Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Mean cost per number needed to treat with tocilizumab plus methotrexate versus abatacept plus methotrexate in the treatment of rheumatoid arthritis in patients previously treated with methotrexate
Authors Benucci M , Ravasio R , Damiani A
Received 11 May 2017
Accepted for publication 14 June 2017
Published 14 July 2017 Volume 2017:9 Pages 403—410
DOI https://doi.org/10.2147/CEOR.S141610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Maurizio Benucci,1 Roberto Ravasio,2 Arianna Damiani1
1Rheumatology Unit, S. Giovanni di Dio Hospital, Florence, 2Health Publishing & Services Srl, Milan, Italy
Introduction: Biological disease-modifying antirheumatic drugs are particularly recommended for use in patients who are poor responders, are intolerant to conventional disease-modifying antirheumatic drugs (cDMARDs), or in whom continued treatment with cDMARDs is deemed inappropriate. We estimated the efficacy and treatment costs associated with the use of tocilizumab (TCZ) plus methotrexate (Mtx) versus abatacept (ABT) plus Mtx in the treatment of rheumatoid arthritis (RA) in patients previously treated with Mtx.
Methods: Clinical data from a Technology Appraisal Guidance published in January 2016 by the National Institute for Health and Care Excellence were used. Pharmacoeconomic comparison between biological agents was carried out to estimate the respective cost for the number needed to treat (NNT) compared to cDMARDs using both American College of Rheumatology (ACR) and European League against Rheumatism (EULAR) criteria. A 6-month period was considered. Direct medical costs including pharmacological therapy, administration, and monitoring were considered.
Results: Using both ACR and EULAR criteria, TCZ subcutaneously (sc) or intravenously (iv) had a lower NNT (higher efficacy) compared to ABT (iv/sc). The most significant differences in favor of TCZ were observed using EULAR criteria. Related to the level of efficacy observed, TCZ (iv/sc) had a lower cost for NNT with both ACR and EULAR criteria compared to ABT (iv/sc). Sensitivity analysis confirmed these results.
Conclusion: TCZ (iv/sc) represents a more cost-effective option than ABT (iv/sc) in the treatment of RA in patients previously treated with Mtx.
Keywords: ACR, EULAR, pharmacoeconomics, rheumatology, economic, bDMARDs
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder characterized by persistent synovitis that affects the peripheral joints and determines a progressive destruction of cartilage and bone erosion, resulting in joint deformation and disability.1,2 Although the progression of RA varies between patients, symptoms such as joint damage and loss of functional status are manifested from an early stage.3,4 Due to the potential involvement of other organs, resulting in severe respiratory and/or cardiovascular complications, RA patients are at greater risk of mortality compared to the general population.5,6
RA affects ~0.5%–1% of the adult population,1,2 although this figure seems to be influenced by the geographic area of origin (Japan 0.2%, the Netherlands 1%–1.5%, Scandinavia 3%, Spain 0.5%, and the United States 1%).7,8 Two regional observational studies conducted in Italy have suggested an estimated RA prevalence rate of ~0.3%.7,9
Although the management of RA may include the use of nonpharmacological interventions, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids, the mainstay is represented by conventional disease-modifying antirheumatic drugs (cDMARDs) such as methotrexate (Mtx). With the aim of further optimizing the response to treatment and reducing the disability in the long-term, in recent years the therapeutic approach has focused increasingly on the use of biological disease-modifying antirheumatic drugs (bDMARDs).10–14 Indeed, these drugs represent a valid therapeutic option and are particularly recommended for use in patients who are poor responders, are intolerant to cDMARDs, or in whom continued treatment with cDMARDs is deemed inappropriate.15,16
The present analysis focuses mainly on the use of the latter class of drugs (bDMARDs), with the aim of providing an estimation of the efficacy and treatment costs associated with the use of tocilizumab (TCZ) plus Mtx versus abatacept (ABT) plus Mtx in the treatment of RA in patients previously treated with Mtx.
