Back to Journals » International Journal of Nanomedicine » Volume 17
Mechanism of Enhanced Oral Absorption of a Nano-Drug Delivery System Loaded with Trimethyl Chitosan Derivatives
Authors Zhao Y, Lin S, Fang R, Shi Y, Wu W, Zhang W, Chen H
Received 18 January 2022
Accepted for publication 18 July 2022
Published 29 July 2022 Volume 2022:17 Pages 3313—3324
DOI https://doi.org/10.2147/IJN.S358832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ebrahim Mostafavi
Ying Zhao,1,* Shiyuan Lin,1,2,* Ruiyue Fang,1 Yaling Shi,1 Wei Wu,1 Wei Zhang,1 Hui Chen1
1College of Pharmacy, Guilin Medical University, Guilin, 541199, People’s Republic of China; 2Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Chen; Wei Zhang, College of Pharmacy, Guilin Medical University, No. 1 Zhiyuan Road, Guilin, 541199, People’s Republic of China, Email [email protected]; [email protected]
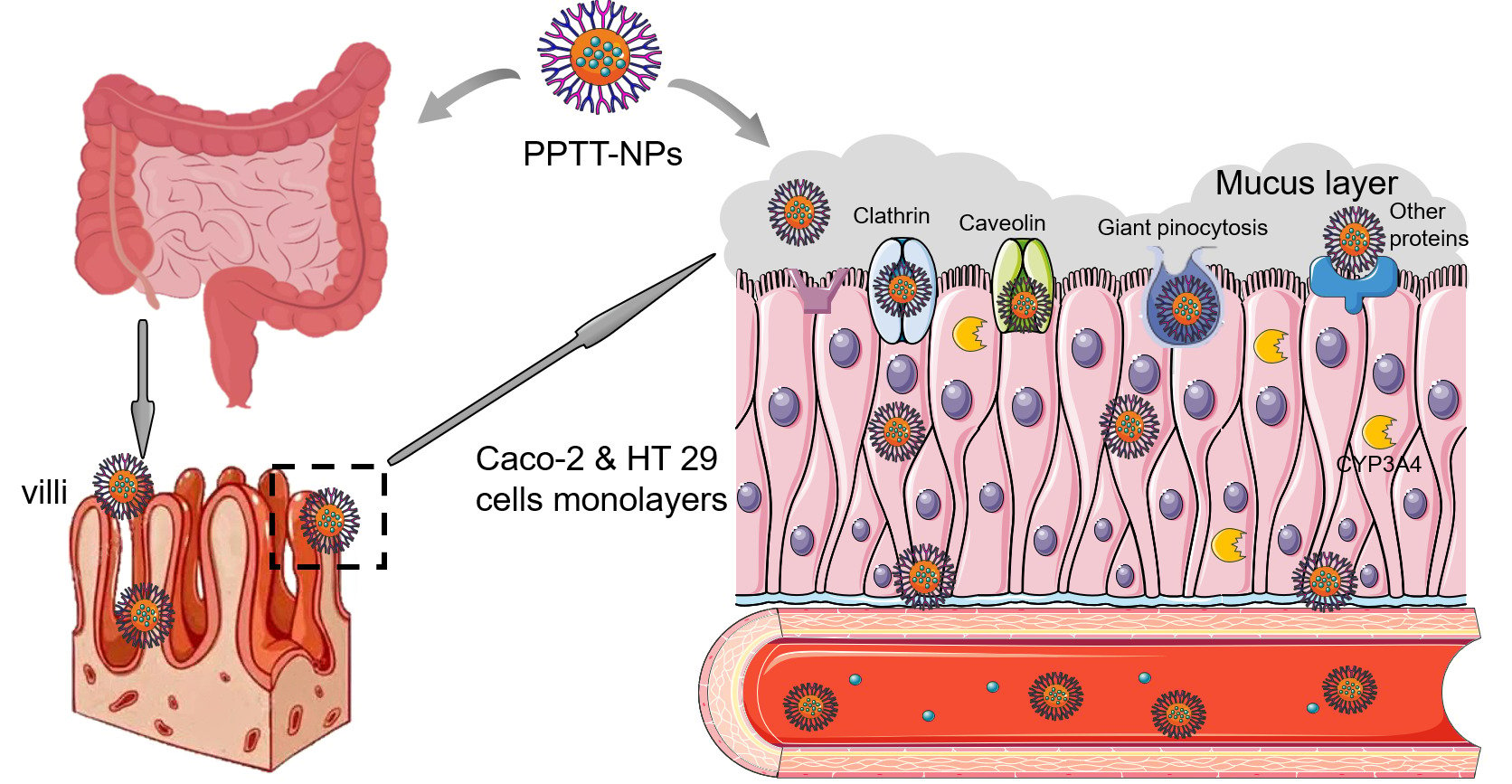
Introduction: In the previous study, nanoparticles coated with trimethyl chitosan (TMC) derivatives (PPTT-NPs) could promote the oral bioavailability of panax notoginseng saponins (PNS). Herein, we chose PPTT-NPs as a model drug to study the property and mechanism of intestinal absorption in vitro and in vivo.
