Back to Journals » Cancer Management and Research » Volume 10
Mechanical ventilation promotes lung metastasis in experimental 4T1 breast cancer lung-metastasized models
Authors Huang YL, Pan L, Helou K, Xia QS, Parris TZ, Li HY, Xu B, Li H
Received 26 May 2017
Accepted for publication 14 November 2017
Published 20 March 2018 Volume 2018:10 Pages 545—555
DOI https://doi.org/10.2147/CMAR.S142650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yinglai Huang,1,2,* Lin Pan,3,* Khalil Helou,2 Qisheng Xia,3 Toshima Z Parris,2 Hongyan Li,3 Bo Xu,3 Hon Li3
1Division of Breast and Endocrine Surgery, Department of Surgery, Borås Hospital, Borås, 2Department of Oncology, Institute of Clinical Sciences, Sahlgrenska Cancer Center, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden; 3Department of Biochemistry and Molecular Biology, China-Japan Friendship Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Background/purpose: The aim of this study was to test the hypothesis that mechanical ventilation (MV) during cancer surgery induces lung stroma/tissue milieu changes, creating a favorable microenvironment for postoperative lung metastatic tumor establishment.
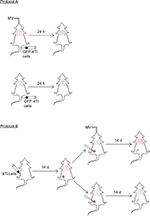
Materials and methods: In Protocol A, female BALB/c mice were divided into an MV group and a control (no MV) group, both of which were anesthetized and subjected to intravenous injection of green fluorescent protein (GFP)-labeled mouse mammary carcinoma cell line (4T1) cells. After 24 h, the lung tissue was removed and the number of GFP-labeled 4T1 cells was calculated. In Protocol B, the clinically relevant mouse model of spontaneous breast cancer lung metastasis was used with surgical resection of the primary tumor to investigate the MV event that dictates postoperative lung metastasis. Female BALB/c mice were inoculated in the mammary fat pad with 4T1 cells. After 14-d growth, mice were anesthetized and divided into an MV group and a control (no MV) group during surgical procedures (mastectomy). Metastatic tumor burden was assessed two weeks after mastectomy by both macroscopic metastatic nodule count, hematoxylin–eosin histology, immunohistochemistry for the macrophage marker (CD68), and epithelial cell adhesion molecule (EpCAM).
Results: MV was associated with a significant increase in the number of circulating breast tumor cells (GFP-labeled 4T1 cells) remaining in the microvasculature of the lung (P<0.01). Immunohistochemical results showed increased infiltration of CD68-positive macrophages within injured lung parenchyma and metastatic tumor as well as increased expression of EpCAM in metastatic nodules. Postoperative metastases were more prevalent in the mechanically ventilated mice group compared to the non-ventilated group (P<0.05).
Conclusion: MV-induced lung metastasis occurs by attracting circulating tumor cells to the site of the lung injury and by accelerating the proliferation of preexisting micro-metastases in the lung. These observations indicate that the metastasis-enhancing effect of MV should be considered in general anesthesia during cancer surgery.
Keywords: breast cancer metastasis, mechanical ventilation, 4T1, surgery, inflammatory cellular response, EpCAM
Introduction
The main cause of high mortality rates due to breast cancer is the development of distant metastasis. Molecular mechanisms controlling metastatic growth and dormancy continue to provide many challenges, despite advances in the understanding of its pathogenesis.1,2 Although there are various treatment options for breast cancer, control of advanced disease often depends on surgical removal of the primary tumor. Unfortunately, both clinical and experimental studies have shown that cancer surgery may increase the risk of metastases and recurrence.2,3 Tumor manipulation during surgery may increase the metastatic process via tumor cell dissemination into the lymphatic network and bloodstream. Moreover, many patients shelter micro-metastases and scattered tumor cells at the time of surgery. Moreover, perioperative factors can inhibit important host defense and promote the development of metastases.3–5
It is well known that anesthesia plays an important role in cancer recurrence.5,6 Retrospective studies suggest that regional anesthesia reduces the risk of tumor metastasis and recurrence compared with general anesthesia.6 It is increasingly recognized that anesthetic drugs administered during general anesthesia can inhibit the cellular immune system and affect cancer recurrence.7–9 MV is mandatory in patients undergoing general anesthesia. Although MV is considered a safe procedure, it can generate pulmonary stress, triggering local inflammation and local coagulation.10–12 We, therefore, hypothesize that MV used in general anesthesia during breast cancer surgical procedures may directly affect lung metastasis.
