Back to Journals » International Journal of Women's Health » Volume 7
Maternal and neonatal tetanus elimination: from protecting women and newborns to protecting all
Authors Khan R, Vandelaer J, Yakubu A, Raza A, Zulu F
Received 8 October 2014
Accepted for publication 20 November 2014
Published 3 February 2015 Volume 2015:7 Pages 171—180
DOI https://doi.org/10.2147/IJWH.S50539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Elie Al-Chaer
Rownak Khan,1 Jos Vandelaer,1 Ahmadu Yakubu,2 Azhar Abid Raza,1 Flint Zulu1
1Health Section, Programme Division, UNICEF, New York, NY, USA; 2Family, Women and Children’s Health Cluster, World Health Organization, Geneva, Switzerland
Abstract: A total of 35 of the 59 countries that had not eliminated maternal and neonatal tetanus (MNT) as a public health problem in 1999 have since achieved the MNT-elimination goal. Neonatal tetanus deaths have decreased globally from 200,000 in 2000 to 49,000 in 2013. This is the result of increased immunization coverage with tetanus toxoid-containing vaccines among pregnant women, improved access to skilled birth attendance during delivery, and targeted campaigns with these vaccines for women of reproductive age in high-risk areas. In the process, inequities have been reduced, private–public partnerships fostered, and innovations triggered. However, lack of funding, poor accessibility to some areas, suboptimal surveillance, and a perceived low priority for the disease are among the main obstacles. To ensure MNT elimination is sustained, countries must build and maintain strong routine programs that reach people with vaccination and with clean deliveries. This should also be an opportunity to shift programs into preventing tetanus among all people. Regular assessments, and where needed appropriate action, are key to prevent increases in MNT incidence over time, especially in areas that are at higher risk. The main objective of the paper is to provide a detailed update on the progress toward MNT elimination between 1999 and 2014. It elaborates on the challenges and opportunities, and discusses how MNT elimination can be sustained and to shift the program to protect wider populations against tetanus.
Keywords: maternal, neonatal, tetanus, elimination, high risk, immunization, vaccination, clean delivery
Background
Tetanus is a noncommunicable disease contracted through exposure to the spores of the bacteria Clostridium tetani. C. tetani exists worldwide in soil and in animal intestinal tracts, and as such can contaminate many surfaces and substances.1 As a result of the ubiquity of the bacterium causing tetanus, the disease cannot be eradicated.1,2 If a wound is contaminated with C. tetani, the neurotoxin produced in anaerobic conditions by the bacteria leads to tetanus.2 Tetanus occurring during pregnancy or within 6 weeks of the end of pregnancy is called maternal tetanus. Neonatal tetanus (NT) is defined as tetanus in the first 28 days of life. Whereas tetanus can occur worldwide and in all age-groups, neonates, who acquire the infection through contamination of the umbilical stump, and their mothers are most at risk, particularly when childbirth takes place under unhygienic conditions, and the mothers have insufficient antitoxins due to no or suboptimal immunization to protect themselves and their newborn babies. The case-fatality rate from tetanus in resource-constrained settings can be close to 100%, but can be reduced to 50% if access to basic medical care with experienced staff is available.1,2 In the 1980s, it was estimated that over 787,000 neonates died annually of tetanus.1 In the early 1990s, 15,000–30,000 women died globally of maternal tetanus (5% of all maternal mortality).1
There is no natural immunity against tetanus, but the disease is preventable through immunization. Recovery from tetanus does not confer immunity.2 Tetanus vaccination at any age will prevent disease, and the duration of protection depends on the number and spacing of doses received.3 Maternal immunization with tetanus toxoid-containing vaccines (TTCVs) protects both the mother and her newborn, and is therefore a cornerstone to prevent both maternal and NT (MNT), in addition to clean deliveries and clean cord-care practices.
In an effort to take advantage of the availability of existing and affordable vaccines, and to reduce the burden caused by NT, the World Health Assembly in 1989 and the World Summit for Children in 1990 called for NT elimination by 1995.4 NT elimination is defined as an incidence of less than one NT case per 1,000 live births in a district or similar administrative unit in a year.4 Later, the target date was extended to 2000 and again to 2005, and maternal tetanus elimination was added as a goal by the World Health Organization (WHO), the United Nations Children Fund (UNICEF), and the United Nations Population Fund (UNFPA) in 1999. In the absence of a definition for maternal tetanus elimination, NT elimination acts as a proxy for maternal tetanus elimination, as the predisposing conditions, risk factors, and prevention measures are the same for maternal tetanus.1
In order to accelerate progress, the WHO, UNICEF, and UNFPA relaunched in 1999 the “Maternal and Neonatal Tetanus Elimination” initiative with a focus on 57 priority countries that had not yet eliminated MNT.4 Due to geopolitical divisions in 2002 and 2011, two more countries (Timor-Leste and South Sudan) were added to the list, thus totaling 59 priority countries.
