Back to Journals » Cancer Management and Research » Volume 12
Mean Platelet Volume Has Prognostic Value in Chronic Lymphocytic Leukemia
Authors Masternak M , Puła B , Knap J , Waszczuk-Gajda A , Drozd-Sokołowska J , Wdowiak K , Grosicki S , Kozłowska I , Kaźmierczak M, Łabędź A, Szukalski Ł, Wiśniewski K , Subocz E, Hałka J, Szymczyk A, Hus M, Jamroziak K, Giannopoulos K
Received 17 January 2020
Accepted for publication 8 September 2020
Published 12 October 2020 Volume 2020:12 Pages 9977—9985
DOI https://doi.org/10.2147/CMAR.S246385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Marta Masternak,1 Bartosz Puła,2 Joanna Knap,1 Anna Waszczuk-Gajda,3 Joanna Drozd-Sokołowska,3 Kamil Wdowiak,4 Sebastian Grosicki,5 Izabela Kozłowska,6 Marta Kaźmierczak,6 Anna Łabędź,7 Łukasz Szukalski,8 Kamil Wiśniewski,2 Edyta Subocz,9 Janusz Hałka,10 Agnieszka Szymczyk,11 Marek Hus,12 Krzysztof Jamroziak,2 Krzysztof Giannopoulos13
1Department of Experimental Hematooncology, Medical University of Lublin, Lublin, Poland; 2Department of Hematology, Institute of Hematology and Transfusion Medicine, Warsaw, Poland; 3Department of Hematology, Oncology and Internal Diseases, Medical University of Warsaw, Warsaw, Poland; 4Department of Internal Medicine and Oncological Chemotherapy, Silesian Medical University, Katowice, Poland; 5Department of Hematology and Cancer Prevention in Chorzow, Faculty of Health Sciences in Bytom, Silesian Medical University, Katowice, Poland; 6Department of Hematology and Cancer Prevention, Municipal Hospital in Chorzów, Chorzów, Poland; 7Department of Hematology, Rydrygier’s Hospital in Cracow, Cracow, Poland; 8Department of Hematology, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, Toruń, Poland; 9Department of Hematology, Military Institute of Medicine, Warsaw, Poland; Clinical Department of Hematology, Independent Public Healthcare Centre of the Ministry of Internal Affairs and Administration with Warmia-Mazury Region’s Oncology Centre in Olsztyn, Olsztyn, Poland; 10Clinical Department of Hematology, Independent Public Healthcare Centre of the Ministry of Internal Affairs and Administration with Warmia-Mazury Region’s Oncology Centre in Olsztyn, Olsztyn, Poland; 11Department of Hematooncology and Bone Marrow Transplantation, Medical University of Lublin, Lublin, Poland; Hematology Department, St John’s Cancer Center, Lublin, Poland; 12Department of Hematooncology and Bone Marrow Transplantation, Medical University of Lublin, Lublin, Poland; 13Department of Experimental Hematooncology, Medical University of Lublin, Lublin, Poland; Hematology Department, St John’s Cancer Center, Lublin, Poland
Correspondence: Krzysztof Giannopoulos Tel +48 81448 6632
Fax +48 81448 6634
Email [email protected]
Purpose: Mean platelet volume (MPV) is a readily accessible and commonly tested hematological indicator. Recent studies revealed a significant impact of MPV on the course and prognosis of many diseases, including some types of cancer, as well as on the incidence of atrial fibrillation and bleeding. The study aimed to perform a retrospective analysis of MPV in terms of time to first treatment (TTFT) and to determine its prognostic value in the group of patients with chronic lymphocytic leukemia (CLL). Moreover, the study includes a retrospective analysis of platelet parameters in patients treated with ibrutinib concerning bleeding and atrial fibrillation.
Patients and Methods: The study included 523 patients with CLL, for 344 the most important cytogenetic aberrations were reported. The Mann–Whitney, Kruskal–Wallis, Kaplan–Meier, chi-squared, log‑rank tests and multivariate Cox proportional hazard regression model were used to analyze collected data.