Metastasis is a complex, coordinated process that depends on the interaction between cancer cells and the tumor micro-environment, involving several cell types, cytokines, and cell-adhering molecular pathways.13–16 During metastasis, cells that are arrested in a capillary bed (intravasation) are able to invade the host-organ parenchyma (extravasation), resulting in the growth of secondary tumor cell colonies.15–18 Animal studies have shown that, following intravenous injection, the vast majority of circulating tumor cells are initially arrested in the lungs, although few cells remain in the lungs after 1–3 days.19 The arrest of circulating cancer cells in the capillary beds of the lungs and their subsequent release have been associated with the anti-metastatic effect of healthy microvascular endothelial barriers17,18 and the host immune system.20 Disruption of the endothelial barrier has been reported to directly facilitate cancer cell intravasation/extravasation, which accelerates cancer metastasis.21
The association between high-airway-pressure MV and pronounced morphological changes at both the epithelial and microvascular endothelial barriers is well defined in animal models.12 There is evidence that subtle endothelial changes and inflammatory reaction within the lungs might be caused by MV at low lung volume.22,23 Macrophages have a central role in the maintenance of immunological homeostasis and host defense. Macrophages can be rapidly activated by MV and, thus, may play an important role as inflammatory mediators in the pathogenesis of lung injury induced by MV.24,25 A number of animal studies have shown that injurious ventilatory strategies are associated with the release of a variety of proinflammatory mediators, which is important in cell signaling. Proinflammatory cytokines such as IL-1β, IL-6, and TNF-α are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions.11,24,25
EpCAM, a transmembrane glycoprotein, is highly expressed in rapidly proliferating carcinomas, including breast cancer.26 Recent data suggest a more multipotent role of EpCAM, such as cell–cell adhesion, cell signaling, migration, inflammation, proliferation, and differentiation.27–29 In addition, it was shown that EpCAM plays a major role in lung metastatic processes.26,30
In the present study, we investigated the role MV plays in promoting lung metastasis. The study was designed to use two protocols (A and B) to assess: 1) the effect of MV on lung 4T1 tumor-cell retention in BALB/c mice, 24 h after 4T1 cell tail-vein injection (Protocol A); 2) the impact of MV on vascular permeability, alveolar damage, and inflammatory cell infiltrates (Protocol A); and 3) effects of MV during surgical resection of the primary tumor on lung metastasis development, macrophage infiltration, and EpCAM expression in lungs two weeks after the operation (Protocol B) using a clinically relevant animal model of spontaneous breast cancer lung metastasis.
Materials and methods
Experimental animals
Female BALB/c mice (6–8 weeks old) were purchased from Beijing HFK Bioscience Co. Ltd. (batch number SCXK 2014-004). Study animals were housed in the animal research facility at the China-Japan Friendships Clinic Medical Research Institute. The treatment and use of animals during the study were in accordance with the regulations of the Guide for the Care and Use of Laboratory Animal of the National Institutes of Health (GB14925-2010). The study protocols were approved by the Committee on the Ethics of Animal Experiments of the China-Japan Friendship Hospital.
Anesthesia
Mice were anaesthetized by intraperitoneal injection of a mixture of pentobarbital sodium (60 mg/mL), diazepam (5 mg/mL), and PBS in the proportion 1:2:1 (0.4 mL/kg body weight).
Mechanical ventilation
MV was undertaken as described elsewhere.13,37 Briefly, after sodium pentobarbital anesthesia and insertion of a tracheotomy tube (1.3 mm outer diameter and 0.8 mm inner diameter), female BALB/c mice were connected to a ventilator (DHX-50; Chengdu Instrument Company, Chengdu, People’s Republic of China) and mechanically ventilated for 60 min (tidal volume, 8 mL/kg; positive end-expiratory pressure, 4 cm H2O; fraction of inspired oxygen, 0.40).22,23,25
Cell culture
The highly metastatic 4T1 breast cancer cell line derived from a spontaneously arising BALB/c mammary tumor was supplied by the Cell Center of the Chinese Academy of Medical Sciences (Beijing, People’s Republic of China). These 4T1 cells (3×105 cells/mL) were plated on six-well plates at a concentration of 3×104 cells/well and transfected using the polybrene method with GFP-containing plasmid (plenti6-EGFP, 8 μg/mL; Invitrogen, NY, USA). The cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% FBS and 1% penicillin–streptomycin (Gibco-Invitrogen, Grand Island, NY, USA), at 37°C in a humidified incubator in 5% CO2.31 The cell-culture medium was changed 16 h after seeding, and cells were split after 48 h.