Methods
In RA, the main criteria used to assess the clinical response to pharmacological treatment are those recommended by the American College of Rheumatology (ACR) or the European League against Rheumatism (EULAR). Both the scientific societies have reached a consensus with regard to the minimum number of active disease variables (core set) to be measured while evaluating the efficacy of a treatment.17 These variables include number of painful and swollen joints, measurement of functional ability, acute phase reactants (erythrocyte sedimentation rate and C-reactive protein [CRP]), assessment of disease activity expressed by both the physician and the patient, as well as intensity of pain perceived by the patient.18 Although quite different, both sets of criteria are routinely used in clinical trials. EULAR criteria are based on absolute values and on variations of Disease Activity Score DAS/DAS28 compared to baseline, classifying response to treatment as good, moderate, or absent,19 whereas ACR criteria assess the improvement manifested (20%, 50%, or 70%) by all core set variables.17
Clinical data
The clinical basis for this economic analysis was provided by the outcome of the Technology Appraisal Guidance published in January 2016 by the National Institute for Health and Care Excellence (NICE).20 Following a systematic literature review of the key databases, 60 randomized, controlled trials (RCTs) were identified and included in the analysis; in six of these, a direct head-to-head comparison was carried out between two bDMARDs; in one case, a comparison was carried out between anti-tumor necrosis factor (TNF) alpha and cDMARDs, whereas the remaining 53 cases compared bDMARDs with either placebo or cDMARDs. ACR and EULAR response criteria were taken as the main outcomes of pharmacological treatments administered. Table 1 summarizes the efficacy data (ACR and EULAR) for both TCZ and ABT in combination with Mtx in the treatment of RA in patients previously treated with Mtx. Both ACR and EULAR criteria confirmed TCZ as the most effective therapeutic option.20
  | Table 1 ACR and EULAR criteria: TCZ + Mtx versus ABT + Mtx Note: Data from a previous study.20 Abbreviations: ACR, American College of Rheumatology; EULAR, European League against Rheumatism; cDMARDs, conventional disease-modifying antirheumatic drugs; ABT, abatacept; Mtx, methotrexate; TCZ, tocilizumab; sc, subcutaneous; iv, intravenous. |
Number needed to treat (NNT)
The present economic analysis was undertaken to estimate the relevant cost per NNT for the two bDMARDs compared to cDMARDs.21,22 The NNT represents the number of patients who need to be treated to obtain a therapeutic benefit. Therefore, in the present study it represents the number of patients who need to be treated with TCZ plus Mtx or with ABT plus Mtx compared to cDMARDs to obtain a positive patient response, in which efficacy is measured on the basis of response to ACR and EULAR criteria. Lastly, by multiplying this indicator by the relative cost of treatment, the cost per NNT for the two bDMARDs investigated is obtained.
Time horizon
While comparing two or more healthcare technologies, a suitable time horizon should be used to ascertain the (most important) differences in terms of both outcome and cost of treatment.23–25 Given that the follow-up period against which efficacy was evaluated in the majority of clinical studies taken into account by the NICE guidance was 24 weeks, also for this analysis a period of 6 months was deemed sufficient to grasp the most important differences in terms of both efficacy and cost of treatment.
Perspective of the analysis
The economic analysis was conducted from the Italian National Health Service (NHS) perspective. Thus, direct medical costs alone were taken into consideration, that is, pharmacological treatment (bDMARDs), administration, and monitoring. Other direct medical costs (ie, adverse events) were excluded as considered to be similar for abatacept and tocilizumab.
Treatments
As indicated in the relative Summaries of Product Characteristics (SPC), both ABT (selective T-cell co-stimulation modulator) and TCZ (interleukin-6 receptor inhibitor) may be administered subcutaneously (sc) or intravenously (iv) in the treatment of RA in patients previously treated with Mtx. However, working hypotheses were adopted in all comparisons due to the difficulty at times of distinguishing efficacy (ACR or EULAR) versus route of administration (sc or iv) based on the estimated outcomes reported in the NICE guidance (Table 1). Using relevant data published in the literature for both bDMARDs, the two routes of administration were found to be equally effective. Indeed, the SUMMACTA study demonstrated a corresponding efficacy between TCZ (sc, weekly 162 mg injection) and TCZ (iv, 8 mg/kg every 4 weeks).26 Likewise, the NCT00559585 trial reported a comparable efficacy and safety for ABT (sc and iv).27 However, data relating to Mtx were excluded from the estimation of the cost of pharmacological treatment, as it was hypothesized that, irrespective of the biological formulation administered, all subjects were treated with an identical mean daily dose.
