Back to Journals » Infection and Drug Resistance » Volume 15
Manual Homogenization Improves the Sensitivity of Microbiological Culture for Patients with Pyogenic Spondylitis
Authors Cui Y , Mi C, Wang B, Zheng B, Sun L , Pan Y, Lin Y, Shi X
Received 19 August 2022
Accepted for publication 28 October 2022
Published 4 November 2022 Volume 2022:15 Pages 6485—6493
DOI https://doi.org/10.2147/IDR.S386148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yunpeng Cui,1 Chuan Mi,1 Bing Wang,1 Bo Zheng,2 Liying Sun,3 Yuanxing Pan,1 Yunfei Lin,1 Xuedong Shi1
1Department of Orthopaedics, Peking University First Hospital, Beijing, People’s Republic of China; 2Department of Clinical Pharmacology, Peking University First Hospital, Beijing, People’s Republic of China; 3Department of Clinical Laboratory, Peking University First Hospital, Beijing, People’s Republic of China
Correspondence: Xuedong Shi, Department of Orthopaedics, Peking University First Hospital, No. 7 Xishiku Street, Xicheng District, Beijing, 100032, People’s Republic of China, Tel +86 28-010-83572970, Email [email protected]
Objective: This study aimed to investigate the effects of manual homogenization on the sensitivity of microbiological culture for patients with pyogenic spondylitis.
Methods: From October 2018 to March 2021, patients undergoing fluoroscopy-guided biopsy or open debridement due to pyogenic spondylitis were recruited. Their demographic data and baseline characteristics were recorded. Tissue samples were obtained through fluoroscopy-guided biopsy or open debridement. Tissue samples were divided into three parts: manual homogenization (MH), manual mixture (MM), and pathological examination. Sterile normal saline was set as the negative control to exclude false-positive culture results. The Chi-square test was used to detect the difference of microbiological culture results.
Results: Twenty-four consecutive patients (33 tissue cultures) with pyogenic spondylitis treated in our department between October 2018 and March 2021 were recruited in this study. The average age was 61.7± 3.2 years old and 10 patients were female. The MH group had a significantly higher positive rate compared with the MM group in aerobic conditions: 78.8% (26 isolates) vs 54.5% (18 isolates), P=0.037 and anaerobic condition: 63.6% (21 isolates) vs 39.4% (13 isolates), P=0.049. The results of subgroup analyses showed that MH could improve the culture sensitivity for patients with previous antibiotics use and without paravertebral abscesses but not reach a significant level on statistics.
Conclusion: Based on the present study, manual homogenization could improve the sensitivity of microbiological cultures for patients with pyogenic spondylitis.
Keywords: tissue culture, pyogenic spondylitis, manual homogenization, blood culture bottle
Introduction
Pyogenic spondylitis (PS) is a nonspecific infectious disease of vertebrae and intervertebral discs caused by various microorganisms.1 The incidence and medical burden of PS had been increasing in the past 20 years.2 PS has a high incidence of morbidity and mortality; 29% of patients develop neurological symptoms with disease progression.3 For selected cases with progressive neurologic deficits, deformity, and spinal instability, surgical treatment with internal fixation is also needed.4 Pathogen identification and appropriate antibiotic use are crucial in the early stage of the PS. However, just like patients with periprosthetic joint infection (PJI), the sensitivity of microbiological culture was low. Previous literature reported that the sensitivity of microbiological culture for patients with PS was lower than 50%.5–7 Suspension of antibiotic pre-culture and soft tissue culture were proven to improve microbiological culture results.9,10 However, difficulty in obtaining the soft tissue and the risk of disease progression limited the implementation of these methods.
The sensitivity of microbiological culture depended on two factors: the number of pathogens released from diseased tissues into the culture medium and the viability of the pathogens.11 The formation of biofilm is an important factor causing a false negative culture of infection related to spinal implantation. Biofilms could limit bacterial release from diseased tissues. Several methods, such as tissue homogenization, DL-dithiothreitol and sonication, which could increase the quantity and viability pathogens released from diseased tissue, had been applied to overcome the biofilm infection among PJI and spinal implant-related infection.12–16 None of these effective methods have been reported to improve the sensitivity of microbiological culture of patients of PS. Among these methods, manual homogenization is simple and easy to operate.
