Back to Journals » Journal of Inflammation Research » Volume 14
MCTR1 Intervention Reverses Experimental Lung Fibrosis in Mice
Authors Pan J, Li X, Wang X, Yang L, Chen H, Su N, Wu C, Hao Y , Jin S , Li H
Received 8 February 2021
Accepted for publication 21 April 2021
Published 11 May 2021 Volume 2021:14 Pages 1873—1881
DOI https://doi.org/10.2147/JIR.S304811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Jingyi Pan,* Xinyu Li,* Xinyang Wang, Lili Yang, Houlin Chen, Nana Su, Chenghua Wu, Yu Hao, Shengwei Jin, Hui Li
Department of Anaesthesia and Critical Care, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Li; Shengwei Jin
Department of Anaesthesia and Critical Care, Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, 325027, People’s Republic of China
Email [email protected]; [email protected]
Purpose: Pulmonary fibrosis (PF) is a progressing lethal disease, effective curative therapies remain elusive and mortality remains high. Maresin conjugates in tissue regeneration 1 (MCTR1) is a DHA-derived lipid mediator promoting inflammation resolution produced in macrophage. However, the effect of MCTR1 on PF remains unknown.
Material and Methods: We established a lung fibrosis model in mice induced by intratracheal administration of bleomycin (BLM). On day 7 after lung fibrosis model establishment, treatment with MCTR1 up to day 21. The body weight of each mouse was recorded every day and survival curves were plotted. Histological staining was used to detect pulmonary inflammation and fibrosis. Lung sections were examined with transmission electron microscope to evaluate the ultrastructure of cells and deposit of collagen. Inflammatory cytokines in lung tissues were tested by ELISA. q-PCR and Western blot were used to evaluate the mRNA and the protein levels of EMT-related markers.
Results: We found that MCTR1 intervention attenuated BLM-induced lung inflammatory and fibrotic response. Furthermore, MCTR1 protected BLM-induced epithelial cell destroy and reversed epithelial-to-mesenchymal transition phenotype into an epithelial one in lung fibrosis mice. Most importantly, post-treatment with MCTR1 restored BLM-induced lung dysfunction and enhanced survival rate significantly.
Conclusion: Posttreatment with MCTR1 attenuated BLM-induced inflammation and fibrosis changes in mice, suggested MCTR1 may serve as a novel therapeutic strategy for fibrosis-related diseases.
Keywords: pulmonary fibrosis, MCTR1, EMT, lung dysfunction
Introduction
Pulmonary fibrosis (PF) is a chronic and life-threatening pulmonary interstitial disease, which can be idiopathic or secondary to various lung diseases.1 The main pathological features of pulmonary fibrosis are apoptosis of alveolar epithelial cells, transformation of epithelial cells to mesenchymal cells (EMT), activation of fibroblasts, resulting in extracellular matrix (EMC) deposition in the lung interstitium, and ultimately affect the normal structure and function of lung tissue.2 Owing to the devastating incidence and mortality, PF has become a major and growing public health problem worldwide.3 While the advances in medicine during the past few decades have improved the care of lung diseases dramatically in clinic, however, the mortality of PF has barely improved.4 Therefore, it is of great significance to clarify the pathogenesis of pulmonary fibrosis and find more suitable therapeutic drugs.
The specialized pro-resolving mediators (SPMs) derived from polyunsaturated fatty acids (PUFA) exert both anti-inflammatory and pro-inflammatory effects.5 When the lung is injured, the inflammatory response initiates, and the uncontrolled inflammatory response can trigger excessive fibrosis and mediate the occurrence of pulmonary fibrotic disease.6 Maresin conjugates in tissue regeneration 1 (MCTR1) as a novel SPM is generated by docosahexaenoic acid (DHA) through 12S-LOX-mediated pathways in macrophages.7 It has been reported that MCTR1 accelerated the resolution of inflammation induced by LPS in mice, improved lung alveolar fluid clearance and restored cardiac function.8–10 However, the effect of MCTR1 on pulmonary fibrosis is not well undefined.
Here, we first evaluated the effect of MCTR1 at different doses on bleomycin (BLM) -induced lung fibrosis via histochemical staining. Additionally, to better understand the potential mechanism of our findings, we also observed that MCTR1 protected the alveolar epithelial cells in fibrotic mice. Finally, we demonstrated that MCTR1 reduced the BLM-induced mortality and improved lung function after the lung fibrosis model establishment.
