Back to Journals » Journal of Inflammation Research » Volume 15
Mannan-Binding Lectin Regulates the Th17/Treg Axis Through JAK/STAT and TGF-β/SMAD Signaling Against Candida albicans Infection
Authors Wang F, Yang Y, Li Z, Wang Y, Zhang Z, Zhang W, Mu Y, Yang J, Yu L, Wang M
Received 22 October 2021
Accepted for publication 8 March 2022
Published 11 March 2022 Volume 2022:15 Pages 1797—1810
DOI https://doi.org/10.2147/JIR.S344489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Fanping Wang,1,2 Yonghui Yang,1,3 Zhixin Li,1,2 Yan Wang,1,4 Zhenchao Zhang,2,5 Wei Zhang,2,5 Yonghui Mu,2,5 Jingwen Yang,1,2 Lili Yu,2,5 Mingyong Wang1,2
1Henan Key Laboratory of Immunology and Targeted Drugs, School of Laboratory Medicine, Xinxiang Medical University, Xinxiang, Henan, 453003, People’s Republic of China; 2Xinxiang Key Laboratory of Immunoregulation and Molecular Diagnostics, Xinxiang, 453003, People’s Republic of China; 3Henan Center for Disease Control and Prevention, Zhengzhou, Henan, 450000, People’s Republic of China; 4Department of Laboratory Medicine, Luoyang Oriental Hospital, Luoyang, Henan, 471000, People’s Republic of China; 5School of Basic Medical Sciences, Xinxiang Medical University, Xinxiang, Henan, 453003, People’s Republic of China
Correspondence: Mingyong Wang; Lili Yu, Email [email protected]; [email protected]
Background: Mannan-binding lectin (MBL) is a key molecule in innate immunity and activates the lectin complement pathway, which plays an important role in resisting Candida albicans (C. albicans) infection. However, the underlying mechanism of this resistance to infection remains unclear.
Methods: In this study, we investigated how MBL regulates the differentiation of CD4+ T cells into T helper type 17 (Th17) and T regulatory (Treg) cells against C. albicans in mice, as well as the underlying mechanisms. We generated MBL double-knockout (KO) mice and infected them with C. albicans by intraperitoneal injection.
Results: Compared with that in wild-type (WT) mice, the percentage of Th17 cells increased in MBL-null mice, whereas Treg cells decreased, indicating that MBL might regulate the Th17/Treg balance. In addition, in MBL-null mice, the expression levels of interleukin (IL)-17A, IL-21, and the master transcription factor of Th17 cells, RORγt, significantly increased. Conversely, IL-10, IL-2, and the Treg-specific transcription factor, Foxp3, decreased. Moreover, we found that the levels of TGF-β and IL-6 upregulated in MBL-null mice. Mechanistically, we found that MBL regulated the TGF-β/SMAD pathway through the inhibition of p-SMAD2 and promotion of p-SMAD3, and mediated the JAK/STAT pathway through the inhibition of p-JAK2 and p-STAT3 and promotion of p-JAK3 and p-STAT5. MBL double-KO mice showed a more severe inflammatory response and significantly lower survival rates with C. albicans infection.
Conclusion: These results suggest that MBL regulates the Th17/Treg cell balance to inhibit inflammatory responses, possibly via IL-6- and TGF-β-mediated JAK/STAT and TGF-β/SMAD signaling, and play an important role in anti-C. albicans infection.
Keywords: mannan-binding lectin, Th17/Treg axis, JAK/STAT, TGF-β/SMAD, Candida albicans
Introduction
Mannan-binding lectin (MBL) is the key recognition molecule in the complement activation pathway. In humans, MBL is encoded by a single gene, MBL2, while in rodents, there are two MBL genes, MBL-A and MBL-C.1 The carbohydrate-recognition domain of MBL senses polysaccharides such as D-mannose, L-fucose, and N-acetylglucosamine on several organisms, including C. albicans, which play a key role in innate immunity.2–4 Recent studies have reported that MBL is also critical for the adaptive immune response.5,6 Moreover, Stefan Müller et al found that MBL-deficient mice exhibit increased antibody production and impairment of the adaptive immune response owing to the absence of the first-line defense function of MBL in innate immunity, which can promote the development of adaptive immune responses in the circulatory system.7–9 Crohn’s disease is susceptible to a complicated clinical phenotype when accompanied by a defective MBL gene, possibly reflecting the delayed clearance of oligomannan-containing microorganisms by the innate immune system in the absence of MBL.10
C. albicans is an opportunistic pathogenic fungus that usually colonizes the human gastrointestinal tract, skin, and mucosa.11 MBL levels increase markedly during the infection process in patients with invasive candidiasis, and serum MBL levels exhibit a dynamic decrease during the 2 days preceding positive blood culture sampling, indicating that MBL may play a role during the early stage invasive candidiasis. It has been shown that MBL is expressed in mouse intestinal epithelial cells, and dextran sodium sulfate-induced colitis promotes C. albicans dissemination to the kidneys and lungs in MBL-deficient mice after intestinal infection.12 The gastrointestinal tract of MBL-null mice infected with C. albicans will have an excessive inflammatory reaction.13,14