Results: The receiver operating characteristic curve analysis was performed to identify optimal cut-off value for MPV. The analysis of survival curves showed that in the group of patients with higher values of MPV TTFT was significantly longer than in the group with lower MPV (17.9 vs 36 months, p=0.0015, cut-off value for MPV= 10.4 fl). In multivariate Cox proportional hazard regression model low MPV, the presence of del11q and del13q provided independent prognostic value for TTFT (HR=0.69, 95%-CI, 0.5293 to 0.9081; p=0.0078; HR=1.76, 95%-CI, 1.3000 to 2.3882, p=0.0003, HR=0.74, 95%-Cl, 0.5674 to 0.9588, p=0.0229, respectively). In the group treated with ibrutinib, 59 patients had no significant correlation between MPV level and the incidence of therapy complications, although in the group of patients with low MPV there was a tendency for more frequent occurrence of atrial fibrillation (p=0.259).
Conclusion: Low MPV values are associated with unfavorable prognosis and might represent a novel, independent prognostic factor in CLL.
Keywords: MPV, chronic lymphocytic leukemia, TTFT
Introduction
The complete blood count (CBC) is basic and usually the first medical test in which abnormalities reflect any pathological phenomena in the organism. Among its components are platelet parameters: platelet count (PLT), platelet distribution width (PDW), mean platelet volume (MPV) and plateletcrit (PCT). The platelets play a significant role in many physiological pathways in the body; therefore, abnormalities in these parameters indicate pathological processes, such as homeostatic disorder, inflammation or tumor growth.1 The role of platelet parameters in oncological patients was first described in 1872, when Leopold Riess observed an increased number of thrombocytes in patients with carcinoma.2 These findings were confirmed and complemented in many further studies, which proved that PLT might represent a potential marker of cancer, and correlate with the severity of the disease.3
Therefore, other platelet indices were considered as potential prognosis-affecting factors. Recent studies revealed a significant impact of MPV value on the course of disease in different types of neoplasms. MPV is a machine-calculated measurement of the average size of platelets found in the blood. The level of MPV seems to have prognostic value in many types of solid tumors – colorectal cancer, gastric cancer, renal cell carcinoma, esophageal cancer, non-small cell lung cancer, invasive breast cancer or papillary thyroid carcinoma.4–11 Single reports on the role of MPV in lymphoproliferative diseases could also be found in the literature. Low MPV values were related to worse outcome in patients with diffuse large B-cell lymphoma (DLBCL).12 Low MPV was also associated with a greater number of venous thromboembolism episodes in DLBCL patients treated with chemotherapy.13 Meanwhile, elevated MPV was associated with worse clinical outcomes in patients with primary or secondary osteomyelofibrosis (OMF).14 However, the prognostic significance of MPV in other lymphoproliferative diseases remains an issue that requires further studies.
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in adult patients in Europe and North America.15 The disease is heterogeneous in both the molecular characteristics and clinical course that varies from stable to rapidly progressive. The survival of patients could range from 1 year to 15 years or more.16 Despite the improvement in the understanding of the pathophysiology of CLL over the past decade, its pathogenesis has not been precisely defined.17 The variety of prognostic factors have been already described; nevertheless, they are not fully efficient in predicting the course of CLL, especially when the disease is diagnosed at an early stage. International Prognostic Index (CLL‑IPI) created in 2016 by the Chronic Lymphocytic Leukemia Working Group included five factors: age (>65 years), stage (Rai I–IV or Binet B-C), IGHV gene mutational status (unmutated), β2-microglobulin level (>3.5 mg/L) and TP53 gene (mutation or deletion).18 The studies conducted in the recent decade have revealed several somatic mutations with a potential impact on the course and prognosis of the disease. Among them: neurogenic locus notch homolog protein 1 (NOTCH1), myeloid differentiation primary response gene 88 (MYD88), and splicing factor 3B subunit 1 (SF3B1).19 Nevertheless, the determination of the mutation is a procedure that requires time, adequate laboratory facilities and generates costs. The potential use of MPV as a prognostic factor would allow a quick assessment of the patient at the time of diagnosis. Moreover, CBC is a fast, cheap, easily accessible and repeatable test.
Besides, MPV could be also useful in assessing the risk of therapy complications. Ibrutinib, which becomes the new standard in CLL treatment, is an inhibitor of Bruton tyrosine kinase (BTK). BTK is also important for signaling via the collagen receptor glycoprotein VI in platelets and thereby ibrutinib affects platelet function. Among the adverse effects of ibrutinib therapy, the most characteristic and recognized are bleeding episodes and atrial fibrillation (AF), that are observed, respectively, in 44% and 9% of treated patients.20,21 Both bleeding episodes and AF are potentially related to platelet parameters.22,23 It seems that testing the dependence between MPV level and occurrence of AF in patients treated with ibrutinib may help to identify the potential predictor of this adverse effect.