MNT-elimination strategies and current status of MNT-elimination program
Substantial progress has been made in the past two decades in reducing NT incidence and deaths from an estimated 490,000 NT deaths in 1994 to 200,000 NT deaths in 2000, and down to 49,000 in 2013.1,5,6 Globally, NT now accounts for only 1% of neonatal deaths, down from 14% in 1993.6,7 A multicountry analysis of neonatal mortality between 2000 and 2013 concluded that of all causes for neonatal deaths, only tetanus, measles, and acquired immunodeficiency syndrome in Sub-Saharan Africa, decreased at an annual rate sufficient to attain the Millennium Development Goal (MDG).4,6,8
Concerted efforts by countries, partners, and donors have made a significant positive impact on the MNT-elimination status of the priority countries. As of June 2014, 35 of the 59 priority countries have achieved MNT elimination validated by the WHO, as shown in Figure 1.9 In addition, several large countries have attained elimination in parts of the country: MNT elimination has been validated in India in 24 of 36 states, in Ethiopia in all regions except the Somali region, and in Indonesia in 30 of 34 provinces.9 The target date for the attainment of elimination in all countries is currently set for 2015.7,10
  | Figure 1 MNT-elimination status of 59 priority countries. |
All MNT-elimination achievements have been due to the ownership by countries and the support by partners and donors to implement the elimination strategies. The following four strategies are key to achieving MNT elimination:4,7
- delivery by skilled birth attendants (SBAs) to ensure clean delivery practices
- immunization of women during pregnancy (at fixed sites or through outreach) with TTCVs
- immunization of women of reproductive age (WRA) with TTCVs, through supplementary immunization activities (SIAs) in high-risk areas
- surveillance for NT.
Delivery by skilled birth attendants to ensure clean delivery practices
Delivery by SBAs11,12 has been proven to be an effective intervention to reduce NT.13–15 The presence of SBAs during delivery ensures that critical components of clean delivery practices, including the use of clean surfaces, clean hands, and clean cord cutting, are applied during the delivery. It also gives the opportunity for the health worker to convey messages on how to practice clean cord care once the mother has returned home, as unclean cord-care practice is a significant risk factor for NT. Evidence suggests that clean delivery practices can reduce the incidence of NT by 55% to 99%.14
Delivery by SBAs has increased from 56% in 2000 to 68% in 2012 at the global level, and from 28% to 48% in the least developed countries for the same time periods.16,17 Wide variations exist between and within countries: skilled birth attendance among the lowest wealth quintile is only 31%, compared to 85% for the highest wealth quintile (global averages); in rural areas it averages at 53% compared to 84% in urban areas in the same period.17 These numbers also indicate that the risk for MNT will be higher among the poor and among rural populations.
Immunization of women during pregnancy (at fixed sites or through outreach) with TTCVs
Since the 1980s, TTCVs, such as TT, have been part of routine immunization programs, especially in developing countries, to protect pregnant women and their future newborns from tetanus.18 Administration of properly-spaced doses of TTCVs during the antenatal period can reduce the incidence of NT by up to 88%–100%, and can help to achieve MNT elimination in countries with a relatively strong and equitable health system.14 The number of doses of TTCV administered during antenatal checkups will depend on the previous vaccination status of the women. Previously unvaccinated women will require five properly-spaced doses of TTCV. Reported TT2+ coverage19 has progressively increased from 62% in 2000 to 75% in 2012 at the global level, but dropped to 65% in 2013 (Figure 2).20 The interpretation of these numbers has to be approached with caution, as precision issues are well recognized21 (Figure 3).