Regulatory T cells (Treg) and T helper type 17 cells (Th17) can eliminate pathogens and reduce tissue damage caused by the inflammatory reaction by inhibiting and promoting inflammatory reactions, respectively.13 During an oral C. albicans infection in mice, Treg cells induced IL-17 cytokines in responder T cells (Tresp), which markedly enhanced fungal clearance and recovery from infection. The Th17 cells, acting largely through IL-17, confers the dominant response to oral candidiasis through neutrophils and antimicrobial factors.14 Moreover, Treg cells could promote acute Th17 cell responses to suppress mucosal fungus infections and had a powerful capability to fight infections besides their role in maintaining tolerance or immune homeostasis.15
Foxp3 is a Treg-specific nuclear transcription factor, and the current understanding of the transcriptional regulation of Foxp3 gene included signaling pathways initiated by TCR, IL-2R/STAT pathway, TGF-beta/Smad pathway, PI3K/Akt/mTOR axis, Notch signal pathway, IFN/IRF and IFN/nitric oxide axis, and epigenetic mechanisms.16,17 The retinoid-related orphan receptor gamma t (RORγt) is mainly expressed in the Th17 cells of CD4+ T cell subsets, which regulate natural immunity and the adaptive immune response by producing inflammatory mediators that can also help remove invading pathogens.16,18 Published studies showed that specific JAK/STAT pathways played a critical role in the functional differentiation of distinct Th subsets, and heme oxygenase-1 exerted its inhibitory effect on Th17 cell differentiation by directly associating and blocking STAT3 phosphorylation.19 In addition, fungal killing by IL-6/23-stimulated human peripheral blood neutrophils was impaired by JAK/STAT inhibitors Ruxolitinib and Stattic and the RORγt inhibitor SR1001.20
Until now, the underlying molecular mechanism of MBL against C. albicans infection remains unclear. In this study, we established an MBL double-knockout (KO) mouse model and clarified how MBL regulates the balance between Treg cells and Th17 cells through intraperitoneal injection of C. albicans in mice.
Materials and Methods
Animals
MBL-null, MBL-A, and MBL-C double-knockout (KO) mice were obtained by CRISPR/Cas9 genome editing on the C57BL/6 background, followed by intercrossing of mice. Laboratory of Immunophenomics performed the genome-editing experiments of mice in Xinxiang Medical University following the protocols as previously described.21 WT C57BL/6 mice were used as controls for all experiments and were purchased from Charles River Laboratories. All mice were housed in the Xinxiang Medical University animal care facility, maintained in pathogen-free conditions, and provided standard laboratory chow and water ad libitum. All experiments were approved and carried out following the Xinxiang Medical University Animal Care Committee guidelines.
Genotyping
DNA was extracted from the tails of MBL double-KO mice using a commercial DNA extraction kit (GENFINE Company) according to the manufacturer’s instructions. Briefly, tails were incubated at 56°C for 6–8 h with lysis buffer solution. Proteinase K. Lysis buffer was then added to the solution. The samples were further incubated at 70 °C for 10 min. After adding ethanol, the samples were applied to the column and centrifuged. DNA was eluted in Buffer TB from the kit and subsequently used for PCR.
The PCR was run in a thermocycler (Bio-Rad, Hercules, CA, USA) using the 2× Taq Plus Master Mix following the manufacturer’s instructions. Thermocycling was performed in a final volume of 10 μL containing 1.2 μL of cDNA and 10 μM of each primer. The amplification conditions comprised an initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 30s, 61°C for 30s, 72°C for 40s, and a final extension at 72°C for 20 min. The following primers were used: m-MBL-A-F-PCR, Forward: 5’- GCT TCC ATT ACT CCC TGT CCT T −3’ and Reverse: 5’- TCC ATC TCC GAA CAC AGA ACA G −3’; m-MBL-C-F-PCR, Forward: 5’- CCT TCT GCT GTG TGT GGT GA-3’ and Reverse: 5’- GCA CAC CTG GTT CTC CCT TT −3’.
C. albicans Strain and Infection
The C. albicans strain used in this study was the international standard strain ATCC90028; WT and MBL double-KO mice were matched by gender and age, and more than 15 mice per genotype were used for each experiment. C. albicans was cultured in a YPD medium (1% yeast extract, 2% bacto peptone, and 2% dextrose) on a shaker at 28 °C for 48 h.22 Before injection, the fungi were washed twice in phosphate-buffered saline (PBS; Gibco BRL). Mice (6-to-9-weeks-old) were challenged intraperitoneally with 5×107 CFUs C. albicans.
Flow Cytometry for Detection of Treg and Th17 Cells
On the 7th day after infection, three mice were randomly selected from each group. After the eyeballs of the mice had been removed, blood was collected into the EP tubes containing anticoagulator EDTA. For CD45, CD5, CD4, RORγt, and Foxp3 staining, in turn, the cells were washed, fixed, and then stained using a flow cytometry staining kit from BD Biosciences, following the manufacturer’s protocol. Data were acquired using Becton Dickinson FACS-Calibur cytometers and analyzed using FlowJo 9.1 software.