The study aimed to perform a retrospective analysis of MPV in terms of time to first treatment (TTFT) and to determine its potential prognostic value in the group of patients with CLL. The study also includes a retrospective analysis of platelet parameters in patients treated with ibrutinib concerning bleedings and AF.
Patients and Methods
The study investigated retrospectively 523 patients in the care of 7 hematological centers in Poland. The cohort consisted of 302 (57.7%) men and 221 (42.3%) women aged from 32 to 89 years of age. The median age was 65 years. Most of them – 363 patients – received chemotherapy, while 160 did not require treatment until the end of the study. The group of 59 patients were treated with ibrutinib. Clinical data of all of the patients were collected before the start of treatment. Distribution of patients according to the Rai classification was as follows: stage 0–44 patients; stage I – 171 patients; stage II – 180 patients; stage III – 55 patients; and stage IV – 65 patients. Clinically, the prognostic significance in terms of the TTFT was assessed for 519 patients with CLL. The TTFT was defined as the time from diagnosis to the start of treatment, for active disease following the criteria proposed by the International Workshop on CLL. In the group of 344 patients, cytogenetic aberrations (del11q, del13q, del17p and tri12) using immunofluorescent in situ hybridization (FISH) were assessed. The detailed clinical characteristics of patients are presented in Table 1.
 |
Table 1 (A) Clinical Characteristics of Patients. (B) Cytogenetic Aberrations in the Study |
The collected data were used to perform statistical analysis with GraphPad Prism 5 (La Jolla, California, United States). The Mann–Whitney, Kruskal–Wallis and chi-squared test tests were used to evaluate the differences between the subgroups. The correlations of variables were computed with the Spearman rank correlation coefficient. The Kaplan–Meier method and the log‑rank test were used to assess TTFT of CLL patients in different prognostic subgroups as well as groups defined by MPV value. Multivariate Cox Proportional Hazard Regression Model was performed using MedCalc (MedCalc Software, Ostend, Belgium) to establish independent prognostic markers for TTFT. Statistical significance was defined as a p-value of less than 0.05.
Results
The MPV value ranged from 6.1 to 14.5 fl. The median was 10.2 fl and the mean was 10.17 (±1.45) fl. MPV characteristics in individual hematological centers involved in the project are presented in Table 2. The receiver operating characteristic (ROC) curve analysis was performed to identify optimal cut-off value, which was established as 10.4 fl (AUC: 0.595, sensitivity 61.02%, specificity 56.60%) (Figure 1). Patients were divided into two groups: patients with MPV ≤ 10.4 fL (low MPV) and patients with MPV > 10.4 fL (high MPV). There were 289 (55.3%) patients with low MPV and 234 (44.7%) patients with high MPV values.
 |
Table 2 MPV Characteristics in Individual Hematological Centers Involved in the Project |
 |
Figure 1 The optimized cut-off value was determined for MPV using standard ROC curve analysis. |
The study revealed that MPV levels are associated with clinical advancement of the disease according to Rai staging, the need for treatment and IGHV mutational status. There were no differences according to age, sex, ZAP-70 or CD-38 levels. The detailed comparison of clinical features in groups with low and high MPV values is shown in Table 3. Moreover, median MPV in patients, who required treatment, was significantly lower than in patients with no need for treatment (median MPV 10.1 fL vs 10.6 fl; p=0.009). There was a tendency for lower median MPV in more advanced clinical stages according to Rai classification; however, this relationship did not apply to stage IV (median MPV, stage 0–10.6; I- 10.3; II-10.15; III-10.1; IV −10.6 [fl]; p=0.35). These results are shown in Figure 2.