Methods: The stability of PPTT-NPs was evaluated using simulated gastric fluid and simulated intestinal fluid. The uptake and transport of PPTT-NPs were investigated in Caco-2 and Caco-2&HT29 co-culture cells. The biosafety, intestinal permeability, adhesion, and absorption mechanism of PPTT-NPs were investigated using SD rats in vivo. The live imaging and biodistribution of PPTT-NPs were observed by IVIS. Furthermore, the effects on CYP3A4 of PPTT-NPs were investigated using testosterone as the probe substrate.
Results: The results of the stability assay showed that PPTT-NPs had a strong tolerance to the pH and digestive enzymes in the gastrointestinal environment. In vitro cell experiments showed that the uptake of drugs exhibited a time-dependent. When the ratio of TMC-VB12 and TMC-Cys was 1:3, the uptake capacity of PPTT-NPs was the highest. PPTT-NPs could enhance the paracellular transport of drugs by reversibly opening a tight junction. Animal experiments demonstrated that PPTT-NPs have good biological safety. PPTT-NPs had good adhesion and permeability to small intestinal mucosa. Meanwhile, PPTT-NPs needed energy and various protein to participate in the uptake of drugs. The live imaging of NPs illustrated that PPTT-NPs could prolong the residence time in the intestine. Moreover, TMC-VB12 and TMC-Cys could reduce the metabolism of drugs by inhibiting CYP3A4 to a certain extent.
Conclusion: The results show that TMC-VB12 and TMC-Cys are effective in the transport of PPTT-NPs. PPTT-NPs can increase the intestinal adhesion of drugs and exert high permeation by intestinal enterocytes which demonstrate significant and efficient potential for oral delivery of the BCS III drugs.
Keywords: Panax notoginseng saponins, TMC derivatives, nanoparticles, oral absorption, Caco-2&HT29 co-culture cells
Graphical Abstract:
Introduction
At present, oral administration is a safe, convenient, economical and widely used route of administration, which has good patient compliance, safety, tolerance and so on.1–4 Due to the physicochemical properties of drugs and gastrointestinal physiological barriers, the drugs cannot be effectively absorbed by the oral route. However, most drugs are absorbed in the small intestine after oral administration.5–7 The intestinal absorption of drugs is affected by many factors, including drug solubility and stability, uptake ability of intestinal epithelial cells, intestinal permeability to drugs, and multiple barriers of the digestive tract. Among them, the intestinal barrier is considered to be an important factor affecting the bioavailability of oral drugs.8–10 Furthermore, some active ingredients of Traditional Chinese Medicine such as Panax noteginseng saponins (PNS), Silymarin, and Puerarin have low permeability of intestinal mucosa, which is considered to be the main reason affecting its oral bioavailability. Due to its poor absorption and low bioavailability after oral administration, its clinical therapeutic effect has been limited.11 Therefore, it is very important to improve the intestinal absorption capacity and oral bioavailability for low-permeability drugs.
As an effective method of drug delivery, the microparticle drug delivery system has been widely concerned.12–15 Nano-carriers have many advantages when carrying drugs, such as higher biocompatibility and lower biotoxicity, controlled drug release, protection of drugs from the external environment, and promotion of oral absorption.16–20 Moreover, the surface of nano-carriers can also be modified with polymers to achieve targeted drug delivery, reduced drug dose, improved physicochemical properties of drugs, and enhanced the therapeutic effect.21–23 Consequently, coating polymer materials on the surface of nanoparticles provides an effective method to solve the oral absorption problem of low-permeability drugs.
In previous studies, our research group prepared PNS nanoparticles coated with TMC-VB12 (Vitamin B12 modified TMC) and TMC-Cys (Cysteine modified TMC) (PPTT-NPs). The size of PPTT-NPs was 144.3 ± 0.99 nm, and the zeta potential was 22.3 ± 0.49 mV. In vivo pharmacokinetics, the Cmax values of Rg1 and Rb1 in PPTT-NPs were greater than those of Xueshuangtong, and the relative bioavailability of Rg1 and Rb1 was 383.06% and 267.40%, respectively. The results preliminarily showed that PPTT-NPs had a certain effect of promoting oral absorption.24 The mechanism of improved oral absorption may be related to the effect of cysteine on the mucus layer, which enhanced the intestinal mucosal adhesion of TMC and promoted more drugs to pass through the mucus layer. Meanwhile, VB12 can bind to intrinsic factor (IF) in the gastrointestinal tract to form a complex that can bind to the VB12-IF receptor on the surface of intestinal cells, thereby promoting the active transport of drugs in the ileum.25,26 In light of the above discussion, the coating proportion of TMC-VB12 and TMC-Cys was tested in cells, and the intestinal absorption capacity of PPTT-NPs and the absorption mechanism was clarified in vitro and in vivo.
In recent years, Caco-2 cell monolayers have been widely used to simulate the intestinal environment.27 Due to the lack of mucus layer, the mucus-producing HT29 cells for co-culture can provide a more biologically relevant model for evaluating the permeability of drugs in vitro.28 Therefore, the Caco-2&HT29 co-culture cell model is effective for exploring the mechanism of drug oral absorption in vitro. Furthermore, Caco-2 cells lack the expression of cytochrome P450 isozymes compared with normal cells.29 In cytochrome P450 isozymes, CYP3A4 has an important active role in the intestinal metabolism of drugs.30–32 Inhibition of CYP3A4 activity can affect drug metabolism in the body to increase its absorption. To further verify the effect of oral absorption, some biological indicators should be evaluated at the animal levels.