Cost of treatment
Table 2 summarizes the mean 6-monthly cost of each individual treatment investigated in the present analysis. The cost per pack of bDMARDs corresponds to the ex-factory price net of any temporary legal discounts (AIFA Determination dated July 3, 2006, Official Gazette n° 156 of July 7, 2006, and subsequent AIFA Determination of February 9, 2007, Official Gazette n° 57 dated March 9, 2007 and extensions thereof), excluding any other discounts granted to the Italian NHS. These costs were calculated on the basis of doses indicated in the respective SPC.
In line with the procedures implemented in Italy, the cost of administration for the NHS was only considered for bDMARDs (iv), whereas no cost was assumed for sc administration. To valorize each individual administration, a proxy cost per intravenous infusion of €11.62 was applied, as indicated by the national healthcare charges for specialist outpatient services.28
The use of healthcare resources associated with monitoring activities was calculated by using data reported by a recent national study comparing TCZ (iv) with adalimumab (sc).29 Table 3 indicates the healthcare resources used and the mean cost of monitoring activities over the 6-month period of observation, with a clear distinction between sc and iv routes of administration. Also in this case, healthcare services were calculated by applying the relative Italian national charges.28
  | Table 3 Monitoring: mean 6-monthly cost Note: Data from a previous study.28 Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; bDMARDs, biological disease-modifying antirheumatic drugs; iv,intravenous; sc, subcutaneous. |
Sensitivity analysis
A sensitivity analysis, performed with the aim of evaluating the degree of uncertainty of the base case results, focused exclusively on the efficacy data (ACR and EULAR) estimated by the NICE guidance, and subsequently was used to calculate mean cost per NNT.23 In line with this objective, ACR and EULAR values for each of the therapeutic options were simultaneously varied considering first the lower and subsequently the upper limit of the respective variability range. This was performed in order to determine the impact of the variation on mean cost per NNT estimated in the base case.
Variability of the costs of monitoring and administration were not subjected to analysis due to the minimal impact produced on overall costs (1.5%–4.8%); indeed, even marked variations compared to baseline values would fail to yield significant differences capable of affecting base case results.
However, the reason why sensitivity analysis was not performed on the purchase cost of biological drugs for healthcare facilities was completely different. The base case had been developed taking into account the purchase cost of medicinal products net of any compulsory legal discounts and gross of any other discounts granted to hospital facilities. However, in view of the difficulty in quantifying and referencing the latter type of discount, we preferred not to provide alternative scenarios to the base case, as these may have failed to reflect the actual situation of individual hospitals. Accordingly, we opted to perform a threshold analysis in order to determine, compared to base case, at what percentage reduction of the purchase cost the biological agent ABT (iv/sc) would become cost-effective.
Results
NNT
Table 4 shows two distinct series of NNT for TCZ (iv/sc) and ABT (iv/sc). The first was calculated using ACR criteria, whereas EULAR criteria were used to calculate the second. In both the cases, TCZ (iv/sc) was characterized by a lower NNT compared to ABT (iv/sc), showing higher efficacy. The most significant differences in favor of TCZ were observed using EULAR criteria.
Cost of treatment
In light of data presented in the “Methods” section, Table 5 summarizes the mean 6-monthly cost per treated patient. This cost comprises the cost of treatment calculated on purchase cost for bDMARDs and cost of administration and monitoring; however, the latter items produce only a minimal impact on the overall cost (range: 1.5%–4.8%). The lowest cost of treatment was obtained by ABT sc + Mtx (€6,398.98), followed by TCZ sc + Mtx (€6,887.62), TCZ iv + Mtx (€7,130.80), and ABT iv + Mtx (€7,342.66). Differences compared to ABT sc + Mtx ranged from a minimum of ~€500 (vs TCZ sc + Mtx) to a maximum of ~€950 (vs ABT iv + Mtx).