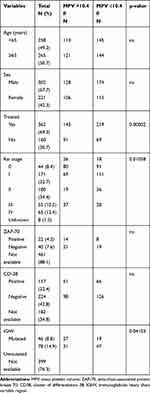 |
Table 3 Baseline Characteristics of the Patients According to MPV Values |
 |
Figure 2 Median MPV depending on clinical characteristics (A and B). |
The median TTFT in patients with low MPV level was significantly shorter compared to the group with higher MPV (17.9 vs 36 months, p=0.0015). The Kaplan-Meier TTFT curves of the low versus high MPV showed a significant separation (Figure 3A). We analyzed the relevance of MPV level and IGHV mutation status to discriminate the group of CLL patients with the longest TTFT. In the case of MPV high/IGHV mutated group showed significantly longer TTFT compared to MPV high/IGHV unmutated, MPV low/IGHV mutated, MPV low/IGHV unmutated (median undefined [more than 92] vs 62.4 vs 42.3 vs 11.9 months, p=0.0007, respectively; Figure 3B). In Multivariate Cox Proportional Hazard Regression Model low MPV, the presence of del11q and del13q provided independent prognostic value for TTFT (HR=0.69, 95%-CI, 0.5293 to 0.9081; p=0.0078; HR=1.76, 95%-CI, 1.3000 to 2.3882, p=0.0003, HR=0.74, 95%-Cl, 0.5674 to 0.9588, p=0.0229).
 |
Figure 3 Kaplan–Meier analysis of TTFT in CLL patients (A and B). |
The group of 59 patients was treated with ibrutinib. In 14 of them complications of the therapy occurred: 7 patients had at least one episode of AF during treatment, 6 patients had an episode of bleeding and one patient had both AF and bleeding episodes. As for bleeding episodes, most of them (n=4) were grade 1 in Common Toxicity Criteria for Adverse Events (CTCAE) scale (version 5.0). There was no significant correlation between MPV level and the incidence of therapy complications, although in the group of patients with low MPV there is a tendency for more frequent occurrence of AF (p=0.259).
According to the fact, that clinical data were obtained from 7 hematological centers, differing in laboratory norms used, we performed analysis including data normalized to the applicable laboratory norms. The analysis of the normalized MPV value is compatible with the analysis of MPV values presented in the article and confirms that the differences in the laboratory norms of individual centers did not affect the homogeneity of the group and the statistical significance of the results. The complete analysis is available as the Supplementary data (Supplementary Table 1, Supplementary Figure 1).
Discussion
According to our knowledge, this is the first analysis to evaluate the association between MPV values and prognosis in CLL. Our findings indicate that patients with lower MPV levels marked before the start of treatment have a worse prognosis. More frequently they need to start therapy, have shorter TTFT and more frequent display co-existence of an unfavorable prognostic factor such as unmutated IGHV status.
The prognostic value of MPV levels has been described in many types of solid tumors. However, in most of them, an elevated MPV level correlated with a worse prognosis. In colorectal carcinoma the MPV level was significantly higher than in patients with colorectal adenoma or healthy participants; moreover, MPV was significantly higher in patients with metastases compared to those with no metastasis.4,24 In gastric cancer, a higher baseline MPV level was correlated with a greater number of metastases and worse response to chemotherapy.25 A similar correlation was described in breast cancer or pancreatic cancer.6,10,26 However, CLL is not the only type of cancer in which low MPV levels correlate with poor outcomes of the disease. In esophageal cancer, survival analysis revealed that both the disease-free survival (DFS) and OS in the lower MPV group (MPV<7.4 fl) were significantly shorter than those in the high MPV group.7 Likewise, in renal cancer and muscle-invasive bladder cancer decreased MPV value was associated with a significantly shorter 5-year OS.8,27,28 There were also ambiguous reports regarding the importance of MPV levels in lymphoproliferative diseases. In DLBCL patients receiving R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) immunochemotherapy low MPV value was related to a worse outcome, while the 2 year-PFS rate was significantly longer in patients with MPV >9.1 fL compared to a group with lower MPV levels.12 Decreased MPV was also associated with the risk of venous thromboembolism and increased mortality in patients treated for DLBCL.13 Whereas, in patients with primary or secondary OMF elevated MPV was associated with worse clinical outcomes and shorter OS in the whole cohort.14