In this study, the intestinal absorption capacity of PPTT-NPs and its mechanism had been preliminarily explored. The stability of PPTT-NPs was investigated using simulated gastric fluid (SGF) and simulated intestinal fluid (SIF). Caco-2 and Caco-2&HT29 co-culture cell models were established to investigate the uptakes and transports of PPTT-NPs. Sprague-Dawley (SD) rats were used to evaluate the biosafety, adhesion, permeability, and absorption mechanisms of PPTT-NPs. The effect of PPTT-NPs on the metabolism of cytochrome P450 isoenzymes (CYP3A4) was investigated, in order to further prove its oral absorption effect.
Materials and Methods
Materials
PPTT-NPs and PP-NPs (PNS-PLGA nanoparticles) were prepared by our laboratory.24 DAPI, PMSF, 4% tissue cell fixative, 1×PBST buffer, 1×PBS buffer, trypsin, anti-fluorescence attenuation mounting tablets, ketoconazole and neutral gum were purchased from Soleibao Biotechnology Co., Ltd. BCA protein concentration determination kit, RAPI lysate and Alcian Blue were purchased from Beyotime Co., Ltd. 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindotricarbocyaine iodide (DiR) was purchased from Yeasen Biotechnology Co., Ltd. HE staining reagent was purchased from Nanchang Yu Lu Experimental Equipment Co., Ltd. The mixed SD rat liver microsomes, CYP450 enzyme metabolism phenotype research kit was purchased from Beijing Huizhi Taikang Pharmaceutical Technology Co., Ltd.
Preparation of Nanoparticles (NPs)
Cou-6/DiR Labeled PPTT-NPs (PPTT-NPs@Cou-6/DiR)
The NPs were prepared by the double emulsion solvent evaporation method and coated twice with TMC derivatives. Cou-6 or DiR, and PLGA were dissolved in ethyl acetate, and an emulsifier was added. The mixture was then sonicated at 190 W for 2 min in an ice bath (5 off for 5 s) to obtain macroemulsion. Then, it was poured into the outer water phase containing 1% PVA and 0.5 mg/mL coating material (TMC-VB12 and TMC-Cys). The phase was fully vortexed and sonicated to obtain a double emulsion. The prepared double emulsion was dispersed in the coating material dispersion liquid. The mixture was stirred at low speed for 2 h at room temperature, and the organic solvent was removed by rotary evaporation under reduced pressure at 30°C for 10 min. The upper layer was a drug-loaded nanoparticle suspension.24
Cou-6/DiR Labeled PP-NPs (PP-NPs@Cou-6/DiR)
PP-NPs@Cou-6/DiR were prepared by the above method. The obtained macroemulsion was poured into the outer water phase containing 1% PVA. Other preparation steps were the same as the preparation of PPTT-NPs@Cou-6/DiR.
Stability Assay in Simulated Gastric Fluid (SGF) and Simulated Intestinal Fluid (SIF)
PNS and PPTT-NPs were dispersed and incubated in SGF (pH1.5 HCl containing 1% (w/v) pepsin) and SIF (pH 6.8 PBS containing 1% (w/v) trypsin) at 37°C. Samples (500 μL) were withdrawn at predetermined intervals (0, 15, 30, 60 and 120 minutes for SGF; 0, 15, 30, 60, 120 and 240 minutes for SIF), and then 1.0 mL methanol was added to terminate the reaction. The concentration of drug was determined by HPLC. The experiments were carried out in triplicate.
Cell Studies in vitro
Caco-2 and HT29 Cells Culture
Caco-2 and HT29 human colon adenocarcinoma cells were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences and cultured in Modified Eagle Medium (MEM) consisting of 100× non-essential amino acids, 1 mL of 100 mM sodium pyruvate and 20%FBS. Cells were incubated in a 5% CO2 incubator at 37°C.
Caco-2 and HT29 (9:1) cells were seeded on transwell polycarbonate membranes (0.4 μm pores, 0.33 cm2 growth area). Caco-2&HT29 cell monolayer could be developed after culture for 21 days. The transepithelial electrical resistance value (TEER) reached a certain range, indicating that the cell monolayer could be used for the transmembrane experiment.33
Cellular Uptake Assay
The qualitative and quantitative evaluation of cellular uptake is necessary for the study of drug absorption in the intestine.8 The Caco-2 cells were combined with Cou-6 solution, PP-NPs@Cou-6 and PPTT-NPs@Cou-6 (The ratio of coating material TMC-VB12: TMC-Cys was 1:0, 5:1, 3:1, 1:1, 1:3, 1:5, 0:1) and incubated for 3 h. Then, 4% paraformaldehyde was used to fix the cells for 15 min. The cell nucleus was stained with DAPI for 8 min, and after that the cells were washed. The uptake of the sample on Caco-2 cells was observed the next day. For quantitative analysis, Caco-2 and HT29 cells (9:1, 1 × 105 cells/well) were transferred to 24-well plates. Caco-2 cells were used for comparison. The cell lysate was added to each well and lysed at 4°C for 30 min. The samples were analyzed by a fluorescence microplate reader (Ex = 466 nm, Em = 504 nm).
At the same time, the uptake of drugs by cells at different times was also investigated.34 The Caco-2 cells were combined with PP-NPs@Cou-6 and PPTT-NPs@Cou-6, and, respectively, incubated for 0.5, 1, 2, 3, and 4 h in the dark at 37°C. Other steps were the same as the above method.