Methods
Animals and Experimental Groups
Six to eight-week-old male C57BL/6 mice were provided by Shanghai Experimental Animal Center of China. All experimental procedures were performed in accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals and approved by the Animal Care and Use Committee of Wenzhou Medical University. Mice were randomly divided into five groups, and the day of modeling was day 0: (1) saline group: mice were treated with the same volume of saline by tracheal intubation; ② BLM (Sigma, Santa Clara, USA)-induced pulmonary fibrosis model group: mice were challenged with BLM (2.5 mg/kg for survival experiment, and 2.0 mg/kg for the other experiments) by tracheal intubation; MCTR1 (Cayman Chemical, Ann Arbor, MI) treatment groups: ③ BLM + MCTR1 1μg group; ④ BLM + MCTR1 100ng group; ⑤ BLM + MCTR1 10ng group. MCTR1 (1μg/mouse, 100 ng/mouse, or 10 ng/mouse) was administered by intraperitoneal injection on day 7 after BLM challenge, then boosted at 1/10 of the initial dose every two days until day 21.
Histological Analysis
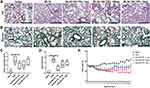
On day 21 after BLM challenge, the lung tissues of mice were removed for analysis. The left lungs were collected and fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-um thick sections. Hematoxylin-Eosin staining and Masson Trichrome staining were used to detect pulmonary inflammation and fibrosis in each group. Lung injury score was used in a blinded fashion to evaluate lung injury, including alveolar structure damage and inflammatory cell infiltration. 0 = no injury; 1 = slight injury (25%); 2 = moderate injury (50%); 3 = severe injury (75%); and 4 = very severe injury. A semi-quantitative assessment of lung fibrosis by Ashcroft score to evaluate the deposit of collage in lung tissues. It was used in a blinded fashion as well. Scores of 0–1 represented no fibrosis, scores of 2–3 represented minimal fibrosis, scores of 4–5 were considered as moderate fibrosis, and scores of 6–8 indicated severe fibrosis.
Transformation Electron Microscope
For transformation electron microscope (TEM), mice left lung samples were removed and dissected. Blocks of each sample were rinsed by PBS and fixed in 2% osmic acid with 1.5% potassium ferricyanide. Next, the blocks were dehydrated with a graded ethanol series, and then embedded with Epoxy resin by using propylene oxide as the transitional solvent. Blocks were cut into 50–70 nm ultrathin sections staining with saturated uranyl acetate and lead citrate. Finally, lung sections were examined with a transmission electron microscope (HITACHIH-7650, Tokoyo, Japan).
ELISA
Lung tissues were harvested, collected, homogenized and sonicated. The levels of cytokines (IL-6, IL-1β, TGF-β and TNF-α) in supernatants were measured with ELISA kits (R&D Systems, USA) according to the manufacturer’s instructions.
Hydroxyproline Assay
Hydroxyproline concentrations in lung tissues were measured using a hydroxyproline assay kit according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China).
Lung Function Test
Lung function was measured in mice as previously described.11 Briefly, we used the flexiVent system (Scireq, Montreal, QC, Canada) to detect the changes of pulmonary function in each group of mice. Deep Inflation perturbation was for assessing inspiratory capacity (IC). Pressure-volume (P-V) curve was constructed by a sequential delivery delivering incremental air into lungs from functional residual (0 mmHg) to total lung capacity to evaluate the distensibility of the respiratory system. Cst (quasistatic compliance), K (shape parameter) and A (hysteresis) were obtained from the analysis of P-V curve. All data obtained were analyzed using the flexi Vent software (version7.6).
RNA Extraction and Quantitative Real-Time PCR
The lung tissues were isolated from mice and then measured mRNA expressions of EMT markers in vivo. Total RNA of lung tissues from mice was extracted with Trizol liquid (Invitrogen, Carlsbad, USA). The primers were obtained from Shenggong company (Shanghai, China) and related mRNA expression was measured with SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan). The expression of target genes was analyzed normalized to GADPH. PCR primer sequences were performed as follows. GAPDH: forward, AAGAAGGTGGTGAAGCAGG; reverse, GAAGGTGGGAAGAGTGGGAGT, mouse. E-cadherin: forward, ATCCTGCTCCTACTG; reverse, CTCCACCTCCTTCTTCATC, mouse. N-cadherin: forward, GGTTTGGAATGGGTCTGT; reverse, ATGTTGGGTGAAGGTGTG, mouse. α-SMA: forward, CTCCCAGCACCATGAAGATCAA; reverse, GGGCGTGACTTAGAAGCATTTG, mouse.