Based on these studies, it is difficult to determine which MPV level is beneficial for the organism and what mechanisms determine the impact of MPV on cancer prognosis. Platelets act as a crucial modulator in tumor development, tumor cell growth, angiogenesis, and metastasis.29,30 The most important role is probably ascribed as the intensity of inflammation associated with the neoplastic process, as thrombopoietin and numerous inflammatory cytokines (eg, IL-1, IL-6 and TNF) regulate thrombopoiesis.31 In the vast majority of solid tumors, the worse prognosis is associated with large platelets. This correlation is likely related to the ability of activated platelets to release soluble factors such as chemokines, proteolytic enzymes, proangiogenic regulatory proteins, and growth factors favoring tumor growth and invasion. Besides, platelets with the participation of integrin αIIbβ3 (glycoprotein IIb/IIIa) are involved in the formulation of tumor cell aggregates and protecting the tumor cells from lysis by the immune system. These aggregates are also more easily transferred into the bloodstream, which promotes metastasis.32,33 Platelet activation may be also associated with oxidative stress.34,35 In CLL, malignant cells are more active in the production of reactive oxygen species (ROS) than normal cells, they present also reduced antioxidant potential as compared to controls.36,37 Oxidative stress promotes the persistence of chronic inflammation, causes immune system dysfunction and may also lead to an increased number of infectious complications.38
Our study proved that in CLL this correlation is opposite, while the worse prognosis is associated with low MPV levels. These findings are consistent with results obtained in the other lymphoproliferative disease, DLBCL.12,13 In hematological neoplasms, it seems that MPV parameters depend on the extent of inflammation. This corresponds to the recent hypothesis for the development of CLL, which assumes that immunologic and inflammatory factors, including antigen stimulation, could be involved in the pathogenesis of CLL.39 Moreover, recent reports confirmed that low levels of MPV are associated with the extent of inflammation.31 Some studies suggest the release rate of small size platelets from the bone marrow increases since excessive pro-inflammatory cytokines interfere with megakaryopoiesis.40 Therefore, there is a hypothesis that lower MPV values could be a consequence of an enhanced consumption of large, hyperactive platelets during inflammation as a result of increased hemostatic demand.31 Defective thrombopoiesis and enhanced destruction and swelling of circulating platelets in an environment rich in activating agents could be responsible for decreased MPV values.
A potential limitation of our study is the fact that the collected data were obtained from several hematological centers, so CBCs were carried out on various laboratory equipment, and MPV norms were different. To avoid those errors, we normalized the laboratory results against the adopted laboratory norm in the given center. The results obtained after data normalization did not differ from the analyses performed on raw data. The standardization methodology and the results of statistical analyses of normalized data are available as supplementary data. We are aware that the clinical usefulness of MPV as a potential prognostic factor depends on the determination of an optimal and universal cut-off level, moreover, its introduction into common clinical practice would require the standardization of hematological analyzers used in hematological centers or the development of a reliable method for normalizing results.
Our study included a group of patients treated with ibrutinib. This first-in-class inhibitor of BTK becomes the new standard in CLL treatment. BTK is a part of the B-cell receptor signaling cascade. It is also expressed in platelets and acts downstream in glycoprotein (GP)VI signaling involved in collagen-mediated platelet aggregation.41 Among the adverse effects of ibrutinib therapy most frequent are bleeding episodes that were observed in 44% of patients in the CLL registration trial and up to 61% of patients after a longer observation period.20 Bleeding is usually mild (grade 1 or 2), corresponding to spontaneous bruising or petechiae. A less frequent, but more dangerous adverse effect of ibrutinib therapy is AF, which occurs in 5–9% of patients.21 Both bleeding episodes and AF are potentially related to platelet parameters. The pooled analysis revealed that MPV level was significantly higher in patients with AF compared to those with sinus rhythm (SR), with a weighted mean difference (WMD) of 0.42 fl.22 Increased MPV level may also predict postoperative AF, as pooled analysis showed that MPV was significantly greater in patients with postoperative AF compared to postoperative SR with WMD of 0.53 fl.23 Our study did not confirm the correlation between MPV level and the incidence of therapy complications, although in the group of patients with low MPV there is a tendency for the more frequent occurrence of AF. The potential limitation of our study was the small size of the group with a low percentage of complications. However, it seems that testing the dependence between MPV levels and AF onset in a bigger study group may help to identify the potential predictor of this adverse effect.
Summarizing, low MPV level is associated with worse prognosis in CLL and it has independent prognostic value. The MPV level may become a new prognostic factor in CLL. There is no significant correlation between MPV level and the incidence of ibrutinib therapy complications, although in the group of patients with low MPV there is a tendency for more frequent occurrence of AF.