Transport Through Caco-2&HT29 Cells Monolayers
Before the study, Caco-2&HT29 cells in the chambers were rinsed with HBSS three times, and then equilibrated with HBSS at 37°C for 30 min. 0.3 mL of PP-NPs@Cou-6 and PPTT-NPs@Cou-6 (Cou-6 at a concentration of 200 ng/mL) were added to the apical chambers. At different time intervals (0.5, 1, 1.5, 2, 3 h), samples (0.1 mL) were removed from the basolateral chamber and 0.1 mL HBSS was supplemented. The samples were analyzed by a fluorescence microplate reader (Ex = 466 nm, Em = 504 nm). The apparent permeability coefficients (Papp, cm/s) of Cou-6 were calculated as follows.
Q: The total quantity of Cou-6 permeated (ng)
A: The surface area of the apical chamber of cell monolayers (cm2)
C: The initial quality of Cou-6 in the apical chamber (ng/cm3)
T: The incubation time of the administration
Animal Evaluations in vivo
Animals
The SD rats, 220 ± 20 grams (Hunan Slack Jingda Experimental Animal Co., Ltd., China), were used in this study. All animal procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Laboratory Animal Ethics Committee of Guilin Medical University of China.
Assessment of Intestinal Membrane Damage
The SD rats were fasted for 12 h prior to the experiment with free access to water. PPTT-NPs (60 mg/kg) were given by oral administration. In the control group, normal saline was instead. After 3 h, the small intestine was taken out. The isolated tissue was fixed in 4% tissue cell fixative, dehydrated and immersed in wax for embedding. According to the operating method of the HE solution kit, the sliced tissue was stained and observed under a microscope.35
Intestinal Permeability Test
The intestinal permeability of NPs was studied using the intestinal permeability ligating model.36 Duodenum, jejunum and ileum (5 cm) were removed from the SD rats and ligated at both ends. PP-NPs@Cou6 and PPTT-NPs@Cou6 (0.4 mL) were injected into the intestinal cavity of three intestine segments, respectively. The experiments were carried out in triplicate. The intestine segments were then placed in 10 mL of oxygenated KR buffer at 37°C and shaken at 100 rpm. At different time intervals (0.5, 1, 1.5, and 2 h), samples (200 μL) were taken out to determine the content. The cumulative permeation amounts were calculated.
Observation of Intestinal Villus Absorption
The intestinal villi absorption of NPs was studied using an in vivo intestinal ligation model.37 The SD rats were randomly divided into three groups of Cou-6, PP-NPs@Cou6 and PPTT-NPs@Cou6. The rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium. Both ends of the 2 cm jejunum were closed with a thin thread. Cou-6, PP-NPs@Cou6 and PPTT-NPs@Cou6 (0.1 mL) were injected into the jejunum segments. After 2 hours, the intestine segments were cut off and rinsed with PBS. Then, the intestinal tissue was fixed in 4% paraformaldehyde for 2 h, and sliced with cryostat. The sliced tissue was stained with DAPI and mounted with an anti-fluorescence quencher. The samples were observed under a fluorescence microscope to evaluate intestinal villus absorption.
Intestinal Absorption Mechanism
The intestinal absorption mechanism of NPs was studied using the everted intestine model.38 Duodenum (about 50–90 mg) was removed from of the SD rats and everted. The intestinal segments were placed in 24 well cell culture plates. Inhibitors (colchicine, chlorpromazine, indometacin and quercetin) were separately added and incubated at 37°C for 45 minutes in an air oscillator. Then, PP-NPs@Cou6 and PPTT-NPs@Cou6 (1.0 mL) were injected into the wells, respectively. After 45 minutes, the reaction was stopped. 300 μL of KR solution was added to each well for homogenization. Samples (100 μL) were taken out to determine the content with a fluorescence microplate reader.
Live Imaging and Biodistribution of NPs
Prior to the experiment, the abdominal skin of the SD rats was shaved and cleaned to eliminate autofluorescence interference of hair and all rats were fasted overnight with free access to water. Rats were randomly divided into two groups of PP-NPs@DiR and PPTT-NPs@DiR. Fluorescence images of animals were captured at predetermined intervals by the IVIS System under anesthesia with isoflurane. At predetermined intervals, the rats were sacrificed by overdose of 2% pentobarbital sodium (i.p.). The whole gastrointestinal tract was then dissected and visualized under IVIS.
Effect of Cytochrome P450 Isoenzyme (CYP3A4) on Metabolism
Testosterone was selected as the probe substrate of CYP3A4. The activity of CYP3A4 was evaluated by determining the amount of the metabolite 6β-hydroxytestosterone (6β-OHT).39 Probe substrate testosterone reference solution 2 μL (10 mg/mL) was added to an ice bath. At the same time, TMC-VB12, TMC-Cys, TMC-VB12: TMC-Cys=1:3, PP-NPs and PPTT-NPs (2 mg/mL) series of solutions (20, 50, 100 μL) were added. Ketoconazole was used as the control group. 10 μL of NADPH(A) solution and 2 μL of NADPH(B) solution were added, and PBS was added to 195 μL. After preincubating for 5 min at 37°C, 5 μL of liver microsome solution was added to start the reaction. After 45 minutes, 1.5 mL of pre-cooled ethyl acetate was added to terminate the reaction. After the samples were centrifuged, the upper ethyl acetate layer was taken out and dried with N2. The samples were redissolved in 200 μL methanol and determined by HPLC. The experiments were carried out in triplicate. Percent of inhibition was calculated using the following formula.