  | Figure 2 Trend in reported TT2+ coverage and estimated neonatal tetanus deaths, 2000–2013. |
  | Figure 3 Measuring tetanus toxoid-immunization coverage. |
Immunization of women of reproductive age with TTCVs through SIAs in high-risk areas
The high-risk approach has been widely used to accelerate MNT elimination in priority countries.1 Under the high-risk approach, countries identify districts at risk for MNT, which are often those with the most vulnerable populations or areas deprived of all essential services, including health services, through a review of subnational (most often district level) data, complemented with local knowledge and often field visits. In these areas, three doses of TTCV are administered to WRA (usually women aged 15–49 years) at recommended intervals, using a campaign approach.9 Since the program was relaunched in 1999, all 59 priority countries have undertaken the district data review, and 52 have implemented the high-risk approach (Figure 4). The scale of implementation of the high-risk approach in these countries has ranged from less than 20% of all areas (eg, Senegal) to nationwide campaigns (eg, Nepal,22 Liberia, and Sierra Leone). More than 128 million WRA in high-risk areas have been reached with at least two doses of TTCV. The approach has also been used to deliver other essential health interventions in these deprived areas, and community involvement through interpersonal communication and focus-group discussions before and during the campaigns has had a positive influence on behavioral change, as demonstrated in Pakistan in early 2000.23
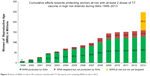  | Figure 4 Status of WRA at risk in 59 countries reached with TT (by years), and remaining WRA at risk in 2013. |
Neonatal tetanus surveillance
Effective surveillance of NT is important to identify areas or populations at high risk for MNT, and to monitor the impact of interventions, as well as maintenance of the elimination status once achieved. Lack of effective surveillance should however not delay the implementation of activities using the high-risk approach aimed at reducing the incidence of MNT.24
Surveillance data are primarily available for NT, whereas maternal tetanus surveillance data are very difficult to obtain. Reported NT data relate to case incidence, while estimated NT burden data relate to mortality. Assuming a case-fatality rate approaching 100% for reported NT cases, notification efficiency since 1988 has been at 11% or less (Table 1). If lower case-fatality rates are assumed, even lower notification efficiencies are observed.
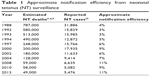  | Table 1 Approximate notification efficiency from neonatal tetanus (NT) surveillance |
In most countries, NT surveillance is passive, and many reported cases are not investigated. Where cases or deaths are investigated, misreporting can occur. The absence of a reliable laboratory technique to confirm the NT diagnosis further complicates the situation. To address the low notification efficiency for NT reporting, countries have started adopting community-based surveillance that integrates surveillance for vaccine-preventable diseases (polio and measles, especially) and vital-events registration that includes neonatal deaths (to investigate and rule out NT), births, and deaths.
Success, opportunities, and challenges to achieving the MNT Elimination Initiative goal of 2015
Success
The MNT Elimination Initiative has made remarkable progress: more than half of the priority countries have attained elimination, and the remaining countries have made steady progress over the years. Some of the factors that have contributed to the success of the initiative follow.
Low-cost intervention
The high-risk approach of the MNT Elimination Initiative is a relatively low-cost intervention compared to other similar initiatives.26 The average cost to reach a woman with three doses of TTCV through the campaign is just under US$2,27 but costs may be higher in some of the more challenging countries, such as Somalia and Afghanistan.
Reaching the unreached
MNT affects the most deprived and poorest populations. The initiative specifically targets these populations through the high-risk approach, and paves the way to deliver other interventions. As such, the MNT Elimination Initiative reduces some of the inequities between rich and poor.
Success in public–private partnership
Since 2000, the initiative has brought a wide array of partners committed to supporting – often in innovative ways – the program technically, by raising awareness and by leveraging funds. Since 2006, Procter and Gamble has been supporting the initiative through cause-related marketing through which the program receives the cost of one dose of vaccine per pack of Pampers sold.28 Similarly, since 2010, Kiwanis International, an international civil society organization, has been supporting the initiative through its worldwide service initiative “The Eliminate Project” by mobilizing funds through its members and partners.29 Additionally, Becton Dickinson, a pioneer manufacturer of injection devices, has provided support in reaching very difficult-to-access areas through supporting technology transfer and making available the TT Uniject device for the program, along with other forms of support.30 These efforts are helping to raise awareness of this disease that is almost forgotten in high-income countries.
Challenges
In spite of the immense success of the MNT Elimination Initiative that has contributed to both the MDGs related to childhood and maternal mortality (MDGs 4 and 5), the program is also facing some challenges as it approaches its ultimate goal.31
Inadequate access
Prevailing complex humanitarian emergencies, compounded by the lack of physical access and increased insecurity, in countries like Afghanistan, Somalia, and South Sudan make it difficult for health workers to reach populations, especially women and children, with health services, including TTCVs.