Biofilms could exist in the surface of soft tissue, cartilage, bone and the medullary cavity, which was detected in more than 60% of chronic osteomyelitis patients.17,18 The low culture sensitivity of patients with PS might be related to biofilms. In addition, the structure of bone tissue is complex and the release of bacteria would be limited in untreated bone tissue.
Manual homogenization is a simple method to increase the number of pathogens released from diseased tissues. This retrospective self-control cohort study aimed to investigate the effects of manual homogenization on the sensitivity of microbiological culture for patients with PS.
Materials and Methods
Study Design, Inclusion, and Exclusion Criteria
This study was a single-centered cohort study. Consecutive patients >18 years of age who underwent fluoroscopy-guided biopsy or open debridement were recruited from October 2018 to March 2021. We excluded patients with the following situations”: 1) Patients with specific pathogen infections such as tuberculosis or Brucella infection; and 2) Negative control (sterile normal saline) indicates that the tissue samples were suspected of contamination. PS was confirmed by pathological examination results that showed acute and chronic inflammatory cell infiltration and no cases of necrosis and granuloma formation.
This was a retrospective study. There was no follow-up requirement in this study. It was not necessary to collect blood samples and other samples of the patients, and no additional examination was required. The research ethics boards of our hospital approved the study protocol (2022 scientific research 081) and required neither patient approval nor informed consent for the routine laboratory procedure and review of patients’ images and medical records. Researchers would strictly keep the personal information of patients confidential. Identifiable information would not be disclosed to persons other than research members unless permission was obtained from the patient. All research members were required to keep the identity of patients confidential. When the research results were published, no personal information of patients would be disclosed. This study was conducted in accordance with the Declaration of Helsinki and its revisions.
Data Collection
Patients’ characteristics, including demographic information, location of the lesion, systemic inflammatory response syndrome (SIRS), paravertebral abscess, c-reactive protein, erythrocyte sedimentation rate, procalcitonin (PCT), interleukin-6, surgical intervention, previous antibiotic use, and culture results were carefully extracted from their electronic medical records.
Surgical Intervention
All patients underwent X-ray, Computer Tomography (CT), or Magnetic Resonance Imaging (MRI) to confirm the lesion’s location. Blood samples were taken for routine laboratory tests before fluoroscopy-guided biopsy and/or open debridement. Patients received routine empiric antibiotic therapy after surgery. Adjustment of antibiotics was based on the culture results. Fluoroscopy-guided biopsy was carried out in two steps. First, the biopsy needle was inserted into the pedicle of the lower vertebral body. We then pulled out the core of the biopsy needle and continued to penetrate the upper endplate. Then we took out the biopsy needle and bone lesion. Second, the biopsy needle was repositioned into the intervertebral space under the guidance of a K-wire. Soft tissue lesions including intervertebral disc and pus were extracted under negative pressure with a 20 mL syringe in some patients.
Open debridement surgery via an anterior or posterior approach was carried out for patients with spinal cord compression or failed treatment of antibiotics alone. The surgery option was determined by multidisciplinary cooperation, composed of an experienced spine surgeon and an infectious diseases specialist.
Tissue Samples Culture
Tissue samples (bone and soft tissue) were divided into three parts: manual homogenization (MH), manual mixture (MM), and pathological examination. For the MH group, tissue samples were placed in a disposable sterile tissue grinder (made of glass) with 5 mL normal saline. This was ground clockwise for 1 minute and the homogenate was diluted to 20 mL with normal saline. For the MM group, tissue samples were placed in the disposable sterile measuring cup with 20 mL normal saline and stirred clockwise for 1 minute. For pathological examination, tissue samples were soaked in formalin solution as soon as possible. Then 20 mL sterile normal saline was used to wash the grinder and measuring cup, and this was set as the negative control to exclude false-positive culture results. We collected 20 mL manual homogenate, manual mixture, and sterile normal saline, respectively. Microbiological aerobic and anaerobic cultures were performed on blood culture bottles (Becton, Dickinson and Company Spark, MD 21152, USA) for 2 weeks.