Western Blot
Total proteins were obtained from lung tissues using RIPA lysis containing PMSF and protein phosphatase inhibitor. Each sample was ultrasonicated for 3 times and centrifuged at 12,000 × g for 30 min at 4°C. Protein concentrations were measured with a BCA protein assay kit. 30μg of protein was added and separated by 10% SDS-PAGE gel and transferred to a 0.45 μm-PVDF membrane. The membranes we got were blocked with 10% skimmed milk for 2.5 h followed by incubating with primary antibodies (CST, Boston, USA) including Anti-alpha smooth muscle actin (α-SMA) and E-cadherin at dilutions indicated overnight at 4 °C. The next day, the membranes were incubated with secondary antibodies at 1:3000 dilution and visualized by Image Quant LAS 4000 mini imager (GE, Sweden). Quantification of the bands was obtained through AlphaEaseFC software (AlphaImager System, San Leandro, USA).
Statistical Analysis
Date were reported as mean ±SEM. All data were analyzed by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. Kaplan-Meier survival curves was used to assess the survival of the three groups with Pairwise Log rank test. Statistical analyses and graphs were performed with Prism 8.0 (San Diego, USA). Statistical significance was determined at the p < 0.05 level.
Results
MCTR1 Ameliorated BLM-Induced Pulmonary Fibrosis in Mice in a Dose-Dependent
To explore the protective effects of MCTR1, we evaluated the effect of MCTR1 at different doses on BLM-induced lung fibrosis. Hematoxylin and eosin staining and Masson trichrome staining of lung sections were used to evaluate lung inflammatory injury and fibrosis (Figure 1A and B). Treatment with MCTR1 diminished infiltration of inflammatory cells and amounts of collagen deposition in a dose-dependent manner in lung tissues (Figure 1C and D). At the same time, we found that the effect of MCTR1 on the loss of body weight was consistent with histologic changes (Figure 1E). As observed, MCTR1 worked best at the dose of 1μg in BLM-induced lung fibrosis in mice, so we chose 1μg/mouse as the dose for the subsequent experiments.
MCTR1 Reduced the Production of Cytokines Related to Inflammation and Fibrosis
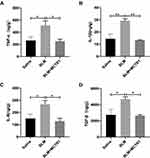
The release of inflammatory and fibrotic factors is essential for the progression of fibrosis. Subsequently, we examined the expression of inflammatory mediators including TNF-α, IL-1β and IL-6 and fibrosis regulatory cytokine TGF-β in the lung tissue in mice in each group. The protein concentrations of cytokines we tested were all much higher than those in lung tissue from the saline group mice. MCTR1 reduced these molecules dramatically after the lung fibrosis model establishment (Figure 2A–D).
MCTR1 Protected BLM-Induced Epithelial Cells Destroy and Reduced Collagen Deposition
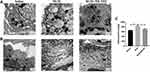
Alveolar Type II epithelial cell (ATII) is critical to maintain the alveolar function. We evaluated the effect of MCTR1 on ultrastructural changes of ATII through transmission electron microscope (TEM). The images showed that BLM-induced ATII structure destroyed including lamellar body swelling and vacuolation (Figure 3A) and amounts of collagen deposition in interstitial (Figure 3B). Treatment with MCTR1 significantly ameliorated BLM-induced ultrastructural changes. In addition, the concentration of hydroxyproline in the lung tissues was detected. As observed, MCTR1 reduced the elevated hydroxyproline level in the lung tissues in mice (Figure 3C).
MCTR1 Inhibited Epithelial-to-Mesenchymal Transition in vivo After BLM Challenge
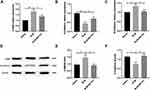
EMT-derived myofibroblasts were believed to be the major contributor to fibrosis. The lung tissue was isolated from mice and then measured the level of EMT markers. BLM reduced E-cadherin (epithelial marker) in mRNA level and enhanced α-SMA and N-cadherin (mesenchymal markers) mRNA expression in the lung tissues and MCTR1 reversed the changes induced by BLM (Figure 4A–C). The alterations of α-SMA and E-cadherin in the lung tissues were also evaluated at the protein level. Immunoprecipitated protein analysis showed MCTR1 had the same effect on those markers (Figure 4D–F).