Sociodemographic and cultural barriers for TT vaccines
Women’s status in the society and local culture play an important role in accessing reproductive health services, which results in a lack of uptake of tetanus vaccine, as the service is provided through antenatal care and immunization platforms.32 In some societies, women’s lack of decision-making power coupled with cultural barriers are hindrances to availing themselves of maternal health services. The situation is made worse in some areas where, because of sociocultural practices, women often are not allowed to visit health centers or access services if these are provided by male health workers.33 Similarly, a study in India shows that poor women and women belonging to lower castes of society are less likely to receive antenatal care including, tetanus vaccines.34 On the other hand, the experience in Pakistan, where female health workers were allowed to administer TTCVs, shows that culturally appropriate changes to the program can increase vaccination coverage and reduce mortality.35
Funding constraints
Although countries have developed plans to eliminate MNT, implementation of such plans is often delayed for lack of sufficient, predictable, and timely funding from external and/or domestic sources. Reaching the remaining 88 million WRA who are still at high risk in the 24 remaining countries with TTCVs through campaigns will cost over $170 million, of which $91 million remains unfunded (as of June 2014).7
Competing priorities and political will
MNT cases mostly occur in remote areas that lack vital registration systems or community surveillance, resulting in cases and deaths not being systematically reported. As tetanus is noncommunicable, outbreaks are rare and limited. The ensuing misperception that tetanus is not a disease of public health importance leads to governments and donors giving a low level of priority to the disease in favor of other visible priorities.
Opportunities
Despite the challenges, there are opportunities to achieve MNT elimination in all countries.
Innovative approach to reach inaccessible areas
From 2003 to 2005, prefilled TT Uniject devices were used in Afghanistan, Burkina Faso, Ghana, Mali, South Sudan, and Somalia to improve access in very hard-to-reach areas. TT Uniject is heat-stable and can be used for extended periods of time, up to 72 days, without refrigeration.36 The evaluation conducted by PATH in Mali showed that community-based volunteers (CBVs) including illiterate CBVs, could be trained to safely and correctly use TT-Uniject,36 and that the communities accepted the practice of CBVs vaccinating women.
Integrated service delivery
Some countries (eg, Liberia, Madagascar, Papua New Guinea, Sierra Leone, and Uganda) have used the high-risk approach to deliver other interventions also besides administration of TTCVs to women and children. This approach, if adequately planned, coordinated, implemented, and monitored, is cost-effective and beneficial for the population, as they receive several essential services from one service point at the same time.
Social mobilization and health education
Social mobilization is critical to inform, sensitize, and prepare high-risk communities for TTCV campaigns. This communication strategy can also deliver a broad range of messages on immunization and reproductive health, including on clean delivery and clean cord-care practices, to reduce the risk of tetanus. In Ethiopia and Mali, TTCV-campaign communications have been used to address false beliefs and rumors against tetanus vaccines, and to overcome sociocultural barriers for women to receive the vaccine.23
Sustaining MNTE: protecting all against tetanus
Once a country has eliminated MNT as a public health problem, the achievement needs to be maintained. Several strategies can be used to maintain MNT elimination, most often in combination.
Strong routine services
Strong “routine” services, ie, the ability of the health system to provide services on an ongoing basis, underpin the success of vaccine-preventable disease programs. Maintaining MNT elimination will therefore heavily rely on the ongoing availability of immunization and clean delivery practices.
Whereas TTCVs administered to adult women (eg, in pregnancy) are mainly intended to prevent MNT, the WHO recommends that schedules be adjusted to include a total of six doses of TTCV administered over several years to all people, in order to provide long-lasting protection throughout life for all: three doses with diphtheria, tetanus, and pertussis (DTP; or DTP-containing) vaccine in infancy, a booster dose with tetanus and diphtheria containing vaccines at 4–7 years and in adolescence, and one dose during the first pregnancy.2
The level to which such six dose schedules have been implemented varies. In all countries, at least three doses of TT (DTP3) are given as part of the infant-immunization program, reaching a global DTP3 coverage of 84% (2013).37 The global DTP3 coverage has been above 70% since 1990, meaning that a large proportion of women now entering childbearing age received three doses of TT in their infancy.38 It has to be stressed, however, that even with high coverage at national level, some areas or populations may have substantially lower coverage.39
As per a WHO position paper, two boosters should be given to children at school age (4–7 years and 12–15 years). With attendance rates for primary school globally at 82% for boys and 79% for girls, and even in the least developed countries at 75%, school-based immunization is a promising approach.17 Indeed, over half of all countries already have a school-based immunization approach, and TT is the most frequently administered antigen within such an approach.40 Lower attendance for secondary schools makes the administration of the dose aimed at adolescents more challenging.17 Reaching children who do not attend school, but who at the same time tend to be at increased risk for diseases like tetanus, is another challenge. The introduction of human papillomavirus vaccination of adolescents, in some places linked with broader adolescent health interventions, may offer opportunities to also provide a booster dose of TTCVs, although preferences for such add-on interventions may vary at the local level.41,42
In the African “meningitis belt”, the use of meningitis A-conjugate vaccine campaigns targeting large parts of the population may provide added protection against tetanus, as the meningitis vaccine has been conjugated with TT, and can possibly act as a TT booster. Further research is needed.