m: The amount of 6β-OHT produced in the test group
m1: The amount of 6β-OHT produced in the blank group
m0: The amount of 6β-OHT produced in the no NADPH(A) solution and no NADPH(B) solution group
Statistical Analysis
All experiment data were expressed as mean ± standard deviation (SD). Paired t-test was used to assess the statistical significance of the differences between the two groups, and variance analysis was used for comparison of multiple groups. P value of less than 0.05 was considered statistically significant.
Results and Discussion
Stability Assay
PNS are unstable and rapidly degrade in gastrointestinal environment.40 In order to evaluate the protective effect of NPs coated with TMC-VB12 and TMC-Cys on PNS, the degradation ratio of drugs under acid conditions and digestive enzyme exposure was studied. As shown in Figure 1, free PNS degraded rapidly, the residual percentages of free PNS were 37.90% for R1, 41.40% for Rg1 and 48.11% for Rb1 after 2 h incubation in the SGF, while 61.12% for R1, 81.55% for Rg1, 79.32% for Rb1 after 4 h incubation in the SIF. For PPTT-NPs, the amount of residual drug significantly increased (P < 0.05). Specifically, the residual percentages of encapsulated PNS were 51.83% for R1, 54.09% for Rg1 and 55.13% for Rb1 after 2 h incubation in the SGF, while 79.63% for R1, 92.29% for Rg1, 89.45% for Rb1 after 4 h incubation in the SIF. These results demonstrated that PPTT-NPs could protect PNS from degradation by gastrointestinal acid and enzymes.
 |
Figure 1 Percentage of R1 (A), Rg1 (B), Rb1 (C) remained after incubation in SGF and percentage of R1 (D), Rg1 (E), Rb1 (F) remained after incubation in SIF. Data are presented as mean ± SD (n=3). |
Cell Studies in vitro
Cellular Uptake Assay
As shown in Figure 2, the cellular uptake of Cou-6 solution, PP-NPs@Cou-6 and PPTT-NPs@Cou-6 (The ratio of coating material TMC-VB12: TMC-Cys was 1:0, 5:1, 3:1, 1:1, 1:3, 1:5, 0:1) was analyzed qualitatively and quantitatively. Figure 2A shows that the green fluorescence intensity of PPTT-NPs (TMC-VB12: TMC-Cys was 1:3) was the highest, indicating that PPTT-NPs (TMC-VB12: TMC-Cys was 1:3) could enhance the uptake of PNS in cells. The quantitative cellular uptake results are shown in Figure 2B and C. The cellular uptake of PPTT-NPs is always higher than that of PP-NPs. In Caco-2 cells, the cellular uptake of PPTT-NPs (TMC-VB12: TMC-Cys was 1: 3) was 2.13 times higher than that of PP-NPs. In Caco-2&HT29 cells, the cellular uptake of PPTT-NPs (TMC-VB12: TMC-Cys was 1: 3) was 3.79 times higher than that of PP-NPs. This indicated that in the presence of mucus layer, PPTT-NPs could still effectively increase the cellular uptake of drugs. Furthermore, it was speculated that PPTT-NPs could adhere to the small intestinal cells, which would increase the residence time to promote the absorption of drugs.
The cellular uptake of PP-NPs@Cou-6 and PPTT-NPs@Cou-6 at different intervals was also investigated. As shown in Figure 2D, the cellular uptake of drugs was time-dependent and increased with the uptake time. At every different time point, the uptake of PPTT-NPs was higher than that of PP-NPs, which further verified that PPTT-NPs could promote the intestinal absorption.
Transport Through Caco-2&HT29 Cells Monolayers
The results of transport through Caco-2&HT29 cells monolayers are shown in Figure 3. As shown in Figure 3A, the growth of Caco-2 and HT29 cells were staggered and the dense cell monolayer was formed. Through alcian blue staining,41 it could be seen that the mucus layer was produced in Caco-2&HT29 cells model. It was reported that, when the TEER value was greater than 300 Ω/cm2, Caco-2&HT29 cells could be used to measure penetration of drugs.42 From Figure 3B, the value of TEER was above 450 Ω/cm2, indicating that the Caco-2&HT29 cells monolayers were successfully constructed. The penetration through the Caco-2&HT29 cells monolayers indicated that the transport of PPTT-NPs was significantly increased as cultivating prolonged and higher than that of PP-NPs (Figure 3C). The Papp value of PPTT-NPs was 1.21-fold higher than that of PP-NPs. It further confirmed that PPTT-NPs could enhance drugs transport through Caco-2&HT29 cells monolayers (Figure 3D). Figure 3E shows that the TEER value of Caco-2&HT29 cells monolayers treated with PPTT-NPs dropped to about 74%. After removing the PPTT-NPs suspension, the TEER value increased gradually. However, the TEER value of Caco-2&HT29 cell monolayers treated with PP-NPs did not change significantly. These phenomena indicated that PPTT-NPs could enhance the paracellular transport of drugs by reversibly opening a tight junction.33
Animal Evaluation in vivo
Assessment of Intestinal Membrane Damage
The microscopic evaluation of intestinal membrane damage is shown in Figure 4A. The structure of the small intestine was clear. The small intestine was composed of four layers including mucosal layer, submucosa, muscle layer and serosal layer. Compared with the control group, no obvious lesions were found in each layer of the small intestine in the PPTT-NPs group. Therefore, it is illustrated that PPTT-NPs have good biocompatibility and biosafety.