Abbreviations
AF, atrial fibrillation; BTK, Bruton tyrosine kinase; CBC, complete blood count; CD38, cluster of differentiation; CLL, chronic lymphocytic leukemia; CLL-IPI, Chronic Lymphocytic Leukemia International Prognostic Index; DLBCL, diffuse large B-cell lymphoma; FISH, immunofluorescent in situ hybridization; IGHV, immunoglobulin heavy chain variable; MPV, mean platelet volume; MYD88, myeloid differentiation primary response gene 88; NOTCH1, neurogenic locus notch homolog protein 1; OMF, osteomyelofibrosis; PDW, platelet distribution width; PCT, plateletcrit; PLT, platelets count; ROC, receiver operating characteristic; SF3B1, splicing factor 3B subunit 1; TTFT, time to first treatment; tri12, trisomy of 12 chromosome ZAP-70, zeta-chain-associated protein kinase 70.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was conducted with the consent of the Bioethics Committee of the Medical University of Lublin (KE-0254/231/2015). Patient consent was not required by the Bioethics Committee of the Medical University of Lublin, as the study represents a retrospective analysis of basic blood parameters performed as a standard procedure during control or hospitalization, no personal data were collected nor analyzed results enabled identification of the patient. The study was conducted with compliance with the Declaration of Helsinki and General Data Protection Regulation.
Consent for Publication
Not applicable.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Supported by NCN OPUS grant 2018/29/B/NZ5/02706 and 2016/DS462.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195–198. doi:10.1007/s10555-017-9677-x.
2. Riess L. Zur pathologischen Anatomie des Blutes. Arch Anat Physiol Wissensch Med. 1872;39:237–249.
3. Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760. doi:10.1002/ijc.27441.
4. Tuncel T, Ozgun A, Emirzeoglu L, et al. Mean platelet volume as a prognostic marker in metastatic colorectal cancer patients treated with bevacizumab-combined chemotherapy. Asian Pac J Cancer Prev. 2014;15:6421–6423. doi:10.7314/apjcp.2014.15.15.6421
5. Kilincalp S, Ekiz F, Basar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25(8):592–594. doi:10.3109/09537104.2013.783689.
6. Lembeck A, Posch F, Klocker EV, et al. Large platelet size is associated with poor outcome in patients with metastatic pancreatic cancer. Clin Chem Lab Med. 2019;57(5):740–744. doi:10.1515/cclm-2018-0016.
7. Shen W, Cui MM, Wang X, Wang RT. Reduced mean platelet volume is associated with poor prognosis in esophageal cancer. Cancer Biomark. 2018;22(3):559–563. doi:10.3233/CBM-181231.
8. Yun ZY, Zhang X, Liu YS, et al. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci Rep. 2017;7(1):6700. doi:10.1038/s41598-017-07168-x.
9. Sakin A, Secmeler S, Arici S, et al. Prognostic significance of mean platelet volume on local advanced non-small cell lung cancer managed with chemoradiotherapy. Sci Rep. 2019;9(1):3959. doi:10.1038/s41598-019-40589-4.
10. Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. 2016;23(5):752–760. doi:10.1007/s12282-015-0635-6.
11. Baldane S, Ipekci SH, Sozen M, Kebapcilar L. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2015;16(7):2671–2674. doi:10.7314/apjcp.2015.16.7.2671
12. Zhou S, Ma Y, Shi Y, et al. Mean platelet volume predicts prognosis in patients with diffuse large B-cell lymphoma. Hematol Oncol. 2018;36(1):104–109. doi:10.1002/hon.2467.
13. Rupa-Matysek J, Gil L, Balcerzak RK, Barańska M, Komarnicki M. Mean platelet volume as a predictive marker for venous thromboembolism and mortality in patients treated for diffuse large B‐cell lymphoma. Hematol Oncol. 2017;35(4):456–464. doi:10.1002/hon.2321.
14. Lucijanic M, Mitrovic Z, Cicic D, et al. Increased mean platelet volume (MPV) is an independent predictor of inferior survival in patients with primary and secondary myelofibrosis. Int J Hematol. 2018;107(2):166–172. doi:10.1007/s12185-017-2348-4.
15. Kipps TJ, Stevenson FK, Wu CJ, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;19(3):16096. doi:10.1038/nrdp.2016.96.
16. Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi:10.1182/blood.V46.2.219.219
17. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic lymphocytic Leukemia updating the National Cancer Institute‑Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi:10.1182/blood-2007-06-093906.