Statistical Analysis
Continuous variables were presented as mean (± standard deviation) when they were in the normal distribution and median (range) when they were not. t-test or Mann–Whitney U-test were used to detect the difference among continuous variables. The differences among the categorical variables were analyzed using the chi-square test (When n≥40 and T≥5, Pearson chi-square test; when n≥40 and 1≤T≤5, continuity adjusted Pearson chi-square test; when n<40, or T<1, Fisher’s Exact Test). All tests were 2-sided. A P-value<0.05 was considered statistically significant. All statistical analyses were performed with the IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY).
Results
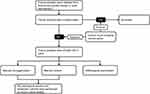
A total of 26 consecutive patients (36 tissue samples) with PS between October 2018 and March 2021 were recruited. The diagnosis of all patients was confirmed by pathological result. Two patients (three tissue samples) were excluded because of tissue samples contamination (positive result of negative control (sterile normal saline). In all, 24 patients (33 tissue samples) were included in this study (Figure 1).
Patients’ general information is shown in Table 1. The average age was 61.7±3.2 years old. Ten patients were female, and 14 were male. Twenty-two patients were involved in the thoracolumbar spine.
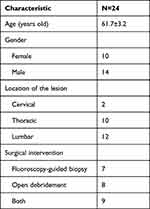 |
Table 1 Patients’ General Information |
The baseline conditions of patients before tissue samples acquired are shown in Table 2. Patients with a positive culture result in the MM group had a higher level of ESR, CRP, IL-6, more paravertebral abscesses and SIRS, and less previous antibiotic use compared with patients with negative culture result, although this did not reach a significant level on statistics. In the MH group, more patients with lower levels of CRP, IL-6, and SIRS had a positive culture result.
 |
Table 2 Characteristics of Patients in MM and MH Groups |
Culture results are shown in Table 3. The positive rates in aerobic conditions were 78.8% (26/33) and 54.5% (18/33) in the MH and MM groups, respectively. The positive rates in anaerobic condition were 63.6% (21/33) and 39.4% (13/33) in the MH and MM groups, respectively. The MH group had a significantly higher positive rate compared with the MM group in aerobic and anaerobic conditions (aerobic condition: 78.8% vs 54.5%, P=0.037; anaerobic condition: 63.6% vs 39.4%, P=0.049). The positive rates in aerobic condition were higher than that in anaerobic condition (MH group: 78.8% vs 63.6%; MH group: 54.5% vs 39.4%).
 |
Table 3 Culture Results |
The microorganisms identified in the present study are shown in Table 4. Five patients had different microorganisms identified in fluoroscopy-guided biopsy and open debridement. Four patients had different microorganisms identified in aerobic and anaerobic blood culture bottles. One patient had two microorganisms identified in the same blood culture bottle. In all, eight patients were identified with polymicrobial infections.
 |
Table 4 Summary of the Causative Microorganisms Identified in Patients with Pyogenic Spondylitis |
Subgroup analyses were carried out depending on previous antibiotic use and paravertebral abscesses. For patients with previous antibiotic use, the positive rates in aerobic condition were 94.4% (17/18) and 66.7% (12/18) in the MH and MM groups, respectively. The positive rates in anaerobic conditions were 66.7% (12/18) and 38.9% (7/18) in the MH and MM groups, respectively. For patients without previous antibiotic use, the positive rates in aerobic and anaerobic conditions were 60.0% (9/15) and 40.0% (6/15) in the MH and MM groups, respectively. For patients with paravertebral abscesses, the positive rates in aerobic condition were 78.9% (15/19) and 63.2% (12/19) in the MH and MM groups, respectively. The positive rates in anaerobic condition were 63.2% (12/19) and 42.1% (8/19) in the MH and MM groups, respectively. For patients without paravertebral abscesses, the positive rates in aerobic and anaerobic condition were 71.4% (5/7) and 42.9% (3/7) in the MH and MM groups, respectively (Table 5).
 |
Table 5 Sub-Group Analysis of Culture Results (Previous Antibiotic Use and Paravertebral Abscess) |
The results showed that MH could improve the culture sensitivity for patients with previous antibiotic use and without paravertebral abscesses, but not reach a significant level on statistics.