Intestinal Permeability Test
The permeability effect of PP-NPs@Cou6 and PPTT-NPs@Cou6 through the isolated small intestine were analyzed by fluorescence quantitative microplate reader (Figure 4B). The permeation amounts of drugs were time-dependent and increased with time. When incubated in the same ileum at the same interval, the permeation of PPTT-NPs was higher than that of PP-NPs. Comparing the permeation of PP-NPs@Cou6 in different intestine segments at 2 h, it was the least in the ileum. On the contrary, the permeation of PPTT-NPs@Cou6 was the highest in the ileum. It illustrated that TMC-Cys could provide strong mucoadhesion and increase the permeability of drugs in the mucus layer.43 In addition, TMC-VB12 can bind to intrinsic factor (IF) to form a complex that binds to specific IF receptors in the ileum, thereby promoting drug absorption in the small intestine.26
Observation of Intestinal Villus Absorption
The intestinal villus absorptions of Cou-6, PP-NPs@Cou6 and PPTT-NPs@Cou6 are shown in Figure 4C. The green fluorescence in the intestinal villus of PP-NPs@Cou6 was weak. PP-NPs might be blocked by the intestinal mucus layer, which could affect its absorption. However, the green fluorescence in the intestinal villus treated with PPTT-NPs@Cou-6 was significantly stronger than that of Cou-6 and PP-NPs@Cou-6 at 1.5 h after administration. PPTT-NPs could increase the adhesion of the mucus layer on the intestinal villus to promote penetration. Combined with cellular uptake, it revealed that PPTT-NPs could act on the mucus layer and increase the adhesion of drugs to the intestine.
Intestinal Absorption Mechanism
To further clarify the absorption pathway of NPs, the intestinal absorption mechanism was studied in vivo (Figure 4D and E). The intestinal absorption of NPs required energy and a suitable temperature. As shown in Figure 4D, the uptake of PP-NPs@Cou-6 in the small intestine treated with colchicine was 84.30% of the control group, and other inhibitors had no obvious inhibitory effect. PP-NPs could only be absorbed by giant pinocytosis. The uptake of PPTT-NPs treated with four inhibitors (colchicine, chlorpromazine, indometacin and quercetin) were 62.32%, 50.49%, 61.84% and 34.74% of the control group (Figure 4E). All these indicated that the intestinal absorption of PPTT-NPs involved giant pinocytosis, clathrin, caveolin, non-clathrin and non-caveolin mediated endocytosis. Therefore, compared with PP-NPs, PPTT-NPs could enter cells through more endocytosis pathways to promote intestinal absorption.
Live Imaging and Biodistribution of NPs
The live imaging and biodistribution of NPs after oral administration are shown in Figure 5. In general, PPTT-NPs@DiR in the abdomen of rats gradually increased in the first 4 hours. At 4 hours, the fluorescence of PPTT-NPs@DiR was significantly stronger than that of PP-NPs@DiR. After 4 hours, the fluorescence of the abdomen in the two groups all gradually weakened. The biodistribution of PP-NPs@DiR reached the ileum at 2 hours, while that of PPTT-NPs@DiR was in the duodenum. At 4 hours, the biodistribution of PPTT-NPs@DiR reached the ileum, and some remained in the duodenum. This indicated that the drugs could still be absorbed at this interval. The fluorescence of PP-NPs@DiR in the intestines after 4 hours was weak, indicating that the drugs had been metabolized. Therefore, PPTT-NPs could prolong the residence time in the intestine to a certain extent.
 |
Figure 5 The live imaging and biodistribution of PP-NPs@DiR and PPTT-NPs@DiR after oral administration in SD rats. |
Effect of Cytochrome P450 Isoenzyme (CYP3A4) on Metabolism
In this study, CYP3A4 activity was studied to investigate the absorption and metabolism of drugs in the body. The inhibition rate of CYP3A4 gradually increased with the concentration of materials/drugs (Figure 6). Among them, both TMC-VB12 and TMC-Cys inhibited CYP3A4, and the inhibition of TMC-VB12&TMC-Cys was stronger than that of TMC-VB12 or TMC-Cys. In different concentrations of drugs, the inhibition of PPTT-NPs was higher than that of PP-NPs. Moreover, the inhibition of PPTT-NPs was 1.21-fold times higher than that of PP-NPs at 1.0 mg/mL. In summary, PPTT-NPs could inhibit CYP3A4 to promote the absorption of drugs in the intestine.
 |
Figure 6 Effects of different components on CYP3A4 enzyme activity. Data are presented as mean ± SD (n=3), ****p<0.0001. |
Conclusion
The intestinal absorption property and mechanism of PPTT-NPs were investigated. The optimal modification ratio of TMC-VB12 and TMC-Cys is 1:3. PPTT-NPs have good gastrointestinal stability and biological safety. Our study evidences that TMC-VB12 and TMC-Cys are effective in the transport of PPTT-NPs. Importantly, PPTT-NPs can increase the intestinal adhesion of drugs, and exert high permeation by intestinal enterocytes. Therefore, PPTT-NPs demonstrate significant and efficient potential for oral delivery of the BCS III drugs.