Cytokine Measurements
ELISA was used to measure the levels of IL-17A, IL-10, IL-6, TGF-β, IL-2, and IL-21 cytokines in the blood of mice. On day 7 after infection, five mice were randomly selected from each group, euthanized, and the eyeballs removed for blood collection. Blood was collected into tubes containing EDTA as an anticoagulant, and the plasma was collected after centrifugation. According to the manufacturer’s instructions, cytokines measurement were performed using ELISA test kits (XinBoSheng Biotechnology Co., Ltd.).
Separation and Purification of CD4+ T Cells
After 7 days of infection, five mice were randomly selected from each group and sacrificed by cervical dislocation. The spleen was removed and ground to obtain a cell suspension. Magnetic spleen cell separation was used for sorting. The MS column was placed in a MACS magnetic sorter (Miltenyi Biotech), and the CD4+ T cells were sorted and purified using a mouse magnetic bead sorting kit (Miltenyi Biotech). All the procedures were performed according to the kit manufacturer’s instructions.
Quantitative RT-PCR
On day 7 after infection, five mice were randomly selected from each group and sacrificed by cervical dislocation. Spleen cells were isolated as described above, and CD4+ T cells were sorted using MACS magnetic beads. Total RNA was isolated from CD4+ T cells of the four groups using TRIzol reagent (TAKARA) and reverse transcribed into cDNA. The qPCR conditions were one cycle at 95 °C for 30s, 95 °C for 5 s, followed by 34 cycles at 60 °C for 30s. In each sample, the expression levels of the target genes were normalized to that of the β-actin housekeeping gene. Data analysis was performed using the 2−∆∆Ct method. The primers used were synthesized by Wuhan JinkaiRui Bioengineering Co., Ltd. The primers used for the qPCR were as follows: Rorγt, sense: 5’-CTG TCC TGG GCT ACC CTA CT-3’ and antisense: 5’-GAA GAA GCC CTT GCA CCC C-3’; Foxp3, sense: 5’-GCG AAA GTG GCA GAG AGG TA-3’ and antisense: 5’-TGT CAG AGG CAG GCT GGA TA-3’; IL-17A, sense: 5’-CCA GGG AGA GCT TCA TCT GTG T-3’ and antisense: 5’-AAG TCC TTG GCC TCA GTG TTT G-3’; IL-10, sense: 5’-GGT TGC CAA GCC TTA TCG GA-3’ and antisense: 5’-GAG AAA TCG ATG ACA GCG CC-3’; IL-6, sense: 5’-CCC CAA TTT CCA ATG CTC TCC T-3’ and antisense: 5’-CAT AAC GCA CTA GGT TTG CCG-3’; TGF-β, sense: 5’-AGC TGC GCT TGC AGA GAT TA-3’ and antisense: 5’-AGC CCT GTA TTC CGT CTC CT-3’; IL-2, sense: 5’-CAA GCA GGC CAC AGA ATT GAA-3’ and antisense: 5’-TCA AAT CCA GAA CAT GCC GC-3’; IL-21, sense: 5’-AGC ACA TAG CTA AAT GCC CTT C-3’ and antisense: 5’-TGT GGG AAC GAG AGC CTA TG-3’; and beta-actin, sense: 5’-AGA TCA AGA TCA TTG CTC CT-3’ and antisense: 5’-ACG CAG CTC AGT AAC AGT CC-3’.
Western Blotting
On day 7 after infection, spleen cells were collected as described above. CD4+ T cells sorted using MACS magnetic beads were lysed on ice for 15 min, centrifuged at 14,000 rpm for 15 min at 4 °C, and the protein concentration in the supernatant was measured Bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. Antibodies against STAT3 (ab68153), p-STAT3Tyr705 (ab267373), p-STAT5Tyr694 (ab278764), SOCS3 (ab280884), RORγt (ab207082), and Foxp3 (ab20034) were purchased from Abcam; antibodies against SMAD2 (5339S), p-SMAD2Ser465+467 (3108S), SMAD3 (9523S), p-SMAD3Ser423+Ser425 (9520S), JAK2 (3230S), JAK3 (8863S), and p-JAK3Tyr980/981 (5031S) were purchased from Cell Signaling Technology; the antibody against SMAD7 (MAB2029-SP) was purchased from R&D Systems, and the anti-β-actin antibody (66009-1-Ig) was purchased from Proteintech. Milk was used for blocking, and the primary antibody was incubated overnight with shaking. After washing with 1×TBST, a horseradish peroxidase-conjugated secondary antibody was added, incubated for 1 h, and staining was developed using an ECL kit (Millipore Corporation, Billerica, MA, USA). Bands were developed using a TANON exposure apparatus, and the software of ImageJ was used to analyze the intensity of western blot bands.
Histology
After 3 days of infection, mice were randomly selected from 4 groups and were euthanized by cervical dislocation. The liver and kidneys were removed, fixed in 4% paraformaldehyde at 4 °C, dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin. The sections were cut into 5-μm slices for hematoxylin-eosin (H&E) and periodic acid-Schiff (PAS) staining.