  | Table 5 Mean 6-monthly cost of treatment per patient Abbreviations: ABT, abatacept; Mtx, methotrexate; TCZ, tocilizumab; sc, subcutaneous; iv, intravenous. |
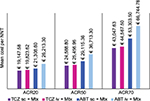
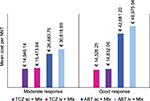
Cost per NNT
Figures 1 and 2 illustrate the mean cost per NNT calculated using ACR or EULAR response criteria. In terms of the percentage of patients achieving ACR response (20%, 50%, or 70%), TCZ (sc) was again characterized by the lowest cost per NNT. Despite the higher costs of administration and monitoring, TCZ (iv) had lower mean costs per NNT compared to ABT (sc) and ABT (iv). In line with ACR criteria, the greater the clinical improvement (20%, 50%, or 70%), the greater the difference between costs calculated per NNT for TCZ (iv/sc) and for ABT (iv/sc).
Mean cost per NNT calculated using EULAR response criteria also indicated TCZ (sc) as the best therapeutic option, followed by TCZ (iv). Significant differences were displayed compared to ABT (iv/sc) and rose in line with degree of efficacy.
Sensitivity analysis
Table 6 shows the results of sensitivity analysis performed in light of changes in efficacy rates related to the limits of corresponding variability ranges. In all comparisons, the option TCZ (sc) + Mtx featured the lowest mean cost per NNT, except when taking into account the upper limit of the confidence interval for criteria ACR20 and ACR50, in which case ABT (sc) + Mtx proved to be the most cost-effective option.
  | Table 6 Sensitivity analysis (efficacy data variation) Note: Data from a previous study.20 Abbreviations: NNT, number needed to treat; ACR, American College of Rheumatology; EULAR, European League against Rheumatism; ABT, abatacept; Mtx, methotrexate; TCZ, tocilizumab; sc, subcutaneous; iv, intravenous; n/a not assessed; MC, mean cost. |
To enhance the cost-effectiveness of ABT (sc) versus other options using ACR criteria, the corresponding purchase cost would need to be reduced by ~20% compared to base case, while the cost of intravenous ABT would need to be reduced by ~37%. However, based on EULAR criteria, the price reductions required to ensure the cost-effectiveness of ABT (iv/sc) correspond to approximately 67% and 73%, respectively.
Discussion
Among the indicators provided by economic assessment in a healthcare context, the Incremental Cost Effectiveness Ratio (ICER) allows decision-makers to ascertain the incremental cost incurred in acquiring an additional unit of result (health), expressed as either life years (LY) or quality-adjusted life years (QALY) gained. To verify acceptability, the ICER should subsequently be compared with a reference threshold value capable of conveying decision-makers’ willingness to pay in order to obtain an additional unit of health (LY/QALY).23 NICE guidance has assessed biological therapies using ACR and EULAR criteria; however, no findings have been provided in terms of LY or QALY.20 As a result, the latter prevented us from adopting an incremental cost-effectiveness analysis in the present study. It would have proved impossible to calculate an ICER expressed in simple LY or QALY for subsequent comparison with a reference threshold value. Conversely, it would have been possible to determine an ICER relating to an intermediate outcome (eg, ICER per patient at therapeutic target), although in the absence of comparison with a reference threshold value.23 Therefore, to overcome the ambiguity of identifying a result indicator but not being able to evaluate its quality, we deemed it more opportune to estimate the cost per NNT of bDMARDs versus cDMARDs,20 as a synthetic indicator of the clinical benefits and costs linked to the use of the biological therapies considered herein.
TCZ (sc) was associated with a lower mean cost per NNT using both ACR and EULAR criteria, while the intravenous formulation had a slightly higher cost. Costs per NNT for both the formulations (iv/sc) of ABT were considerably higher. Therefore, overall, the greater the improvement yielded, in line with both ACR (20%, 50%, or 70%) and EULAR criteria (moderate or good response), the greater the difference in cost per NNT in favor of TCZ (iv/sc).