Patients with previous antibiotics use had higher positive rates than patients without previous antibiotics use (MH group and aerobic condition: 94.4% vs 60.0%; MM group and aerobic condition: 66.7% vs 40.0%). Patients with previous antibiotics use had a higher level of ESR, IL-6, and CRP. The levels of ESR, CRP, and IL-6 of patients with previous antibiotic use were higher than patients without antibiotic use, the difference of IL-6 reached a significant level on statistics (Figure 2).
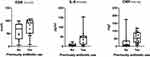 |
Figure 2 ESR, IL-6, and CRP level of patients with and without previous antibiotic use. ESR, IL-6, and CRP. |
Discussion
The low sensitivity of microbiological culture for patients with PS remained a difficult problem for clinicians. The present study demonstrated that MH could significantly improve the sensitivity of microbiological culture of patients of PS (Table 3).
There were several factors which affected the sensitivity of microbiological culture, such as the level of CRP, ESR, paravertebral abscess, pre-culture antibiotic use, and the source of tissue (soft tissue or bone).8,9 A lower level of CRP and ESR, pre-culture antibiotic use, and without abscesses were correlated with poor culture sensitivity.9,19 Our result showed that more patients with a lower level of CRP, ESR and IL-6 could get a positive culture result in the MH group. Sub-group analysis demonstrated that MH could improve the culture sensitivity for patients with previous antibiotics use and without abscesses, although not reach a significant level on statistics. The 2015 Infectious Diseases Society of America Clinical Practice Guidelines suggested that antibiotics be suspended for 1–2 weeks unless the patient was hemodynamically compromised or accompanied by neurologic symptoms.20 Russo et al9 reported that a rigorous UDIPROVE protocol (including Withholding antibiotics 2 weeks) could increase the culture-positive rate to 73.6%, almost twice the rate reported in the other literature.5–7 However, suspension of antibiotic pre-culture was not suitable for all patients and might cause delayed treatment or disease progression, particularly in critically ill patients. Antibiotic use of patients was not interrupted in the present study.
Biofilm was a colony colonized on the surface of tissues or plants in vivo with strong adhesion. It was composed of bacteria, proteins, and matrices. Biofilm was detected in more than 60% of chronic osteomyelitis patients.18 Biofilm was an important factor that causes bacterial resistance and false negative of culture.21,22 The results of this study showed that the microorganisms isolated were mainly gram-positive cocci, such as Staphylococcus. For the same tissue sample, most microorganisms that were isolated in the MH group and not isolated in the MM group were Staphylococcus (Table 4). Staphylococcus easily form biofilms.17,23,24 The result suggested that biofilm might play a role in the false negative culture results for patients with PS. MH could destroy the biofilm and increase the sensitivity of culture.
Disputes still exist between bone and soft tissue. Kim et al10 reported that the culture sensitivity of soft tissue (intervertebral discs, paraspinal abscesses, or psoas abscesses) was higher than bone (63.5% vs 39.7%). However, the significantly higher level of CRP in the soft tissue group (P=0.032) might bias the results. Chang et al25 reported that culture sensitivity was not statistically significantly different between bone and soft-tissue. Compared with soft tissue, especially abscesses, bone tissue biopsy also had another advantage, the additional tissue could be sent for pathologic evaluation and make the pathological diagnosis.26,27 In the present study, patients mainly underwent fluoroscopy-guided bone tissue biopsy. MH was simple and easy to operate. However, MH had limitation when applied in patients of PS. MH was difficult to fully homogenize bone tissue, especially bone tissue containing sclerosing components. Additional methods needed to make up the inadequate homogenization for bone, such as DL-dithiothreitol. DL-dithiothreitol was simple to use and could increase pathogen release from disease tissue.13,14 Combination of MH and DL-dithiothreitol was theoretically feasible to further improve on the culture sensitivity. Moreover, an especial homogenizer might be another choice to fully achieve homogenization of bone.