Determination of Survival Rate and Fungal Burden
WT and MBL double-KO mice were simultaneously intraperitoneally infected with 200 μL of a C. albicans suspension (1 × 108 CFUs) according to a previous study,23 and the survival rates were monitored for 14 days. In addition, to compare differences in tissue load, WT and MBL double-KO mice were infected intraperitoneally with a sublethal dose of C. albicans (5 × 107 CFUs) after 3 days of infection. The liver, kidneys, and spleen were sterilely dissected from each mouse, weighed, and homogenized in sterile PBS. Serial dilutions of the organ homogenate were spread onto agar plates. After 48 h of incubation at 37 °C, the CFUs were counted and determined as CFUs/g of tissue.
Statistical Analysis
All data were processed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). The SPSS statistical package (SPSS for Windows 16; SPSS Inc., Chicago, IL, United States) was utilized to apply Duncan’s multiple range test and one-way analysis of variance (ANOVA) to determine the statistical significance of biological research data, such as the change in the quantity of Treg and Th17 and the expression of cytokines. The survival periods were compared using the Kaplan–Meier method. Tests of the differences among groups were conducted, and the threshold value of P < 0.05 indicated that the difference was statistically significant.
Results
Generation of MBL Double-Knockout Mice
As described in published articles,24 agarose electrophoresis, genotyping, and sequencing analyses were used to confirm that mice homozygous for MBL-A and MBL-C gene double-KO were successfully obtained.
Establishment of a Mouse Model of C. albicans Infection
The mouse abdominal cavity was opened 3 days after infection, and the infection foci were identified in the liver and kidneys (S1 Fig A, B). When the liver and kidney suspensions were coated on Sabouraud’s medium, white colonies with a smooth surface were observed (S1 Fig C, D). Single colonies were transferred to new plates for green-color colony Candida selection, and green colonies could be observed (S1 Fig E, F). Moreover, periodic acid-Schiff (PAS) staining showed that both types of C. albicans cells (yeast and branched hyphae) were present in the livers and kidneys of both WT (WT) and MBL double-KO mice (S1 Fig G, H). These results showed that the mouse model of systemic C. albicans infection had been successfully established.
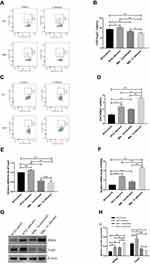
The Change of Treg, Th17 Cells and the Expression of Foxp3 and RORγt in MBL Double-KO Mice
To explore the roles of MBL in Treg and Th17 cell differentiation, the proportion of Treg and Th17 cells in peripheral blood and the expression levels of the nuclear transcription factors Foxp3 and RORγt were compared between WT and MBL double-KO mice by flow cytometry (S2 Fig) and western blotting. The results showed that the proportion of Treg cells decreased significantly (P < 0.01) in MBL double-KO mice, and the percentage of Treg cells in C. albicans infected mice was significantly higher (P < 0.05) than that in uninfected mice while MBL double-KO could inhibit the increase of Treg cells (Figure 1A and B), indicating that MBL can induce the differentiation of CD4+ T cells into Treg cell subsets, especially, during C. albicans infection. Simultaneously, the proportion of Th17 cells in the MBL double-KO mice was significantly higher (P < 0.05) than that in WT mice, and the number of Th17 cells in C. albicans infected mice was significantly higher (P < 0.05) than that in uninfected mice and MBL double-KO could promote the increase of Th17 cells (Figure 1C and D), suggesting that MBL can inhibit the differentiation of CD4+ T cells into Th17 cell subsets in both normal and pathogen-infected conditions. Foxp3 and RORγt are important transcriptional Treg and Th17 cell differentiation and function regulators. Therefore, we also compared the protein and mRNA levels of Foxp3 and RORγt between WT and MBL double-KO mice. We found that Foxp3 expression decreased, while that of RORγt was increased, in MBL double-KO mice compared with that of WT mice in both normal and pathogen-infected conditions (Figure 1F–H). These results revealed that MBL positively regulates Treg differentiation and Foxp3 expression, negatively regulating Th17 differentiation and RORγt expression.
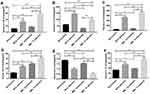
The Change of the Serum Levels of IL-17A, IL-10, IL-6, TGF-β, IL-2, and IL-21 in MBL Double-KO Mice
Th17 and Treg cells are known to be involved in fungal infection-associated immunoreactions. Furthermore, IL-17 and IL-21 are hallmarks of Th17 cells,25 while IL-10 and IL-2 are hallmarks of Treg cells.26,27 TGF-β induces the differentiation of Tregs. However, when combined with IL-6, TGF-β can differentiate CD4+ T cells into Th17 cells.28 As previously described, we observed dynamic changes in the Treg/Th17 cell balance in mouse peripheral blood. Next, we measured the serum levels of the related cytokines by ELISA. The measured cytokines included IL-17A, IL-10, IL-6, TGF-β, IL-2, and IL-21. The results of ELISA showed that, following C. albicans infection, the secretion of cytokines related to Th17 and Treg cells in the WT group was significantly different from that of the MBL-null group. The levels of IL-17A (Figure 2A), IL-6 (Figure 2C), TGF-β (Figure 2D), and IL-21 (Figure 2F), in the MBL double-KO mice, was significantly higher (P < 0.01) than that in the WT with both normal and C. albicans infected conditions, respectively, whereas the levels of IL-10 (Figure 2B) and IL-2 (Figure 2E) showed a significant decrease (P < 0.01). Collectively, these findings indicate that the changes in serum cytokine levels were consistent with the changes in Treg and Th17 cell proportions.