Acknowledgments
This work was supported by the Science and Technology Base and Talent Project of Guangxi Province (AD19110070), Natural Science Foundation of Guangxi Province (2018GXNSFBA281044, 2020GXNSFAA297133), National Natural Science Foundation of China (81760718).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Deng J, Zhu X, Chen Z, et al. A review of food-drug interactions on oral drug absorption. Drugs. 2017;77(17):1833–1855. doi:10.1007/s40265-017-0832-z
2. Khatun Z, Nurunnabi M, Cho KJ, Byun Y, Bae YH, Lee YK. Oral absorption mechanism and anti-angiogenesis effect of taurocholic acid-linked heparin-docetaxel conjugates. J Control Release. 2014;177:64–73. doi:10.1016/j.jconrel.2013.12.034
3. Reinholz J, Landfester K, Mailander V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018;25(1):1694–1705. doi:10.1080/10717544.2018.1501119
4. Date T, Paul K, Singh N, Jain S. Drug-lipid conjugates for enhanced oral drug delivery. AAPS PharmSciTech. 2019;20(2):41. doi:10.1208/s12249-018-1272-0
5. Luo Z, Liu Y, Zhao B, et al. Ex vivo and in situ approaches used to study intestinal absorption. J Pharmacol Toxicol Methods. 2013;68(2):208–216. doi:10.1016/j.vascn.2013.06.001
6. Cao SJ, Xu S, Wang HM, et al. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech. 2019;20(5):190. doi:10.1208/s12249-019-1325-z
7. Perucca E, Bialer M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs. 2020;34(8):795–800. doi:10.1007/s40263-020-00741-5
8. Yike H, Suya D, Xinxin L, et al. Evaluation of intestinal absorption mechanism and pharmacokinetics of curcumin-loaded galactosylated albumin nanoparticles. Int J Nanomedicine. 2019;14:9721–9730. doi:10.2147/IJN.S229992
9. Nicolas JM, Bouzom F, Hugues C, Ungell AL. Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions. Biopharm Drug Dispos. 2017;38(3):209–230. doi:10.1002/bdd.2052
10. Heikkinen AT, Korjamo T, Monkkonen J. Modelling of drug disposition kinetics in in vitro intestinal absorption cell models. Basic Clin Pharmacol Toxicol. 2010;106(3):180–188. doi:10.1111/j.1742-7843.2009.00504.x
11. Gökçe EH, Okur NÜ, Siafaka PI. Challenges in oral drug delivery and applications of lipid nanoparticles as potent oral drug carriers for managing cardiovascular risk factors. Curr Pharm Biotechnol. 2021;22(7):892–905. doi:10.2174/1389201021666200804155535
12. Liu Y, Zhao J, Wang L, et al. Nanocrystals technology for transdermal delivery of water-insoluble drugs. Curr Drug Deliv. 2018;15(9):1221–1229. doi:10.2174/1567201815666180518124345
13. Zar MK, Qiannan Y, Yongmei X, et al. Bioavailability and antioxidant activity of nanotechnology-based botanic antioxidants. J Food Sci. 2021;86(2):284–292. doi:10.1111/1750-3841.15582
14. Korani S, Korani M, Bahrami S, et al. Application of nanotechnology to improve the therapeutic benefits of statins. Drug Discov Today. 2019;24(2):567–574. doi:10.1016/j.drudis.2018.09.023
15. Zhou F, Teng F, Deng P, Meng N, Song Z, Feng R. Recent progress of nano-drug delivery system for liver cancer treatment. Anticancer Agents Med Chem. 2018;17(14):1884–1897. doi:10.2174/1871520617666170713151149
16. Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther. 2019;34:1. doi:10.1515/dmpt-2018-0032
17. Jenjob R, Phakkeeree T, Seidi F, Theerasilp M, Crespy D. Emulsion techniques for the production of pharmacological nanoparticles. Macromol Biosci. 2019;19(6):e1900063. doi:10.1002/mabi.201900063
18. Erdogar N, Akkin S, Bilensoy E. Nanocapsules for drug delivery: an updated review of the last decade. Recent Pat Drug Deliv Formul. 2018;12(4):252–266. doi:10.2174/1872211313666190123153711
19. Liu Y, Yang G, Jin S, Xu L, Zhao CX. Development of high-drug-loading nanoparticles. Chempluschem. 2020;85(9):2143–2157. doi:10.1002/cplu.202000496
20. Covarrubias-Zambrano O, Yu J, Bossmann SH. Nano-inspired technologies for peptide delivery. Curr Protein Pept Sci. 2020;21(4):379–400. doi:10.2174/1389203720666191202112429
21. Chaudhary D, Joshi G, Sawant K, Patel M. Preparation and surface modification of polymeric nanoparticles for drug delivery: state of the art. Recent Pat Drug Deliv Formul. 2020;14(3):201–213. doi:10.2174/1872211314666200904105036
22. O Elzoghby A, M Abd-Elwakil M, Abd-Elsalam K, T Elsayed M, Hashem Y, Mohamed O. Natural polymeric nanoparticles for brain-targeting: implications on drug and gene delivery. Curr Pharm Des. 2016;22(22):3305–3323. doi:10.2174/1381612822666160204120829