Countries may also choose alternative strategies to administer TTCVs. Periodic intensified routine immunization activities, in which a campaign-style approach is used on an intermittent basis to improve routine immunization coverage, has been shown to have the potential to extend the reach of regular immunization and other interventions in children and adults (mostly women).43 Many higher-income countries recommend TT booster doses later in life in the form of a 10- or 20-yearly booster, and sometimes these are given in opportunistic ways, such as after trauma.44,45
Visits to antenatal clinics are often used in developing countries to provide TTCVs to pregnant women. With antenatal clinic attendance (at least one visit) reaching 81% globally (up from 72% in the year 2000)46 and in every geographic region above 70%,15 this platform must be more extensively used not only for vaccination but also to educate women about hygienic delivery, clean cord practices, and postnatal newborn care.47
The provision of clean delivery practices remains another pillar to maintain MNT elimination, and to protect mothers and children from a variety of infections and health risks. Since 1997, WHO has been promoting the idea that professional SBAs assist during childbirth.48 As a result, national governments have increasingly been adopting the policy of delivery by SBAs and investing in improving access to SBAs.49 Investments range from creating new cadres, such as community-based midwives,50 training of SBAs, improving referral systems from the community to health facilities,51 and equipping and upgrading health facilities. Additionally, national governments have established various schemes, including cash-based incentives to reduce barriers to access to SBAs and institutional delivery.52–55
In the period 2007–2012, globally 61% of deliveries took place in an institution, and 68% were assisted by a skilled attendant, but in the least developed countries both figures were less than 50%.17 Seventy-eight percent of births take place in 73 of the world’s poorest countries, yet these countries are home to only 42% of the world’s physicians, nurses, and midwives.49 Increasing human-resource capacity and availability and access to institutions will take time, but such examples as the one in India show that with political will and incentives, it is possible to increase the number of institutional deliveries in a short time.56
Monitoring risk
NT surveillance is generally weak, but can benefit from integrated surveillance for vaccine-preventable diseases and/or active acute flaccid paralysis surveillance for polio.57 Active surveillance, where hospital records are searched for suspected polio, measles, or other vaccine-preventable diseases, can especially offer better case detection for NT.
In addition, as interest in vital registration increases, neonatal death audits or verbal autopsies can provide better insights into the causes of early deaths, including from tetanus.58 Community surveillance, for which volunteers selected by the communities are trained in using a very simplified reporting format with pictorials for recording events, has been used in Ghana under the umbrella of a community-based health planning and services scheme.59 Some countries, including Myanmar, Nigeria, and Uganda, are considering introducing or strengthening their community surveillance as a complement to their existing vaccine-preventable disease-surveillance systems. It is essential that tetanus is considered as a possible cause of death during such audits.
A better understanding of the proportion of the population that is protected against tetanus will require monitoring of the proportion of women delivering with an SBA, as well as the implementation of the “protection at birth”60 methodology to better measure the impact of immunization (and to ultimately replace TT2+ monitoring) (Figure 3).
While many of these measures will take time to be implemented, it is imperative that countries regularly monitor whether their MNT-elimination status is at risk. An annual review of a set of indicators that include the core (NT rate, TT2+ coverage in pregnant women, SBA coverage) and the surrogate (DTP3 coverage, antenatal care coverage, previous tetanus SIA coverage) indicators, district by district, will allow for assessment of whether in certain districts MNT incidence is possibly on the increase, and will help to identify low-performing districts, where children and women are at risk for tetanus, but most often also for other diseases.
Occasional serosurveys can complement data on protection levels from tetanus in the community. This is especially useful where the routinely reported immunization data are very unreliable, card retention is very low, and/or where history of doses received is questionable.
Responses to increased risks
In the short term, several measures can be implemented to maintain MNT elimination. The distribution of clean delivery kits can prevent the use of contaminated materials during home-based childbirth, and have an important impact on infections and mortality.61,62 Application of chlorhexidine on the umbilical cord has been shown to prevent infections.63
Health education about the advantages of hygienic delivery practices is another essential component. Incentives to promote institutional delivery, such as are used in India, can be a motivational factor for women to improve their delivery practices.56
In areas where the annual review shows that MNT risks are increasing, health systems are weak, MNT elimination was heavily dependent on SIAs, and/or protection levels are wearing off, periodic small-scale TTCV campaigns targeting age-groups at risk may be required to reach girls who were too young to be included in an earlier campaign and/or to give an additional (booster) dose. Broader campaigns, administering more antigens, or more regular periodic intensified routine immunization approaches can also be used.