Blood culture bottles were used in the present study. Blood culture bottles could increase the culture sensitivity of patients with PJI.28,29 In the present study, polymicrobial infections were identified in eight patients. Among them, one patient with polymicrobial were identified in the same blood culture bottle. Usually, only one dominant bacterium will reproduce in the blood culture bottle, although culture was the gold standard for microorganism identification. Compared with blood culture bottle, polymerase chain reaction and metagenomic next generation sequencing had the advantage on the identification of polymicrobial infections.30,31
We acknowledge limitations to the present study. The main limitation was that this was a single-center study with a relatively small sample size. A large-scale case should be collected to validate these results, especially for patients with a different risk factor, such as the level of infectious indicator (ESR, CRP), paraspinal abscess, and pre-culture antibiotics use. However, the strength of the study was that it was self-control designed in that it provided essential information regarding applying a simple and effective method to improve culture sensitivity for patients with PS.
Conclusions
Based on the present study, MH could improve the sensitivity of microbiological culture for patients with pyogenic spondylitis.
Data Sharing Statement
The data that support the findings of this study is available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethical Approval
The research ethics boards of Peking University First Hospital approved the study protocol (2022 scientific research 081) and required neither patient approval nor informed consent for the routine laboratory procedure and review of patients’ images and medical records. This study was conducted in accordance with the Declaration of Helsinki and its revisions.
Consent to Participate
According to the local institutional review board, for this type of retrospective study, informed consent is not required.
Consent for Publication
All the authors listed have approved the enclosed manuscript and agree with its content.
Funding
National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of Peking University First Hospital) 2022CR14.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Babic M, Simpfendorfer CS. Infections of the spine. Infect Dis Clin North Am. 2017;31:279–297. doi:10.1016/j.idc.2017.01.003
2. Kim YS, Kim JG, Yi J, et al. Changes in the medical burden of pyogenic and tuberculous spondylitis between 2007 and 2016: a nationwide cohort study. J Clin Neurosci. 2020;73:89–93. doi:10.1016/j.jocn.2020.01.023
3. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–17. doi:10.1016/j.semarthrit.2008.03.002
4. Dennis Hey HW, Nathaniel NLW, Tan CS, et al. Spinal implants can be inserted in patients with deep spine infection: results from a large cohort study. Spine. 2017;42:E490–E495. doi:10.1097/BRS.0000000000001747
5. Hirschfeld CB, Kapadia SN, Bryan J, et al. Impact of diagnostic bone biopsies on the management of non-vertebral osteomyelitis: a retrospective cohort study. Medicine. 2019;98(34):e16954. doi:10.1097/MD.0000000000016954
6. Hoang D, Fisher S, Oz OK, La Fontaine J, Chhabra A. Percutaneous CT guided bone biopsy for suspected osteomyelitis: diagnostic yield and impact on patient’s treatment change and recovery. Eur J Radiol. 2019;114:85–91. doi:10.1016/j.ejrad.2019.01.032
7. Kim BJ, Lee JW, Kim SJ, Lee GY, Kang HS. Diagnostic yield of fluoroscopy-guided biopsy for infectious spondylitis. AJNR Am J Neuroradiol. 2013;34:233–238. doi:10.3174/ajnr.A3120
8. Dai G, Li S, Yin C, et al. Culture-negative versus culture-positive in pyogenic spondylitis and analysis of risk factors for relapse. Br J Neurosurg. 2021;8:1–5. doi:10.1080/02688697.2021.1896677
9. Russo A, Graziano E, Carnelutti A, et al. Management of vertebral osteomyelitis over an eight-year period: the UDIPROVE (UDIne PROtocol on VErtebral osteomyelitis). Int J Infect Dis. 2019;89:116–121. doi:10.1016/j.ijid.2019.10.010