The results obtained in the present study were compared with the findings of a previous economic analysis conducted to determine the cost per NNT of a series of biological agents in the treatment of Crohn’s disease, psoriasis, and RA.30 Namely, with regard to the treatment of RA, in the previous international study both NNT and cost per NNT were calculated for TCZ (iv) plus Mtx and ABT (iv) plus Mtx according to ACR50 and ACR70 criteria. In both the comparisons, TCZ achieved both the best NNT and lowest mean cost per NNT (Table 7). Although relating solely to the iv formulation, the findings of the international study confirm the results obtained in the present analysis.
  | Table 7 Cost per NNT – comparison at 24 weeks of treatment Note: Data from a previous study.30 Abbreviations: NNT, number needed to treat; ACR, American College of Rheumatology; ABT, abatacept; Mtx, methotrexate; TCZ, tocilizumab. |
In addition to ascertaining the results of an analysis, the validity of the same should also be contextualized and any limitations discussed. Although the NICE guidance20 was based on the investigation of ~60 RCTs, the efficacy data (ACR and EULAR) illustrated in Table 1 are characterized by a wide range of variability. This variability is inevitable while comparing patients from different RCTs. In view of this limitation, a sensitivity analysis was undertaken to evaluate the impact on final outcome. Indeed, even when taking into account simultaneously the lower and upper limits of the respective variability range (ACR20, 50, 70, moderate, or good response), the base case results were largely confirmed.
The present analysis was based on published data from only RCTs. As with any RCT, there is a concern that patient enrollment may not reflect the type of patients observed elsewhere in general practice. For this reason, the present results should be interpreted with caution to derive conclusions to the real-world rheumatology practices.
Moreover, the time horizon considered may constitute a further limitation to the comparison performed. An extension of the analysis to 56 or 124 weeks would render the base case results more robust (6 months). Unfortunately, to date, no studies yielding results beyond a 6-month period of observation for the two therapeutic options considered herein have been described in the scientific literature.
Adverse events costs were excluded because there are no head to head trials that compare TCZ and ABT from a clinical point of view. However, meta-analysis has been performed to assess the efficacy and safety of biologic therapy in RA,31,32 and it was found that the numbers of adverse events for TCZ and ABT were not statistically significantly different from those observed in the control groups.
Lastly, the use of ACR and EULAR response criteria may represent a further limitation of the analysis. As mentioned in the NICE guidance, the importance of applying caution in interpreting the efficacy of TCZ should be underlined in view of the direct effect exerted by the agent on CRP values compared to TNF inhibitor, particularly as the latter is a component of the ACR and EULAR composite endpoints on which the comparison was based.
In view of the scarce impact on overall costs, no sensitivity analysis was performed on monitoring and administration costs. However, with regard to pharmacological costs, a threshold analysis was performed to determine, compared to base case, at what percentage reduction of the purchase cost the biological agent ABT (iv/sc) would represent a cost-effective option. This was seen to occur only following marked reductions.
Conclusion
Taking into account the potential of applying a synthetic indicator of the efficacy and cost of treatment such as NNT, from an NHS perspective, TCZ (iv/sc) represents a more cost-effective option than ABT (iv/sc) in the treatment of RA in patients previously treated with Mtx. The findings of this study should encourage the undertaking of future clinical observational and real-world studies aimed at promoting a more efficient use of biological therapies in the treatment of RA.
Acknowledgment
This research was supported by an educational grant from Roche S.p.A.
Disclosure
The authors report no conflicts of interest in this work.