Conclusion
Progress toward MNT elimination has been the result of a combination of strategies, both long- and short-term. However, a handful of countries, including countries with acute or chronic humanitarian crises or with sociocultural barriers, are still to achieve the goal. The international community has a shared responsibility in helping to achieve and sustain this global goal.
With more countries achieving MNT elimination, efforts to sustain the achievement become ever more important. Such efforts will not only affect the incidence of MNT, but have the potential to reduce tetanus in the whole population, as well as other maternal and newborn infections.
Disclosure
The authors report no conflicts of interest in this work.
References
Roper MH, Vandelaer J, Gasse F. Maternal and neonatal tetanus. Lancet. 2007;370:1947–1959. | ||
[No authors listed]. Tetanus vaccine. Wkly Epidemiol Rec. 2006;81:198–208. | ||
Borrow R, Balmer P, Roper MH. The Immunological Basis for Immunization Series. Module 3: Tetanus Update 2006. Geneva: World Health Organization; 2007. Available from: http://whqlibdoc.who.int/publications/2007/9789241595551_eng.pdf. Accessed September 30, 2014. | ||
Vandelaer J, Birmingham M, Gasse F, Kurian M, Shaw C, Garnier S. Tetanus in developing countries: an update on the Maternal and Neonatal Tetanus Elimination Initiative. Vaccine. 2003;21:3442–3445. | ||
Centers for Disease Control and Prevention (CDC). Progress toward elimination of neonatal tetanus – Egypt, 1988–1994. MMWR Morb Mortal Wkly Rep. 1996;45:89–92. | ||
Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–2013, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. Epub 2014 Sep 30. | ||
World Health Organization, United Nations Children’s Fund, United Nations Population Fund. Achieving and Sustaining Maternal and Neonatal Tetanus Elimination: Strategic Plan 2012–2015. Geneva: WHO; 2013. Available from: http://apps.who.int/immunization_monitoring/MNTEStrategicPlan_E.pdf. Accessed June 11, 2014. | ||
Lawn JE, Kinney MV, Black RE, et al. Newborn survival: a multi-country analysis of a decade of change. Health Policy Plan. 2012;27 Suppl 3:iii6–iii28. | ||
World Health Organization. Maternal and neonatal tetanus (MNT) elimination. 2014. Available from: http://www.who.int/immunization/diseases/MNTE_initiative/en. Accessed August 7, 2014. | ||
United Nations Children’s Fund. Global Vaccine Action Plan 2011–2020. 2013. Available from: http://www.unicef.org/immunization/index_27089.html. Accessed June 11, 2014. | ||
Harvey SA, Blandón YC, McCaw-Binns A, et al. Are skilled birth attendants really skilled? A measurement method, some disturbing results and a potential way forward. Bull World Health Organ. 2007;85:783–790. | ||
World Health Organization. Making Pregnancy Safer: The Critical Role of the Skilled Attendant. Geneva: WHO; 2004. Available from: http://whqlibdoc.who.int/publications/2004/9241591692.pdf. Accessed September 2, 2014. | ||
Khan AA, Zahidie A, Rabbani F. Interventions to reduce neonatal mortality from neonatal tetanus in low and middle income countries – a systematic review. BMC Public Health. 2013;13:322. | ||
Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–988. | ||
Blencowe H, Cousens S, Mulluny LC, et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11 Suppl 3:S11. | ||
United Nations Children’s Fund. The State of the World’s Children 2001. New York: UNICEF; 2000. Available from: http://www.unicef.org/publications/files/pub_sowc01_en.pdf. Accessed September 2, 2014. | ||
United Nations Children’s Fund. The State of the World’s Children 2013. New York: UNICEF; 2013. Available from: http://www.unicef.org/sowc2013/files/SWCR2013_ENG_Lo_res_24_Apr_2013.pdf. Accessed September 2, 2014. | ||
World Health Organization. Second and subsequent doses of tetanus toxoid: reported estimates of TT2+ coverage. 2014. Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragett2plus.html. Accessed September 2, 2014. | ||
Deming MS, Roungou J, Kristiansen M, et al. Tetanus toxoid coverage as an indicator of serological protection against neonatal tetanus. Bull World Health Organ. 2002;80:696–703. | ||
World Health Organization. Global and regional immunization profile. 2014. Available from: http://www.who.int/immunization/monitoring_surveillance/data/gs_gloprofile.pdf?ua=1. Accessed September 14, 2014. | ||