10. Kim CJ, Kang SJ, Choe PG, et al. Which tissues are best for microbiological diagnosis in patients with pyogenic vertebral osteomyelitis undergoing needle biopsy? Clin Microbiol Infect. 2015;21:931–935. doi:10.1016/j.cmi.2015.06.021
11. Askar M, Ashraf W, Scammell B, Bayston R. Comparison of different human tissue processing methods for maximization of bacterial recovery. Eur J Clin Microbiol Infect Dis. 2019;38:149–155. doi:10.1007/s10096-018-3406-4
12. Fang X, Zhang L, Cai Y, et al. Effects of different tissue specimen pretreatment methods on microbial culture results in the diagnosis of periprosthetic joint infection. Bone Joint Res. 2021;10:96–104. doi:10.1302/2046-3758.102.BJR-2020-0104.R3
13. Drago L, Romanò CL, Mattina R, Signori V, De Vecchi E. Does dithiothreitol improve bacterial detection from infected prostheses? A pilot study. Clin Orthop Relat Res. 2012;470:2915–2925. doi:10.1007/s11999-012-2415-3
14. Sambri A, Cadossi M, Giannini S, et al. Is treatment with dithiothreitol more effective than sonication for the diagnosis of prosthetic joint infection? Clin Orthop Relat Res. 2018;476:137–145. doi:10.1007/s11999.0000000000000060
15. Bürger J, Akgün D, Strube P, Putzier M, Pumberger M. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J. 2019;28:768–774. doi:10.1007/s00586-019-05881-x
16. Carlson BC, Hines JT, Robinson WA, et al. Implant Sonication versus Tissue Culture for the Diagnosis of Spinal Implant Infection. Spine. 2020;45:E525–E532. doi:10.1097/BRS.0000000000003311
17. Coscia MF, Denys GA, Wack MF. Propionibacterium acnes, coagulase-negative Staphylococcus, and the “Biofilm-like” intervertebral disc. Spine. 2016;41(24):1860–1865. doi:10.1097/BRS.0000000000001909
18. Magana M, Sereti C, Ioannidis A, et al. Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev. 2018;31(3):e00084–16. doi:10.1128/CMR.00084-16
19. Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi:10.1056/NEJMcp0910753
20. Berbari EF, Kanj SS, Kowalski TJ, et al.; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26–e46. doi:10.1093/cid/civ482
21. Del Pozo JL. Biofilm-related disease. Expert Rev Anti Infect Ther. 2018;16(1):51–65. doi:10.1080/14787210.2018.1417036
22. Yin W, Wang Y, Liu L, He J. Biofilms: the microbial “Protective Clothing” in extreme environments. Int J Mol Sci. 2019;20(14):3423. doi:10.3390/ijms20143423
23. Nasser A, Dallal MMS, Jahanbakhshi S, Azimi T, Nikouei L. Staphylococcus aureus: biofilm formation and strategies against it. Curr Pharm Biotechnol. 2022;23(5):664–678. doi:10.2174/1389201022666210708171123
24. Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33(26):5967–5982. doi:10.1016/j.biomaterials.2012.05.031
25. Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-year retrospective review of CT-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol. 2015;205:123–129. doi:10.2214/AJR.14.13545
26. Howard CB, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br. 1994;76:311–314. doi:10.1302/0301-620X.76B2.8113300
27. White LM, Schweitzer ME, Deely DM, Gannon F. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology. 1995;197:840–842. doi:10.1148/radiology.197.3.7480765
28. Peel TN, Dylla BL, Hughes JG, et al. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. mBio. 2016;7:e01776–e01815. doi:10.1128/mBio.01776-15
29. Portillo ME, Salvadó M, Trampuz A, et al. Improved diagnosis of orthopedic implant-associated infection by inoculation of sonication fluid into blood culture bottles. J Clin Microbiol. 2015;53:1622–1627. doi:10.1128/JCM.03683-14
30. Zhang C, Fang X, Huang Z, et al. Value of mNGS in sonication fluid for the diagnosis of periprosthetic joint infection. Arthroplasty. 2019;1:9. doi:10.1186/s42836-019-0006-4
31. He R, Wang Q, Wang J, Tang J, Shen H, Zhang X. Better choice of the type of specimen used for untargeted metagenomic sequencing in the diagnosis of periprosthetic joint infections. Bone Joint J. 2021;103-B:923–930. doi:10.1302/0301-620X.103B5.BJJ-2020-0745.R1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.