23. Frank LA, Contri RV, Beck RC, Pohlmann AR, Guterres SS. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):623–639. doi:10.1002/wnan.1334
24. Chen H, Zhao Y, Li R, et al. Preparation and in vitro and in vivo evaluation of panax notoginseng saponins-loaded nanoparticles coated with trimethyl chitosan derivatives. J Pharm Sci. 2022;111(6):1659–1666. doi:10.1016/j.xphs.2021.11.002
25. He H, Wang P, Cai C, Yang R, Tang X. VB12-coated Gel-Core-SLN containing insulin: another way to improve oral absorption. Int J Pharm. 2015;493(1–2):451–459. doi:10.1016/j.ijpharm.2015.08.004
26. Wang J, Tan J, Luo J, et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J Nanobiotechnology. 2017;15(1):18. doi:10.1186/s12951-017-0251-z
27. Keemink J, Bergstrom CAS. Caco-2 cell conditions enabling studies of drug absorption from digestible lipid-based formulations. Pharm Res. 2018;35(4):74. doi:10.1007/s11095-017-2327-8
28. Liu J, Werner U, Funke M, et al. SEDDS for intestinal absorption of insulin: application of Caco-2 and Caco-2/HT29 co-culture monolayers and intra-jejunal instillation in rats. Int J Pharm. 2019;560:377–384. doi:10.1016/j.ijpharm.2019.02.014
29. van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1(2):175–185. doi:10.1517/17425255.1.2.175
30. Takenaka T, Kazuki K, Harada N, et al. Development of Caco-2 cells co-expressing CYP3A4 and NADPH-cytochrome P450 reductase using a human artificial chromosome for the prediction of intestinal extraction ratio of CYP3A4 substrates. Drug Metab Pharmacokinet. 2017;32(1):61–68. doi:10.1016/j.dmpk.2016.08.004
31. Papp-Jámbor C, Jaschinski U, Forst H. Cytochrome P450 enzymes and their role in drug interactions. Anaesthesist. 2002;51(1):2–15. doi:10.1007/s101-002-8365-5
32. Mano Y, Sugiyama Y, Ito K. Use of a physiologically based pharmacokinetic model for quantitative prediction of drug-drug interactions via CYP3A4 and estimation of the intestinal availability of CYP3A4 substrates. J Pharm Sci. 2015;104(9):3183–3193. doi:10.1002/jps.24495
33. Li M, Sun Y, Ma C, Hua Y, Zhang L, Shen J. Design and investigation of penetrating mechanism of octaarginine-modified alginate nanoparticles for improving intestinal insulin delivery. J Pharm Sci. 2021;110(1):268–279. doi:10.1016/j.xphs.2020.07.004
34. Zhang P, Zhao S, Yu Y, Wang H, Yang Y, Liu C. Biocompatibility profile and in vitro cellular uptake of self-assembled alginate nanoparticles. Molecules. 2019;24(3):555. doi:10.3390/molecules24030555
35. Wang B, Huang Q, Li S, et al. [Changes of guanylate cyclase C in colon tissues of rats with intestinal injury associated with severe acute pancreatitis]. Journal of Southern Medical University. 2021;41(3):376–383. Chinese. doi:10.12122/j.issn.1673-4254.2021.03.09
36. Li H, Chen M, Su Z, Sun M, Ping Q. Size-exclusive effect of nanostructured lipid carriers on oral drug delivery. Int J Pharm. 2016;511(1):524–537. doi:10.1016/j.ijpharm.2016.07.049
37. Yuan H, Chen CY, Chai GH, Du YZ, Hu FQ. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm. 2013;10(5):1865–1873. doi:10.1021/mp300649z
38. Lian H. The construction and evaluation of oral drug delivery system based on functional thiolated polymeric micelles. [Doctoral thesis]. Shenyang Pharmaceutical university; 2014.
39. Usmani KA, Tang J. Human cytochrome P450: metabolism of testosterone by CYP3A4 and inhibition by ketoconazole. Curr Protoc Toxicol. 2004;20(1). doi:10.1002/0471140856.tx0413s20
40. Fu W, Liang Y, Xie Z, Wu H, Zhang Z, Lv H. Preparation and evaluation of lecithin/zein hybrid nanoparticles for the oral delivery of Panax notoginseng saponins. Eur J Pharm Sci. 2021;164:105882. doi:10.1016/j.ejps.2021.105882
41. Pan F, Han L, Zhang Y, Yu Y, Liu J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int J Food Sci Nutr. 2015;66(6):680–685. doi:10.3109/09637486.2015.1077792
42. Xia F, Chen Z, Zhu Q, et al. Gastrointestinal lipolysis and trans-epithelial transport of SMEDDS via oral route. Acta Pharmaceutica Sinica B. 2021;11(4):1010–1020. doi:10.1016/j.apsb.2021.03.006
43. Köllner S, Dünnhaupt S, Waldner C, Hauptstein S, Pereira de Sousa I, Bernkop-Schnürch A. Mucus permeating thiomer nanoparticles. Eur J Pharm Biopharm. 2015;97(PtA):265–272. doi:10.1016/j.ejpb.2015.01.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.