World Health Organization, United Nations Children’s Fund. Ad Hoc Committee on Maternal and Neonatal Tetanus: Meeting Report – Geneva, 25–26 March 2003. Geneva: WHO; 2003. Available from: http://libdoc.who.int/hq/2004/WHO_IVB_04.11.pdf. Accessed September 14, 2014. | ||
Vandelaer J, Partridge J, Suvedi BK. Process of neonatal tetanus elimination in Nepal. J Public Health. 2009;31:561–565. | ||
Boggs MK, Bradley PM, Storti CZ. Saving Newborn Lives: Tools for Newborn Health. Washington: Save the Children Federation; 2006. Available from: http://www.savethechildren.org/atf/cf/%7B9def2ebe-10ae-432c-9bd0-df91d2eba74a%7d/communication-for-immunization-campaigns-for-maternal-and-neonatal-tetanus-elimination.pdf. Accessed September 30, 2014. | ||
World Health Organization. Neonatal tetanus. In: WHO-Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases. Geneva: WHO; 2003:22–27. Available from: http://whqlibdoc.who.int/hq/2003/who_v&b_03.01.pdf. Accessed September 14, 2014. | ||
World Health Organization. Neonatal tetanus. Available from: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/neonatal_tetanus/en. Accessed September 2, 2014. | ||
Gandhi G, Lydon P. Updating the evidence base on the operational costs of supplementary immunization activities for current and future accelerated disease control, elimination and eradication efforts. BMC Public Health. 2014;14:67. | ||
Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010;39 Suppl 1:i102–i109. | ||
Pampers [homepage on the internet]. Available from: http://www.pampers.co.uk/unicef. Accessed September 2, 2014. | ||
Kiwanis. The Eliminate Project. 2014. Available from: http://sites.kiwanis.org/Kiwanis/en/theELIMINATEproject/home.aspx. Accessed September 2, 2014. | ||
Becton Dickinson. U.S. fund for UNICEF and BD partner to accelerate elimination of maternal and neonatal tetanus [press release]. New York: BD; 2002 [July 22]. Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=64106&p=irol-newsArticle&ID=320103&highlight=. Accessed August 24, 2014. | ||
United Nations. Millennium Development Goals and beyond 2015. Available from: http://www.un.org/millenniumgoals. Accessed August 24, 2014. | ||
Pallikadavath S, Foss M, Stones WR. Antenatal care: provision and inequality in rural north India. Soc Sci Med. 2004;6:1147–1158. | ||
Nisar N, White E. Factors affecting utilization of antenatal care among reproductive age group women (15–49 years) in an urban squatter settlement of Karachi. J Pak Med Assoc. 2003;53:47–53. | ||
Navaneetham K, Dharmalingam A. Utilization of maternal health care services in southern India. Soc Sci Med. 2002;55:1849–1869. | ||
Khan A, Kinney MV, Hazir T, et al. Newborn survival in Pakistan: a decade of change and future implications. Health Policy Plan. 2012;27:iii72–iii87. | ||
United Nations Children’s Fund, PATH. Introducing TT-Uniject in Maternal and Neonatal Tetanus Elimination: A Guide for Program Managers. Seattle: PATH; 2003. Available from: http://www.path.org/publications/files/TS_introduce_tt.pdf. Accessed September 2, 2014. | ||
United Nations Children’s Fund. Immunization. 2014. Available from: http://data.unicef.org/child-health/immunization. Accessed September 2, 2014. | ||
World Health Organization. Progress Towards Global Immunization Goals – 2012. Geneva: WHO; 2013. Available from: http://www.who.int/immunization/monitoring_surveillance/SlidesGlobalImmunization.pdf?ua=1. Accessed June 14, 2014. | ||
World Health Organization. WHO vaccine-preventable diseases: monitoring system – 2014 global summary. Available from: http://apps.who.int/immunization_monitoring/globalsummary/indicators?. Accessed June 14, 2014. | ||
Vandelaer J, Olaniran M. Using a school-based approach to deliver immunization – global update. Vaccine. 2015;33:719–725. | ||
Broutet N, Lehnertz N, Mehi G, et al. Effective health interventions for adolescents that could be integrated with human papillomavirus vaccination programs. J Adolesc Res. 2013;53:6–13. | ||
MacPhail C, Venables E, Rees H. Using HPV vaccination for promotion of an adolescent package of care: opportunity and perspectives. BMC Public Health. 2013;13:493. | ||