The Levels of Cytokine mRNA Expression in Mouse Splenocytes
We carried out qRT-PCR to compare the mRNA expression levels of IL-17A, IL-10, IL-6, TGF-β, IL-2, and IL-21 among WT mice, WT mice with infection C. albicans, MBL double-KO mice, and MBL double-KO mice with infection C. albicans. The results showed that, with C. albicans infection, the mRNA expression of Th17- and Treg-related factors in the WT mice was significantly different from that in MBL double-KO mice (Figure 3). Compared with the infected WT mice, the mRNA expression of IL-10 (Figure 3B) and IL-2 (Figure 3E) of the mice in the infected MBL double-KO group were decreased, whereas that of IL-17A (Figure 3A), IL-6 (Figure 3C), TGF-β (Figure 3D), and IL-21 (Figure 3F) was increased, and the difference was significant (P < 0.01). These results suggested that MBL has anti-inflammatory activity by regulating the expression of proinflammatory and anti-inflammatory cytokines during CD4+ T cell differentiation.
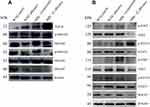
MBL Mediates Treg and Th17 Cell Differentiation Through the JAK/STAT and TGF-β/SMAD Signaling Pathways
The JAK/STAT and TGF-β/SMAD signaling pathways contribute to the differentiation of T helper cells.29,30 To assess the potential mechanism involved in how MBL modulates the differentiation of CD4+ T cells in C. albicans-infected mice, we measured the protein expression levels of key factors in the two signaling pathways using western blot analysis. Compared with WT mice infected with C. albicans, the expression of Foxp3, p-JAK2, p-JAK3, p-STAT5, and p-SMAD3 was decreased, whereas that of RORγt, p-STAT3, and p-SMAD2 was increased, in MBL double-KO mice with C. albicans infected conditions, respectively. Moreover, compared with infected WT mice, JAK2, JAK3, and STAT3 were decreased, and SMAD2 was increased in MBL double-KO mice infected with C. albicans. However, no significant changes were found in the levels of β-actin and total STAT5and SMAD3 proteins. The levels of SOCS331 and SMAD7,32 regulators of the JAK/STAT and TGF-β/SMAD signaling pathways, were also decreased (Figure 4). Therefore, the results revealed that MBL regulates the phosphorylation status of the STAT and SMAD protein families during Treg and Th17 cell differentiation.
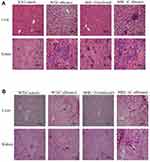
MBL Ameliorated the Inflammatory Response of the Liver and Kidney in Mice with C. albicans Infection
To evaluate the effects of MBL on immune inflammation in vivo, we generated a murine model of C. albicans infection and compared tissue inflammatory responses between C. albicans-infected WT and MBL-null mice. The results of hematoxylin and eosin (H&E) staining showed that, compared with that of infected WT mice, the liver and kidney of infected MBL-null mice presented a greater inflammatory response, wherein most renal units disappeared in the kidney, and multifocal necrotic tissue appeared in the renal medulla and cortex. In addition, C. albicans spores were observed and were surrounded by a large number of inflammatory cells. Moreover, liver tissue infection and peripheral hepatocyte water-like degeneration and fibrosis were more apparent in infected MBL-null mice. In contrast, the inflammation status of infected WT mice was relatively light, showing the disappearance of some of the nephrons, the appearance of a few inflammatory cells, and the ballooning of a small number of hepatocytes (Figure 5A). The results of the PAS staining showed that the liver and kidney of infected MBL double-KO mice were infiltrated with more hyphae and thick-film spores compared with the infected WT mice (Figure 5B).
MBL Double-KO Mice are Susceptible to C. albicans Infection
To investigate the role of MBL in C. albicans infection, we challenged C57BL/6 WT and MBL double-KO mice with C. albicans and monitored the survival rates and tissue burden of the two groups. The survival rates of the C. albicans-infected MBL-deficient mice were decreased compared with those of the WT controls (Figure 6A). On the 14th day of infection, the mortality rate of MBL double-KO mice was 100%, whereas that of WT mice was 80%. In addition, after 3 days of infection, the fungal burden of the liver, kidney, and spleen was higher in MBL double-KO mice than in WT mice (Figure 6B–D), which indicates that MBL is critically important for host defense against C. albicans infection.
Discussion
Plasma MBL, a secreted pattern recognition molecule, is an intrinsic component of the complement system. MBL deficiency is related to a variety of infectious conditions, including fungal infections. Although the role of MBL in the innate immune system has been widely studied, its role in adaptive immune responses remains poorly understood. Our current research aimed to increase our understanding of how MBL affects CD4+ T cell differentiation in the adaptive immune system under the condition of infection.