18. The International CLL‑IPI working group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL‑IPI): a meta‑analysis of individual patient data. Lancet Oncol. 2016;17:779–790. doi:10.1016/S1470-2045(16)30029-8.
19. Cortese D, Sutton LA, Cahill N, et al. On the way towards a ’CLL prognostic index’: focus on TP53, BIRC3, SF3B1, NOTCH1 and MYD88 in a population‑based cohort. Leukemia. 2013;28:710–713. doi:10.1038/leu.2013.333.
20. Kazianka L, Drucker C, Skrabs C, et al. Ristocetin-induced platelet aggregation for monitoring of bleeding tendency in CLL treated with ibrutinib. Leukemia. 2017;31(5):1117–1122. doi:10.1038/leu.2016.316.
21. Thompson PA, Lévy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–466. doi:10.1111/bjh.14324
22. Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, et al. Prediction of new-onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta-analysis. Med Sci Monit Basic Res. 2017;23:179–222. doi:10.12659/msmbr.903320
23. Weymann A, Ali-Hasan-Al-Saegh S, Popov AF, Sabashnikov A. Hematologic indices as predictors of atrial fibrillation following isolated coronary artery bypass grafting, valvular surgery or combined procedures: a systematic review with meta-analysis. Kardiol Pol. 2018;76(1):107–118. doi:10.5603/KP.a2017.0179
24. Zhu X, Cao Y, Lu P, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer. Sci Rep. 2018;8(1):11814. doi:10.1038/s41598-018-29293-x
25. Lian L, Xia YY, Zhou C, et al. Mean platelet volume predicts chemotherapy response and prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015;10:3419–3424. doi:10.1038/s41598-018-29293-x
26. Zhang K, Gao HF, Mo M, et al. A novel scoring system based on hemostatic parameters predicts the prognosis of patients with advanced pancreatic cancer. Pancreatology. 2019;19(2):346–351. doi:10.1016/j.pan.2018.12.010.
27. Seles M, Posch F, Pichler GP, et al. Blood platelet volume represents a novel prognostic factor in patients with nonmetastatic renal cell carcinoma and improves the predictive ability of established prognostic scores. J Urol. 2017;198(6):1247–1252. doi:10.1016/j.juro.2017.07.036.
28. Wang X, Cui MM, Xu Y, et al. Decreased mean platelet volume predicts poor prognosis in invasive bladder cancer. Oncotarget. 2017;8(40):68115–68122. doi:10.18632/oncotarget.19242.
29. Karpatkin S, Pearlstein Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastases. Ann N Y Acad Sci. 1981;370:101–118. doi:10.1111/j.1749-6632.1981.tb29726.x
30. Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65–76. doi:10.1016/j.thromres.2016.01.006.
31. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation. Curr Pharm Des. 2011;17:47–58. doi:10.2174/138161211795049804
32. Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115:3427–3436. doi:10.1182/blood-2009-10-247296.
33. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009.
34. Fuentes E. Palomo I: role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016;148:17–23. doi:10.1016/j.lfs.2016.02.026.
35. Manda G, Isvoranu G, Comanescu MV, et al. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015;5:347–357. doi:10.1016/j.redox.2015.06.014.
36. Xavier O, Montserrat G, Marta R, et al. Oxidative stress in patients with early stage chronic lymphocytic leukemia, assessment and correlation with prognostic factors. J Hematol. 2012;1(4–5):77–88. doi:http://dx.doi.10.4021/jh34w.
37. D’Arena G, Vitale C, Perbellini O, et al. Prognostic relevance of oxidative stress measurement in chronic lymphocytic leukaemia. Eur J Haematol. 2017;99:306–314. doi:10.1111/ejh.12918.
38. Gaman AM, Buga AM, Gaman MA, Popa-Wagner A. The role of oxidative stress and the effects of antioxidants on the incidence of infectious complications of chronic lymphocytic leukemia. Oxid Med Cell Longev. 2014;2014:158135. doi:10.1155/2014/158135.
39. Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med. 2008;264(6):549–562. doi:10.1111/j.1365-2796.2008.02030.x.
40. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the CCENT I trial. Gut. 2014;63:1721–1727. doi:10.1136/gutjnl-2012-304094.
41. Jones JA, Hillmen P, Coutre S, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017;178(2):286–291. doi:10.1111/bjh.14660.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