Histology
Mice were euthanized, and the lungs were excised. The lung tissues were fixed in 4% paraformaldehyde at room temperature for 12 h, then dehydrated overnight in 30% sucrose at 4°C, and embedded in optimal cutting temperature compound (OCT compound). Serial 8-µm tissue sections were sliced throughout the entire lungs on a cryostat cryomicrotome (Leica 3050S) and mounted on positively charged microscope slides (Super Frost Plus; ThermoFisher Scientific, Waltham, MA, USA). The frozen lung sections were subjected to standard H&E and immunohistochemical staining. For H&E staining, sections were stained by following standard procedure. The Olympus IX71 Inverted Fluorescence Microscope, equipped with a blue excitation cube, was used to detect GFP signals in the frozen lung sections. The total number of 4T1 cells per lung section (n=8, 8–10 sections) was counted and averaged among the animals of each group.32
Immunohistochemical procedures
For EpCAM staining, frozen tissue sections were washed in TBS (25 mM Tris, 150 mM NaCl, and 3mM KCl, adjusted to pH 8.0 with 1N NaOH) and incubated in 0.6% H2O2 and 0.06% NaN2 for 30 min to quench endogenous peroxidase activity. After 30-min incubation in 1% BSA-TBS, the slides were incubated with a rabbit polyclonal anti-EpCAM, 1:100 (c-terminus, ab71916; Abcam, Cambridge, UK) or with a rabbit polyclonal anti-CD68, 1:100 (Abcam, Shanghai, People’s Republic of China) primary antibody in 0.1% BSA-TTBS (TBS containing 0.1% Triton X-100) at 4°C overnight, followed by a second incubation with biotinylated goat anti-rabbit-IgG, 1:200 (DAKO, Denmark) for 60 min at room temperature. The sections were developed in GDN solution for 5–20 min and counterstained with hematoxylin,30 before being dehydrated through a gradient of alcohol, cleared with xylene, and mounted with Mountex (Histolab, Gothenburg, Sweden). Images were taken with an Olympus microscope (BX51; Olympus, Japan).
Quantification of EpCAM immunostaining was undertaken by digital image analysis with IPP 6.0 (Media Cybernetics). A total of five fields selected from hotspot areas (metastatic nodules) in the digital image (×400) were acquired per slice, and five slices from separate animals within each group (four animals) were examined. The IOD of all positive staining in each field as well as the AOP were measured.
For macrophage quantitative analysis, the number of CD68-positive cells was manually counted in five random fields at ×20 magnification of view per section from four animals per group.
Animal experiment protocols
The summary of the experiments is depicted in Figure 1. The number of mice studied in each experiment is described in each protocol.
Protocol A
Mice were injected with 1×105 4T1-GFP cells dissolved in 100 µL sterile PBS via the median tail vein, followed by MV for 60 min under anesthesia. Control mice were only injected intravenously with 1×105 GFP-labeled 4T1 cells without MV after intraperitoneal administration of pentobarbital sodium. Mice were killed after 24 h, and the lungs were excised and fixed in 10% neutral buffered formalin overnight. The tissue samples were dehydrated, embedded, and cryosectioned for fluorescence imaging to visualize the surviving circulating tumor cells. Furthermore, adjacent frozen sections were stained with H&E and used for EpCAM immunohistochemistry staining (MV and no MV groups, n=8).32
Protocol B
The effect of MV on breast cancer lung metastasis in a 4T1 orthotopic mouse model was assessed using BALB/c mice. Briefly, mice were lightly anesthetized, the fur over the lateral thorax was shaved, and a 5 mm-long incision was made to reveal the mammary fat pad. The mammary fat pad was injected with 5×105 4T1 breast tumor cells dissolved in 100 µL sterile PBS and the incision was closed with a wound clip. Tumor volume was measured once a week using an electronic caliper. Two weeks after 4T1 inoculation, the mice were randomized into two groups (six mice per group). In Group 1, a complete resection of the mammary primary tumor was carried out in combination with MV for 60 min (under anesthesia). In Group 2, only the surgical excision was done under anesthesia with pentobarbital for a period of 60 min without MV. Two weeks after operation (four weeks after 4T1 tumor cell implantation), the mice were euthanized and the lungs were excised and fixed in 10% Bouin’s solution for 24 h to count the number of lung tumor nodules under a dissecting microscope. Furthermore, the lungs were washed in PBS, placed in 4% formalin solution for 48 h, then dehydrated, embedded, and cryosectioned for H&E and EpCAM immunohistochemical staining, and subjected to double-confirmed quantitative analysis under a microscope (BX51; Olympus). Results are reported as the average number of nodules per group (MV and no MV groups, n=6).33
Statistical analyses
Data are presented as the mean±SD. Statistical significance was calculated by Student’s t test using the Graph Pad Prism statistical program (version 6.0; Graph Pad Software, Inc., San Diego, CA, USA). P<0.05 was considered to be statistically significant.