Noha M. Hammad showed that MBL could be used as a candidate antifungal agent against Candida infection.33 In our previous cytological studies in vitro, we found that a high concentration of MBL inhibited LPS-stimulated TNF-α and IL-12 production in THP1/CD14 cells, peptidoglycan-stimulated TNF-α and IL-6 production in THP1/CD14 cells, and C. albicans-stimulated TNF-α and IL-8 production in THP1/CD14 cells.2–4 It has been reported that two-phase states of C. albicans can stimulate dendritic cells and monocytes to produce cytokines such as IL-8, IL-6, MCP-1, and TNF-α.34 In this study, our results demonstrated that, under the conditions of this mouse model, MBL has a role in limiting excessive pro-inflammatory responses in tissues and resisting C. albicans infection.
Protection against C. albicans is determined not only by host immune resistance but also by the ability to control Candida-induced immunopathology appropriately. Excessive inflammatory responses can be damaging, and the overproduction of proinflammatory cytokines (eg, TNF-α, IL-12, IFN-γ, and IL-18) results in sustained sepsis, shock, and even death. Recent studies35–39 have pointed towards a surprisingly complex relationship between Th17 and Treg responses during C. albicans infections, wherein Th17-mediated immunity is crucial for protection against C. albicans infections, especially mucocutaneous infections, while Treg mainly restrains immunopathology. Our study found that the proportion of Th17 and Treg cells in WT mice infected with C. albicans was significantly higher than that in control mice, which indicated that Th17 and Treg cells played an important role in mice anti-C. albicans. However, excessive Th17 cells or a decrease of Treg cells could reduce the ability against C. albicans infection in mice. So, it is important to maintain the balance between Th17 and Treg cells in the process of anti-pathogen microbial infection, but the mechanism needs further study.
There is some evidence indicating that pattern recognition receptors on antigen-presenting cells recognize C. albicans, leading to the secretion of IL-1β, IL-23, and IL-6, as well as other specific cytokines.40,41 The release of these cytokines facilitates the differentiation of CD4+ T cells into Th17 cells, which expresses IL-17 (also known as IL-17A), IL-17F, and IL-22. In addition to Th17 cells, Tregs are also an important subpopulation of CD4+ T cells. In a stable state, Tregs suppress immunity by consuming the cytokine IL-2. Under inflammatory conditions, however, Treg cells show more potent immunosuppressive effects. This inducible iTreg may be mediated by IL-10 or TGF-β in an immunosuppressive environment, leading to antigen-specific or bypassing immunosuppression.42,43
Accumulating evidence has indicated that the IL-6-mediated JAK/STAT and TGF-β-mediated TGF-β/SMAD signaling pathways play important roles in naive CD4+ T cell differentiation.17,44–46 Moreover, the IL-6/JAK/STAT and TGFβ/SMAD pathways may influence the Th17/Treg balance. Consequently, we investigated the involvement of JAK/STAT and TGF-β/SMAD signaling in mice infected with C. albicans. The cytokine environment is a major determinant of the Th17/Treg balance. IL-6 is an important inflammatory cytokine that activates the tyrosine/serine phosphorylation of STAT3 via the cytokine receptor pathway, and the phosphorylation of STAT3 promotes the synthesis of RORγt. The combined action of IL-2 and TGF-β can promote the tyrosine phosphorylation of STAT5, while the phosphorylation of STAT5 promotes the synthesis of Foxp3.
Moreover, TGF-β has a joint function in differentiating both Th17 and Treg. TGF-β first binds to the TGF-β receptor (TGF-βR), mainly activating the SMAD transcription factors SMAD2 and SMAD3, and the common SMAD mediator, SMAD4. The activated SMAD complexes translocate into the nucleus and regulate the transcription of RORγt and Foxp3, critical transcriptional factors of Th17 and Treg. The increase in the expression of RORγt promotes the differentiation of CD4+ T cells into the Th17 subgroup, while the increase in Foxp3 expression promotes the differentiation of CD4+ T cells into the Treg subgroup.
Therefore, we investigated whether MBL regulates the Th17/Treg balance through the JAK/STAT and TGF-β/SMAD cytokine receptor signaling pathways. The results showed that, compared with infected WT mice, the levels of p-STAT3Y705, p-SMAD2S465+467, and RORγt increased significantly in infected MBL-deficient mice, while the protein levels of SOCS3 and SMAD7 decreased. Furthermore, in MBL double-KO mice infected with C. albicans, the levels of p-STAT5Y694, p-JAK2Y1007+Y1008, p-JAK3Tyr980/981, p-SMAD3S423+S425, and Foxp3 were lower than those in infected, WT mice, indicating that MBL can regulate the Th17/Treg balance through the JAK/STAT and TGF-β/SMAD cytokine receptor pathways.
Conclusion
We determined the MBL-related effects on adaptive immune responses to C. albicans infection, as well as the associated mechanisms, focusing on the differentiation of naive CD4+ T cells. Our results suggested that MBL could regulate the Th17/Treg cell balance by IL-6- and TGF-β-mediated JAK/STAT and TGF-β/SMAD signaling, and Th17/Treg cell balance might maintain an appropriate inflammatory response against pathogen infection (Figure 7). This study extends our knowledge of how innate immunity-related molecules regulate the inflammatory response and provide a potential way to prevent and treat fungal diseases by regulating the level of MBL.