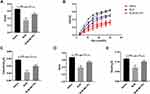
MCTR1 Restored Lung Function in BLM-Induced Lung Fibrosis in Mice
In clinical, lung function test was often used to evaluate condition change in patients with pulmonary fibrosis. In our experiment, two kinds of respiratory system patterns were used to assess the respiratory function of mice. Compared with the saline group, IC of the fibrosis group mice was reduced significantly, whereas MCTR1 increased IC (Figure 5A). Pressure-volume curve (P-V loop) is an important indicator to assess lung compliance. Our date showed that the P-V loop curve in lung fibrosis mice was markedly reduced compared with the saline group (Figure 5B). At the same time, Cst, A and K were decreased significantly in fibrotic mice. Moreover, these changes were reversed after MCTR1 treatment (Figure 5C–E).
MCTR1 Enhanced Survival Rate of Lung Fibrotic Mice
Finally, we assessed the protective effect of MCTR1 via survival rate. Mice were administrated with BLM at a higher dose (2.5 mg/kg) to get a more severe lung fibrosis mice model. Our result showed that BLM induced fibrotic mice death from day 11 after BLM administration, and MCTR1 improved lung fibrotic mice survival rate significantly (p=0.0023) (Figure 6).
Discussion
MCTR1 is a macrophage-derived lipid mediator which promotes the resolution of inflammation. In our previous study, we determined that lung fibrosis was initially formed on day 7 after BLM administration with the evidences of lung histological fibrotic changes and lung dysfunction.11 Here, MCTR1 was administrated by intraperitoneal injection on day 7 after BLM challenge to explore its potential effects in the fibrotic phase. We demonstrated post-treatment with MCTR1 showed beneficial effects on BLM-induced mice model of lung fibrosis.
The formation of fibrosis is essential against pathogens and in normal wound healing in the body.12 In a pathological fibrosis process, pro-inflammatory triggers often exaggerate cascades of inflammatory and fibrotic changes, resulting in downstream fibrotic tissue abnormal remodeling and extracellular-matrix deposition, finally promote fibrosis formation and organ dysfunction.13 It is well known that PF is an irreversible response, but recently, a growing number of studies have shown that fibrosis could be reversed to some extent in experimental lung fibrosis animal models.14–17 This could be due to the fact that fibrosis is a dynamic process including a reversal stage and an irreversible stage. Our results showed that experimental lung fibrosis mice are subjected to early intervention efficiently, promoting fibrosis reversal.
Our results showed that MCTR1 reversed lung fibrosis in mice according to the results of histological staining analysis. Histomorphology change of lung sections is an important indicator for evaluating fibrosis. It was used to assess the efficacy of MCTR1 at different doses in our study. We performed the experiment without the MCTR1 only group, because our previous study has identified treatment with MCTR1 alone in mice showed little difference from the control group mice.8
Tissue fibrosis is a common outcome in chronic inflammatory diseases.18 Previous studies showed that many innate pro-inflammatory cytokines have crucial roles in the pathogenesis of fibrosis.19,20 The expression of several cytokines strongly related to inflammatory was significantly reduced by MCTR1 in BLM-induced mice, including IL-6, IL-1β and TNF-α. The mechanism could be MCTR1 remit the lung fibrosis by promoting the resolution of inflammation in BLM mice model.
One hallmark of fibrosis is excessive deposition of the extracellular matrix. TGF-β is a key mediator associated with pulmonary fibrosis, which contributes to activate the fibroblasts and promote EMT on alveolar epithelial cells, stimulating ECM synthesis and deposit.21 In our results, the level of TGF-β was decreased by MCTR1 in fibrotic mice on the basis of ELISA. The result agrees with the above evidences that MCTR1 could reverse lung fibrosis in BLM-induced mice, partly by reducing the expression of TGF-β.
The EMT program of alveolar epithelial cells leads to a vicious cycle of damage and host response, leading to chronic lung fibrosis.22 We explored whether it could inhibit BLM-induced EMT. In our work, we proved that the expressions of mesenchymal markers α-SMA and N-cadherin were increased and the expression of epithelial marker E-cadherin was decreased in BLM-induced mice and MCTR1 reversed these changes after BLM administration. It is suggesting that MCTR1 performs a beneficial effect on fibrosis, probably through regulating the process of EMT.
Most importantly, we identified that post-treatment with MCTR1 ameliorated lung dysfunction and enhanced the survival rate of experimental lung fibrosis mice significantly. Lung function test is vital for assessment of respiratory disease for clinical.23 The patients and animal models with lung fibrosis are characterized by ventilatory-limitation, specific to IC, compliance and P-V loop.24,25 Consistently, we found that MCTR1 improved fibrotic respiratory dysfunction induced by BLM.