References
Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 2014;20(7 Suppl): S128–S135. | ||
Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012; 18(13 Suppl):S295–S302. | ||
Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–639. | ||
Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(Suppl 1):1–12. | ||
De Maria AN. Relative risk of cardiovascular events in patients with rheumatoid arthritis. Am J Cardiol. 2002;89(6A):33D–38D. | ||
Lubeck DP. Patient-reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics. 2004;22(Suppl 1):27–38. | ||
Cerra C, Ravasio R, Polcaro F. Il costo dell’Artrite Reumatoide: l’esperienza dell’ASL della Provincia di Pavia. Giornale Italiano di Health Technol Assess. 2009;2(3):111–117. | ||
Shichikawa K, Inoue K, Hirota S, et al. Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda. Wakayama, Japan. 1965–1996. Ann Rheum Dis. 1999;58:751–756. | ||
Cimmino MA, Parisi A, Moggiana G, et al. Prevalence of rheumatoid arthritis in Italy: the Chiavari study. Ann Rheum Dis. 1998;57:315–318. | ||
Nell VP, Machold KP, Ebrel G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–914. | ||
Emery P, Breedveld FC, Dougados M, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence-based development of a clinical guide. Ann Rheum Dis. 2002;61:290–297. | ||
Kuek A, Hazleman BL, Östor AJK. Immune mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–260. | ||
Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. | ||
Caporali R, Conti F, Alivernini S, et al. Recommendations for the use of biologic therapy in rheumatoid arthritis: update from the Italian Society for Rheumatology I. Efficacy. Clin Exp Rheumatol. 2011; 29(Suppl 66):S7–S14. | ||
Thwaites C, Finney A. Rheumatoid arthritis. 2: exploring treatment options to achieve early control and remission. Nurs Times. 2010;106(10):18–20. | ||
National Institute for Health and Clinical Excellence. Rheumatoid arthritis (CG79): full guideline. London. UK: NICE; 2009. Available from: http://guidance.nice.org.uk/CG79/Guidance. Accessed March 1, 2017. | ||
Conti F, Scrivo R. Il follow-up del paziente con artrite reumatoide di lunga Durata. [The follow - up of the patient with Rheumatoid Arthritis long duration]. In Focus Anno XIV. N 1. Febbraio 2011. Italian. Available from: https://elearning2.uniroma1.it/pluginfile.php/114873/mod_resource/content/1/Artrite%20reumatoide.pdf. Accessed March 1, 2017. | ||
Aletaha D, Smolen JS. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(Suppl 39): S100–S108. | ||
Prevoo ML, van‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. | ||
NICE Guidance. Adalimumab, etanercept, Inflfliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. Technology appraisal guidance. Published: 26 January 2016. Available from: http://nice.org.uk/guidance/ta375. Accessed March 2017. | ||
Kristensen LE, Christensen R, Bliddal H, Geborek P, Danneskiold-Samsøe B, Saxne T. The number needed to treat for adalimumab, etanercept, and infliximab based on ACR50 response in three randomized controlled trials on established rheumatoid arthritis: a systematic literature review. Scand J Rheumatol. 2007;36(6):411–417. | ||
Barra L, Pope JE, Payne M. Real-world anti-tumor necrosis factor treatment in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: cost-effectiveness based on number needed to treat to improve health assessment questionnaire. J Rheumatol. 2009;36(7):1421–1428. | ||
Gruppo di lavoro AIES (coordinato da G. Fattore). Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. Proposed guidelines for the economic evaluation of health interventions in Italy. PharmacoEconomics. 2009;11(2):83–93. Italian. | ||
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. Available from: http://www.nice.org.uk/aboutnice/howwework/devnicetech/guidetothemethodsoftechnologyappraisal.jsp. Accessed October 2014. | ||
Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 3rd Edition. 2006. Available from: https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed March 2017. | ||
Burmester GR, Rubbert-Roth A, Cantagrel A, et al. A randomized, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73(1):69–74. | ||
Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb non inferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63(10):2854–2864. | ||
Prestazioni di assistenza specialistica ambulatoriale. [Outpatient specialist services]. Supplemento ordinario n. 8 alla Gazzetta Ufficiale. Serie generale n. 23. Available from: http://www.crob.it/crob/files/docs/10/63/33/DOCUMENT_FILE_106333.pdf. Accessed January 28, 2013. Italian. | ||
Batticciotto A, Ravasio R, Riva M, Sarzi-Puttini P. Efficacy and treatment costs of monotherapy with bDMARDs in the treatment of rheumatoid arthritis in patients intolerant to or inappropriate to continue treatment with methotrexate. Adv Ther. 2016;33(8):1360–1373. | ||
Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn’s disease, psoriasis, and rheumatoid arthritis. Adv Ther. 2012;29(7):620–634. | ||
Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2:CD008794. | ||
Pierreisnard A, Issa N, Barnetche T, Richez C, Schaeverbeke T. Meta-analysis of clinical and radiological efficacy of biologics in rheumatoid arthritis patients naive or inadequately responsive to methotrexate. Joint Bone Spine. 2013;80(4):386–392. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.