Results
MV induces lung tissue abnormalities and inflammatory responses in the metastatic 4T1 breast cancer model
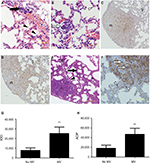
The initial objective was to identify a reasonable volume of MV that would have a measurable experimental effect in the 4T1 spontaneous lung metastasis model without a significant increase in mortality two weeks after surgical excision of the primary tumor (data not presented). Preliminary experiments confirmed previous data, in which MV using clinically relevant ventilation settings (tidal volume, 8 mL/kg; positive end-expiratory pressure, 4 cm H2O; fraction of inspired oxygen, 0.21; 60 min) induced mild lung injury (local alveolar injury, increase in vascular permeability, and influx of inflammatory cells) in BALB/c mice (Figure 2A and B). The results suggested that MV induced morphological changes in pulmonary microvascular endothelial structures before the appearance of definite metastases.
MV affects arrest and retention of 4T1 in pulmonary microvasculature
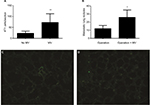
Localization of GFP-4T1 cells in the lungs was examined 24 h after MV treatment. A significantly larger number of intravenously injected GFP-4T1 tumor cells were retained in the lungs of MV-treated mice (Figure 3A and D) than in the lungs of controls without MV (Figure 3A and C), indicating tumor cell survival in the lungs at an early stage after seeding from the blood system. The majority of 4T1 tumor cells were localized in the injured lung areas and formed microtumor emboli in the mice after MV treatment (Figure 2A and B). The integrity of the pulmonary vasculature was found to be disrupted, the microvasculature wall was no longer smooth and regular, and capillary dilatation was apparent (Figure 2A). Furthermore, the penetration of circulating tumor cells in the injured lung areas was more severe than in the normal lungs (Figure 2B).
Furthermore, H&E staining revealed that the lung tissues of the MV-treated mice had inflammatory cellular infiltrates characterized by prominent lymphocytic cells (Figure 2A), suggesting that MV altered the microenvironment of the lung by triggering an inflammatory response. These results suggest that even low-volume MV can induce mild lung injury, while producing an attractive microenvironment for circulatory tumor cells to arrest and survive in the lung at an early stage after seeding from the bloodstream. In addition, these results show that MV modulated the pulmonary microenvironment by disrupting vascular integrity and altering vascular endocrine morphology (Figures 2A, B, and 3D).
MV affects postoperative lung metastases of 4T1 cells
To determine whether MV under surgery affects postoperative pulmonary metastases, we implanted 5×105 4T1 cells subcutaneously into the flanks of female BALB/c mice. After 14 d, the entire primary tumor (diameter, 8–15 mm, n=6) receded with or without MV (control) under mastectomy. Lung tissues were removed from the mice 28 d postoperatively and soaked in Bouin’s solution to count metastatic nodules. Tumor metastatic nodules were more prevalent in the MV-treated lungs than in the normal lungs (Figure 3B). We found that 4T1 tumor cell localization and metastatic growth in the lung were dramatically enhanced following surgical removal of the primary tumor with MV than without MV (control, n=6; Figure 3B). In the MV-treated group, the number of pulmonary nodules was 2.22-fold that of the control group (P<0.05).