Ethics Approval and Consent to Participate
All experiments were approved and carried out following the Xinxiang Medical University Animal Care Committee guidelines (Reference No. 2015016).
Acknowledgments
We are very grateful to Professor Yinming Liang of Laboratory of Mouse Genetics, Xinxiang Medical University, for providing MBL double-KO mice and analyzing the flow cytometry result.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81803607 and 81373120), Key Scientific and Technological Projects of Henan Province (No. 202102310032), the Science and Technology Innovative Research Team in Higher Educational Institutions of Henan Province (No. 20IRTSTHN030), Outstanding Youth Project of Henan Natural Science Foundation (212300410013) and Foundation for University Key Teacher of Henan Province (No. 2018GGJS101).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Hansen S, Thiel S, Willis A, Holmskov U, Jensenius JC. Purification and characterization of two mannan-binding lectins from mouse serum. J Immunol. 2000;164(5):2610–2618. doi:10.4049/jimmunol.164.5.2610
2. Wang M, Chen Y, Zhang Y, Zhang L, Lu X, Chen Z. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol. 2011;8(3):265–275. doi:10.1038/cmi.2011.1
3. Wang M, Wang F, Yang J, et al. Mannan-binding lectin inhibits Candida albicans-induced cellular responses in PMA-activated THP-1 cells through Toll-like receptor 2 and Toll-like receptor 4. PLoS One. 2013;8(12):e83517. doi:10.1371/journal.pone.0083517
4. Wang F, Li Y, Yang C, et al. Mannan-binding lectin suppresses peptidoglycan-induced TLR2 activation and inflammatory responses. Mediators Inflamm. 2019;2019:1349784. doi:10.1155/2019/1349784
5. Juul-Madsen HR, Norup LR, Jorgensen PH, Handberg KJ, Wattrang E, Dalgaard TS. Crosstalk between innate and adaptive immune responses to infectious bronchitis virus after vaccination and challenge of chickens varying in serum mannose-binding lectin concentrations. Vaccine. 2011;29(51):9499–9507. doi:10.1016/j.vaccine.2011.10.016
6. Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230(1):9–21. doi:10.1111/j.1600-065X.2009.00789.x
7. Guttormsen HK, Stuart LM, Shi L, et al. Deficiency of mannose-binding lectin greatly increases antibody response in a mouse model of vaccination. Clin Immunol. 2009;130(3):264–271.
8. Muller S, Schaffer T, Flogerzi B, et al. Mannan-binding lectin deficiency results in unusual antibody production and excessive experimental colitis in response to mannose-expressing mild gut pathogens. Gut. 2010;59(11):1493–1500. doi:10.1136/gut.2010.208348
9. Ruseva M, Kolev M, Dagnaes-Hansen F, et al. Mannan-binding lectin deficiency modulates the humoral immune response dependent on the genetic environment. Immunology. 2009;127(2):279–288. doi:10.1111/j.1365-2567.2008.03016.x
10. Schoepfer AM, Flogerzi B, Seibold-Schmid B, et al. Low Mannan-binding lectin serum levels are associated with complicated Crohn’s disease and reactivity to oligomannan (ASCA). Am J Gastroenterol. 2009;104(10):2508–2516. doi:10.1038/ajg.2009.315
11. Ho J, Camilli G, Griffiths JS, Richardson JP, Kichik N, Naglik JR. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology. 2021;162(1):11–16. doi:10.1111/imm.13255
12. Fiane AE, Videm V, Lingaas PS, et al. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108(7):849–856. doi:10.1161/01.CIR.0000084550.16565.01
13. Pandiyan P, Conti HR, Zheng L, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34(3):422–434. doi:10.1016/j.immuni.2011.03.002
14. Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. doi:10.1084/jem.20081463
15. Bhaskaran N, Weinberg A, Pandiyan P. Th17 inflammation model of oropharyngeal candidiasis in immunodeficient mice. JoVE. 2015;96:56.