Conclusion
In conclusion, our results elucidate that the beneficial effect of MCTR1 on BLM-induced lung fibrosis in mice. We found that post-treatment with MCTR1 reversed BLM-induced inflammatory and fibrotic response, protected BLM-induced epithelial cells destroy and EMT in lung tissue, and improved fibrosis mice lung function and survival rate. These results have significant meaning to future efforts in developing a novel approach for treating lung fibrosis by targeting MCTR1 actions.
Acknowledgments
This work was supported in part by the grants from the Natural Science Foundation of Zhejiang Province (Nos. LQ20H150003), and the Wenzhou Science and Technology Bureau Project (Nos. Y20190087 and Y20190118), Zhejiang Science and Technology department key research and development program (Nos. 2019C03011). Jingyi Pan and Xinyu Li share the first authorship.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Meyer KC. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med. 2017;11(5):343–359. doi:10.1080/17476348.2017.1312346
2. Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi:10.1016/S0140-6736(17)30866-8
3. Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383(10):958–968. doi:10.1056/NEJMra2005230
4. Maher TM, Wuyts W. Management of fibrosing interstitial lung diseases. Adv Ther. 2019;36(7):1518–1531. doi:10.1007/s12325-019-00992-9
5. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27(3):200–215. doi:10.1016/j.smim.2015.03.004
6. Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. doi:10.1038/nri.2015.4
7. Dalli J, Vlasakov I, Riley IR, et al. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc Natl Acad Sci U S A. 2016;113(43):12232–12237. doi:10.1073/pnas.1607003113
8. Li H, Hao Y, Yang LL, et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol. 2020;235(10):7283–7294. doi:10.1002/jcp.29628
9. Yang Y, Zhu Y, Xiao J, et al. Maresin conjugates in tissue regeneration 1 prevents lipopolysaccharide-induced cardiac dysfunction through improvement of mitochondrial biogenesis and function. Biochem Pharmacol. 2020;177:114005. doi:10.1016/j.bcp.2020.114005
10. Han J, Li H, Bhandari S, et al. Maresin conjugates in tissue regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury. J Cell Mol Med. 2020;24(8):4736–4747. doi:10.1111/jcmm.15146
11. Li H, Hao Y, Zhang H, et al. Posttreatment with Protectin DX ameliorates bleomycin-induced pulmonary fibrosis and lung dysfunction in mice. Sci Rep. 2017;7:46754. doi:10.1038/srep46754
12. Thannickal VJ, Zhou Y, Gaggar A, et al. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673–4677. doi:10.1172/JCI74368
13. George PM, Spagnolo P, Kreuter M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020;8(9):925–934. doi:10.1016/S2213-2600(20)30355-6
14. Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi:10.1146/annurev-physiol-012110-142225
15. Rangarajan S, Bone NB, Zmijewska AA, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24(8):1121–1127. doi:10.1038/s41591-018-0087-6
16. Yu G, Tzouvelekis A, Wang R, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39–49. doi:10.1038/nm.4447
17. Kheirollahi V, Wasnick RM, Biasin V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10(1):2987. doi:10.1038/s41467-019-10839-0
18. LaRocca G, Aspelund T, Greve AM, et al. Fibrosis as measured by the biomarker, tissue inhibitor metalloproteinase-1, predicts mortality in Age Gene Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study. Eur Heart J. 2017;38(46):3423–3430. doi:10.1093/eurheartj/ehx510
19. Oh K, Park HB, Byoun OJ, et al. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011;208(8):1707–1719. doi:10.1084/jem.20101457
20. Kolb M, Margetts PJ, Anthony DC, et al. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107(12):1529–1536. doi:10.1172/JCI12568
21. Su J, Morgani SM, David CJ, et al. TGF-beta orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577(7791):566–571. doi:10.1038/s41586-019-1897-5
22. Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21(9):998–1009. doi:10.1038/nm.3902
23. Vanoirbeek JA, Rinaldi M, De Vooght V, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42(1):96–104. doi:10.1165/rcmb.2008-0487OC
24. Pillow JJ, Musk GC, McLean CM, et al. Variable ventilation improves ventilation and lung compliance in preterm lambs. Intensive Care Med. 2011;37(8):1352–1359. doi:10.1007/s00134-011-2237-x
25. Darrah RJ, Mitchell AL, Campanaro CK, et al. Early pulmonary disease manifestations in cystic fibrosis mice. J Cyst Fibros. 2016;15(6):736–744. doi:10.1016/j.jcf.2016.05.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.