MV alters EpCAM expression patterns in the pulmonary in the metastatic 4T1 breast cancer model
In the MV group, immunohistochemical staining of EpCAM in the postoperative pulmonary metastases showed strong membrane staining and moderate-to-strong staining in the cytoplasm (Figure 2D and F), indicating tumor cell survival and growth. In the control group (without MV under mastectomy), lung metastasis nodules demonstrated weak immunohistochemical staining. In addition, low EpCAM expression was found at the periphery of the tumor, suggesting functional inactivation of EpCAM (Figure 2C). EpCAM staining was not only pronounced in metastatic foci, in which 4T1 tumor cells were observed with strong membrane or cytoplasmic staining (Figure 2D and F), but also labeled the surrounding lung parenchyma in the mechanically ventilated group (Figure 2D and F). In contrast, sections from lungs without MV treatment showed no changes in apparent EpCAM immunoreactivity in surrounding normal lung parenchyma of metastatic foci (Figure 2C).
Histologic examination of H&E staining of lung sections showed extensive destruction of lung architecture in the MV group (Figure 2E). Moreover, in conditions of the disordered repair of epithelium, the normal relationship of epithelial cells and basal membrane was altered. Surprisingly, a strong cytoplasmic linear EpCAM reaction was seen in the epithelial cells in injured lung tissues (proliferative phase) surrounding the metastatic nodules (Figure 2F), while alveolar epithelium exhibited normal weak basolateral membranous expression patterns in lung parenchyma in the group without MV (Figure 2C).
The IOD value, representing the intensity of expression of EpCAM, and the AOP value, presenting the area of positive EpCAM staining, were measured using IPP 6.0. The IOD increased markedly in the MV group compared with the no MV group (P<0.01). In addition, the AOP was larger in the MV group than in the no MV group (P<0.01; Figure 2G and H).
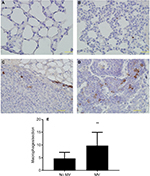
MV increases macrophage infiltrates in lung parenchyma and lung metastatic nodules in the metastatic 4T1 breast cancer model
CD68 (macrophage marker) immunohistochemical staining of lung sections showed increased macrophage infiltration in the lung parenchyma of MV-treated mice when compared to the without MV-treated mice (Figure 4A, B, and E), indicating that MV altered the microenvironment of the lung by triggering a host inflammatory response. In addition, we found that macrophages in the lung metastatic nodules were dramatically enhanced following surgical removal of the primary tumor with MV than without MV (Figure 4C and D), suggesting that macrophages also play an important role in this increased metastatic growth.
Discussion
The tumor-metastasis-enhancing effect of general anesthesia has been previously demonstrated5–7; however, the mechanisms by which general anesthesia increases tumor growth have remained unclear.7–9 Recent reports have shown that pulmonary inflammation and the peripheral immune system can be modified by MV.10–12 This led us to hypothesize that MV during general anesthesia may have a direct effect on lung metastasis. Results from this current study have demonstrated that MV promoted lung metastasis on three aspects. First, we demonstrated that MV promotes tumor establishment during the process of pulmonary metastasis by attracting the circulating 4T1 cells to the site of local inflammation and tissues with lung damage induced by MV. Second, the most important clinically relevant finding was that MV promotes postoperative lung metastases in the 4T1 primary tumor model of spontaneous breast cancer lung metastasis. Third, analysis of CD68 (macrophage marker) and EpCAM expression after MV demonstrated increased infiltration of macrophages within the metastatic tumor and increased EpCAM expression in lung metastases and surrounding lung tissues.
To our knowledge, this is the first report showing an association between MV and the development of lung metastases. Tumor cells require a favorable microenvironment to survive and proliferate at distant sites. Cell–cell interactions among tumor cells, platelets, leukocytes, and endothelial cells have been shown to contribute to enhanced tumor cell survival and to the facilitation of metastasis.13–16 This study demonstrates that MV modulated the pulmonary microenvironment by disrupting vascular integrity, altering vascular endocrine morphology, triggering an inflammatory response, and, then, this altered microenvironment dictates to extravasate, survive, and ultimately facilitate breast cancer cell proliferation in the lung. These findings support the concept that structural disruption of endothelial-capillary permeability and exposure of the basal membrane matrix increases cancer cell attachments after MV.17,18
We were interested in examining the modulation of EpCAM expression because it has been implicated in multiple cellular functions including cell adhesion, nuclear signaling, migration, proliferation, and regeneration in normal and inflammatory tissues26,29 and has been shown to promote cancer progression, invasion, and metastasis.26–30 EpCAM, a cell–cell adhesion molecule, is typically overexpressed in a variety of epithelial cancers, for example, breast carcinomas.30 Normal epithelial cells express EpCAM at variable, but generally lower, levels in situ at intercellular basolateral interfaces than in carcinoma cells. Active proliferation in a number of epithelial tissues is associated with elevated EpCAM expression levels.29 Therefore, we suppose that EpCAM plays a crucial role in the breast cancer lung metastatic cascade after MV. In this study, we demonstrated that the increase in metastatic burden coincided with 4T1 tumor cell EpCAM overexpression within lung metastases, and increased EpCAM expression in epithelial cells has also been found in surrounding lung parenchyma in the MV group. Overexpression of EpCAM has been found to be associated with enhanced transcription and translation of the proto-oncogene c-myc.34 Moreover, it is known that EpCAM stimulates the inflammatory process by activating and attracting macrophages, which can promote tumor invasion.35 EpCAM seems to be involved in the increased metastatic growth after MV in this study.
