric cancer cells. J Cancer. 2016;7(4):453. doi:10.7150/jca.13548
55. Qian Y, Lu S, Shi Y, et al. Celastrus orbiculatusextracts induce apoptosis and inhibit invasion by targeting the maspin gene in human gastric adenocarcinoma cells:. Oncol Lett. 2018;15(1):243–249. doi:10.3892/ol.2017.7341
56. Lu Z, Long Y, Cun X, et al. A size-shrinkable nanoparticle-based combined anti-tumor and anti-inflammatory strategy for enhanced cancer therapy. Nanoscale. 2018;10:9957–9970.
57. Kolb WP, Granger GA. Lymphocyte in vitro cytotoxicity: characterization of human lymphotoxin. Proc Natl Acad Sci U S A. 1970;1(1):122–132.
58. Ruddle NH, Waksman BH. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968;128(6):1267–1279. doi:10.1084/jem.128.6.1267
59. Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies.. Nat Rev Rheumatol. 2016;12(1):49. doi:10.1038/nrrheum.2015.169
60. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. Aaps J. 2013;15(1):195–218. doi:10.1208/s12248-012-9432-8
61. Ji J, Wang HS, Gao YY, et al. Synergistic anti-tumor effect of KLF4 and curcumin in human gastric carcinoma cell line. Asian Pac J Cancer Prev. 2014;15(18):7747–7752. doi:10.7314/apjcp.2014.15.18.7747
62. Hamzehzadeh L, Atkin SL, Majeed M, et al. The versatile role of curcumin in cancer prevention and treatment: a focus on PI3K/AKT pathway. J Cell Physiol. 2018;2:825.
63. Shakibaei M, Kraehe P, Popper B, et al. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15(1):1–15. doi:10.1186/1471-2407-15-1
64. Xie J, Lin Z, Xian Y, et al. (-)-Patchouli alcohol protects against Helicobacter pylori urease-induced apoptosis, oxidative stress and inflammatory response in human gastric epithelial cells.. Int Immunopharmacol. 2016;35:43. doi:10.1016/j.intimp.2016.02.022
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