16. Shen Z, Chen L, Hao F, Wu J. Transcriptional regulation of Foxp3 gene: multiple signal pathways on the road. Med Res Rev. 2009;29(5):742–766. doi:10.1002/med.20152
17. Pang N, Zhang F, Ma X. TGF-beta/Smad signaling pathway regulates Th17/Treg balance during Echinococcus multilocularis infection. Int Immunopharmacol. 2014;20(1):248–257. doi:10.1016/j.intimp.2014.02.038
18. Lee S, Kim J, Min H, Seong RH. RORgammat-driven TH17 cell differentiation requires epigenetic control by the Swi/Snf chromatin remodeling complex. iScience. 2020;23(5):101106. doi:10.1016/j.isci.2020.101106
19. Lin XL, Lv JJ, Lv J, et al. Heme oxygenase-1 directly binds STAT3 to control the generation of pathogenic Th17 cells during neutrophilic airway inflammation. Allergy. 2017;72(12):1972–1987. doi:10.1111/all.13216
20. Taylor PR, Roy S, Meszaros EC, et al. JAK/STAT regulation of Aspergillus fumigatus corneal infections and IL-6/23-stimulated neutrophil, IL-17, elastase, and MMP9 activity. J Leukoc Biol. 2016;100(1):213–222. doi:10.1189/jlb.4A1015-483R
21. Voisinne G, Kersse K, Chaoui K, et al. Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat Immunol. 2019;20(11):1530–1541. doi:10.1038/s41590-019-0489-8
22. Ashkanane A, Gomez GF, Levon J, Windsor LJ, Eckert GJ, Gregory RL. Nicotine upregulates coaggregation of candida albicans and streptococcus mutans. J Prosthodontics. 2019;28(7):790–796. doi:10.1111/jopr.12643
23. Carlson E. Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infect Immun. 1983;42(1):285–292. doi:10.1128/iai.42.1.285-292.1983
24. Wu M, Wang F, Yang J, et al. The responses of the gut microbiota to MBL deficiency. Mol Immunol. 2020;122:99–108. doi:10.1016/j.molimm.2020.03.008
25. Yasmin S, Dixon B, Olivares-Villagomez D, Algood HMS. Interleukin-21 (IL-21) downregulates dendritic cell cytokine responses to helicobacter pylori and modulates T Lymphocyte IL-17A expression in Peyer’s patches during Infection. Infect Immun. 2019;87(11). doi:10.1128/IAI.00237-19
26. Net EMG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172(6):3712–3718. doi:10.4049/jimmunol.172.6.3712
27. Spaccapelo R, Del Sero G, Mosci P, Bistoni F, Romani L. Early T cell unresponsiveness in mice with candidiasis and reversal by IL-2: effect on T helper cell development. J Immunol. 1997;158(5):2294–2302.
28. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi:10.1002/eji.201040391
29. Durant L, Watford WT, Ramos HL, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32(5):605–615. doi:10.1016/j.immuni.2010.05.003
30. Zhang S, Zhang G, Wan YY. SKI and SMAD4 are essential for IL-21-induced Th17 differentiation. Mol Immunol. 2019;114:260–268. doi:10.1016/j.molimm.2019.07.029
31. Li MZ, Lai DH, Zhao HB, Chen Z, Huang QX, Situ J. SOCS3 overexpression enhances ADM resistance in bladder cancer T24 cells. Eur Rev Med Pharmacol Sci. 2017;21(13):3005–3011.
32. Malonis RJ, Fu W, Jelcic MJ, et al. RNF11 sequestration of the E3 ligase SMURF2 on membranes antagonizes SMAD7 down-regulation of transforming growth factor beta signaling. J Biol Chem. 2017;292(18):7435–7451. doi:10.1074/jbc.M117.783662
33. Hammad NM, El Badawy NE, Ghramh HA, Al Kady LM. Mannose-binding lectin: a potential therapeutic candidate against candida infection. Biomed Res Int. 2018;2018:2813737. doi:10.1155/2018/2813737
34. Hsueh PR, Graybill JR, Playford EG, et al. Consensus statement on the management of invasive candidiasis in intensive care units in the Asia-Pacific region. Int J Antimicrob Agents. 2009;34(3):205–209. doi:10.1016/j.ijantimicag.2009.03.014
35. Bichele R, Karner J, Truusalu K, et al. IL-22 neutralizing autoantibodies impair fungal clearance in murine oropharyngeal candidiasis model. Eur J Immunol. 2018;48(3):464–470. doi:10.1002/eji.201747209
36. Gil ML, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front Biosci. 2009;14:570–582. doi:10.2741/3263
37. Kong X, Leng D, Liang G, et al. Paeoniflorin augments systemic Candida albicans infection through inhibiting Th1 and Th17 cell expression in a mouse model. Int Immunopharmacol. 2018;60:76–83. doi:10.1016/j.intimp.2018.03.001
38. Luisa gil M, Murciano C, Yanez A, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front Biosci. 2016;21:278–302. doi:10.2741/4388
39. Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi:10.1016/j.immuni.2010.05.001
40. Cheng SC, Chai LY, Joosten LA, et al. Candida albicans releases soluble factors that potentiate cytokine production by human cells through a protease-activated receptor 1- and 2-independent pathway. Infect Immun. 2010;78(1):393–399. doi:10.1128/IAI.01041-09
41. Zhu LL, Zhao XQ, Jiang C, et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39(2):324–334. doi:10.1016/j.immuni.2013.05.017
42. Feldhoff LM, Rueda CM, Moreno-Fernandez ME, et al. IL-1beta induced HIF-1alpha inhibits the differentiation of human FOXP3(+) T cells. Sci Rep. 2017;7(1):465. doi:10.1038/s41598-017-00508-x
43. Wei F, Zhang Y, Zhao W, Yu X, Liu CJ. Progranulin facilitates conversion and function of regulatory T cells under inflammatory conditions. PLoS One. 2014;9(11):e112110. doi:10.1371/journal.pone.0112110
44. Malhotra N, Robertson E, Kang J. SMAD2 is essential for TGF beta-mediated Th17 cell generation. J Biol Chem. 2010;285(38):29044–29048. doi:10.1074/jbc.C110.156745
45. Yang EJ, Lee J, Lee SY, et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PLoS One. 2014;9(2):e86062. doi:10.1371/journal.pone.0086062
46. Yao R, Ma YL, Liang W, et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7(10):e46082. doi:10.1371/journal.pone.0046082
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.