EpCAM is a type I transmembrane glycoprotein with its N-terminus facing the outer cellular space, extracellular domain (EpEx), single-transmembrane domain, and with the C-terminus directed to the cytoplasm, intracellular domain (EpICD). EpCAM-mediated proliferative signaling has been linked to proteolytic cleavage, RIP. After RIP, EpICD is released in the cytoplasm and shuttles into the cell nucleus on forming a complex with β-catenin to participate in upregulation of EpCAM gene transcription.26,27 Furthermore, shedding of EpEx has been shown to contribute to cancer progression by acting as modulator of cell adhesion, through inducing proteolytic activity and facilitating cell signaling.35
The TACE/ADAM17 is a metalloprotease that has been reported to initiate EpCAM cleavage at the extracellular site.35 There is increasing evidence that mechanical stress affects the release and activation of metalloprotease.12 The increased level of metalloprotease in injured tissue induced by MV appears to be involved in the regulation of EpCAM signaling of 4T1 and epithelial cells. Furthermore, it is interesting to note that we found increased infiltration of CD68-positive macrophages within the injured lung parenchyma and the metastatic tumor. A number of animal studies have shown that injurious ventilatory strategies are associated with the release of a variety of proinflammatory mediators,24,25 which are important in cell signaling. These proinflammatory mediators, including TNFα and CD44 which have been identified as substrates for RIP, are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions.25,36,37
It has been identified that β-catenin activation induced the expression of EpCAM. The Wnt/β-catenin pathway is activated when a Wnt ligand binds to a seven-pass transmembrane Frizzled (Fz) receptor and its co-receptor and results in cytosolic accumulation of β-catenin, which then translocates to the nucleus and participates in upregulation of EPCAM gene transcription.26,27 Recently, it has been shown that MV increased protein levels of Wnt5a (Wnt ligands) and non-phosphorylated (threonine and serine) β-catenin in lungs. In addition, several studies have reported synthesis of Wnt ligands by macrophages.37,38 It may, therefore, be reasonable to hypothesize that Wnt/catenin signaling plays a crucial role in modulating EpCAM expression following MV.
Accordingly, lung-protective strategies should be carried out in breast cancer patients during surgery. Novel strategies per-operation may include approaches aimed not only at decreasing pressures or volumes during MV, but also directed at the development of interventions that are aimed directly at preventing the initiation and/or propagation of the inflammatory response. Perioperative usage of nonsteroidal anti-inflammatory drugs to inhibit matrix metalloproteinases33 and inhaled hydrogen can act as an antioxidant and may be useful to reduce MV-associated inflammatory responses39; prophylactic inhalation of anti-inflammatory cytokines and pretreatment with intratracheal anti-TNF-α or anti-EpCAM antibody may reduce postoperative cancer metastasis and improve survival.26,40
Conclusion
The present results are significant in at least two major aspects. First, the present findings strongly indicate that lung injury inflammation after MV can alter the micro-environmental conditions within the lung to provide a favorable environment for metastasis. Second, MV-induced lung metastasis occurs by attracting the circulating tumor cells to the site of the lung injury and by accelerating the proliferation of preexisting micro-metastases in the lung. These observations indicate that the metastasis-enhancing effect of MV should be considered, which may provide novel strategies per-operation to prevent postoperative cancer metastasis.
Acknowledgments
This work was supported in part by an international research grant from the China-Japan Friendships Hospital and China-Japan Friendships Clinical Research Institute. Dr. Yinglai Huang is the recipient of an international research staff exchange foundation from the Swedish Society of Medicine. The authors are thankful to Professors Tiande Zhao and Zhihua Chen for their valuable assistance and advice during this project. The authors are also indebted to Weiliang Sun and Jing Gou for technical assistance and image analysis.