Immunization Basics, USAID, World Health Organization. Periodic intensification of routine immunization – lessons learned and implications for action. 2009. Available from: http://www.mchip.net/sites/default/files/PIRI%20monograph_Feb09_0.PDF. Accessed September 2, 2014. | ||
Centers for Disease Control and Prevention (CDC). 2014 Recommended Immunizations for Adults by Age. Atlanta: CDC; 2014. Available from: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read.pdf. Accessed September 2, 2014. | ||
Ministre des Affaires Sociales, de la Santé et des Droits des Femmes. Le Guide Calendrier des Vaccinations des Adolescents et des Adultes. Paris: Ministre des Affaires Sociales, de la Santé et des Droits des Femmes; 2013. Available from: http://www.sante.gouv.fr/IMG/pdf/Guide_vaccinations_adolescents_adultes_2013.pdf. Accessed September 2, 2014. | ||
World Health Organization, United Nations Children’s Fund. Antenatal Care in Developing Countries: Promises, Achievements and Missed Opportunities. Geneva: WHO; 2003. Available from: http://www.childinfo.org/files/antenatal_care.pdf. Accessed 2 September 2014. | ||
World Health Organization. Provision of Effective Antenatal Care. Geneva: WHO; 2006. Available from: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/effective_antenatal_care.pdf. Accessed September 2, 2014. | ||
Stanton C. Steps towards achieving skilled attendance at birth. Bull World Health Organ. 2008;86:241–320. | ||
United Nations Population Fund. The State of the World’s Midwifery 2014. A Universal Pathway. A Woman’s Right to Health. New York: UNFPA; 2014. Available from: http://unfpa.org/sites/default/files/pub-pdf/EN_SoWMy2014_complete.pdf. Accessed September 2, 2014. | ||
Murray SF, Pearson SC. Maternity referral systems in developing countries: current knowledge and future research needs. Soc Sci Med. 2006;62:2205–2215. | ||
Sabde Y, De Costa A, Diwan V. A spatial analysis to study access to emergency obstetric transport services under the public private “Janani Express Yojana” program in two districts of Madhya Pradesh, India. Reprod Health. 2014;11:57. | ||
Gopalan SS, Varatharajan D. Addressing maternal healthcare through demand side financial incentives: experience of Janani Suraksha Yojana program in India. BMC Health Serv Res. 2012;12:319. | ||
Powell-Jackson T, Morrison J, Tiwari S, Neupane BD, Costello AM. The experiences of districts in implementing a national incentive program to promote safe delivery in Nepal. BMC Health Serv Res. 2009;9:97. | ||
Agha S. Impact of a maternal health voucher scheme on institutional delivery among low income women in Pakistan. Reprod Health. 2011;8:10. | ||
Brenner S, Muula AS, Robyn PJ, et al. Design of an impact evaluation using a mixed methods model – an explanatory assessment of the effects of results-based financing mechanisms on maternal healthcare services in Malawi. BMC Health Serv Res. 2014;14:180. | ||
Prasad AM, Bhatia S, Agrawal R. The effect of the National Rural Health Mission on health services and outcomes for childbirth in India: a retrospective analysis of survey data. Lancet. 2013;382:11. | ||
World Health Organization. WHO-Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases. Geneva: WHO; 2003. Available from: http://whqlibdoc.who.int/hq/2003/who_v&b_03.01.pdf. Accessed September 2, 2014. | ||
Oomman N, Mehl G, Berg M, Silverman R. Modernising vital registration systems: why now? Lancet. 2013;381:1336–1337. | ||
Kyei-Faried S, Appiah-Denkyira E, Brenya D, Akuamoa-Boateng A, Visser L. The role of community-based surveillance in health outcomes measurement. Ghana Med J. 2006;40:26–30. | ||
[No authors listed]. Protection-at-birth (PAB) method, Tunisia. Wkly Epidemiol Rec. 2000;75:203–206. | ||
Darmstadt GL, Hassan M, Balsara ZP, Winch PJ, Santosham M, Gipson R. Impact of clean delivery-kit use on newborn umbilical cord and maternal puerperal infections in Egypt. J Health Popul Nutr. 2009;27:746–754. | ||
Seward N, Osrin D, Costello A, et al. Association between clean delivery kit use, clean delivery practices, and neonatal survival: pooled analysis of data from three sites in South Asia. PLoS Med. 2012;9:e1001180. | ||
Imdad A, Mullany LC, Baqui A, et al. The effect of umbilical cord cleansing with chlorhexidine on omphalitis and neonatal mortality in community settings in developing countries: a meta-analysis. BMC Public Health. 2013;13 Suppl 3:S15. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