Abbreviations
4T1 cells, mouse mammary carcinoma cell line
AOP, area of positive staining
BSA, bovine serum albumin
BSA-TTBS, TBS containing 0.1% Triton X-100
EpCAM, epithelial cell adhesion molecule
EpEx, EpCAM extracellular domain
EpICD, EpCAM intracellular domain
FBS, fetal bovine serum
GDN, glucose-diaminobenzidine-nickel
GFP, green fluorescent protein
H&E, hematoxylin and eosin
IgG, immunoglobulin G
IL, interleukin
IOD, integrated optical density
IPP, Image-Pro Plus
MV, mechanical ventilation
PBS, phosphate-buffered saline
RIP, regulated intramembrane proteolysis
SD, standard deviation
TACE, TNFα converting enzyme
TNFα, tumor necrosis factor alpha
TBS, Tris-buffered saline
Disclosure
The authors report no conflicts of interest in this work.
References
Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2752–2823. | ||
Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102(9):1327–1334. | ||
Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26(1):99–114. | ||
Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. | ||
Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–115. | ||
Sessler DI. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev. 2008;17(3):269–272. | ||
Santamaria LB, Schifilliti D, La Torre D, Flodale V. Drugs of anaesthesia and cancer. Surg Oncol. 2010;19(2):63–81. | ||
Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–277. | ||
Tavare AN, Perry NJ, Bensonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. | ||
Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005:11(1):82–86. | ||
Halbertsma FJ, Vaneker M, Scheffer GJ, Van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med. 2005;63(10):382–392. | ||
Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med. 2010;38(10 Suppl):539–548. | ||
Hart IR. “Seed and soil” revisited: mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982;1(1):5–16. | ||
Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. | ||
Nguyen DX, Bos PD, Massaqué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. | ||
Mathot L, Stenninger J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 2012;103(4):626–631. | ||
Mierke CT. Role of the endothelium during tumor cell metastasis: is the endothelium a barrier or a promoter for cell invasion and metastasis? J Biophys. 2008;2008:183516. | ||
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2002;6(1):100–102. | ||
Weiss L, Ward PM. Arrest and retention of circulating cancer cells in the lungs of animals with defined metastatic status. Cancer Res. 1982;42(5):1898–1903. | ||
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420(6917):860–867. | ||
Orr FW, Adamson IY, Young L. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res. 1986;46(2):891–897. | ||
Vaneker M, Halbertsma FJ, van Egmond J, et al. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology. 2007;107(3):419–426. | ||
Vaneker M, Joosten LA, Heunks LM, et al. Low-tidal-volume mechanical ventilation induces a toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology. 2008;109(3):465–472. | ||
Pugin J, Dunn I, Jolliet P, et al. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275(6 Pt 1): L1040–L1050. | ||
Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1191–L1198. | ||
van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31(11):1913–1921. | ||
Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11(2):162–171. | ||
Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69(14):5627–5629. | ||
Martowicz A, Seeber A, Untergasser G. The role of EpCAM in physiology and pathology of the epithelium. Histol Histopathol. 2016;31(4):239–255. | ||
Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol. 2011;64(5):415–420. | ||
Misteli T, Spector DL. Applications of green fluorescent protein in cell biology and biotechnology. Nat Biotechnol. 1997;15(10):961–964. | ||
DuPré SA, Redelman D, Hunter KW Jr. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88(5):351–360. | ||
Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. | ||
Münz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23(34):5748–5758. | ||
van Kilsdonk JW, van Kempen LC, van Muijen GN, Ruiter DJ, Swart GW. Soluble adhesion molecules in human cancers: sources and fates. Eur J Cell Biol. 2010;89(6):415–427. | ||
Zhang Y, Liu G, Dull RO, Schwartz DE, Hu G. Autophagy in pulmonary macrophages mediates lung inflammatory injury via NLRP3 inflammasome activation during mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L173–L185. | ||
Cosín-Roger J, Ortiz-Masiá D, Calatayud S, et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One. 2013;8(10):e78128. | ||
Villar J, Cabrera NE, Valladares F, et al. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One. 2011;6(9):e23914. | ||
Huang CS, Kawamura T, Lee S, et al. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care. 2010;14(6):R234. | ||
Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Med. 2013;11:85. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.