Back to Journals » Chronic Wound Care Management and Research » Volume 3
Managing severe burn injuries: challenges and solutions in complex and chronic wound care
Received 27 February 2016
Accepted for publication 1 April 2016
Published 11 June 2016 Volume 2016:3 Pages 59—71
DOI https://doi.org/10.2147/CWCMR.S86762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Alan D Rogers, Marc G Jeschke
Ross Tilley Burn Centre, Division of Plastic and Reconstructive Surgery, Department of Surgery, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada
Abstract: Encountered regularly by health care providers across both medical and surgical fields and an increasing socioeconomic burden globally, wound care is severely neglected. Practice is heavily influenced by anecdote rather than evidence-based protocols and industry-biased literature rather than robust randomized controlled trials. Burn units are well placed to address this considerable need, as a result of their infrastructure, their multispecialty staffing, and their need to evolve in light of the declining incidence of major burn injury in developed countries. The aim of this review is to evaluate some of the ideological and practical challenges facing wound practitioners and burn surgeons while managing chronic and complex wounds. It also includes an approach to wound assessment and how to conceptualize and implement dressing strategies and new and existing multimodal therapies.
Keywords: negative pressure wound therapy, instillation, antiseptic solutions, dressings, multidisciplinary wound care, stem cells, surgery, autograft, allograft, reconstructive ladder
Background
Wounds constitute a major socioeconomic burden globally, accounting for substantial health care expenditure and resources. While wounds are encountered in many spheres of both surgical and medical practices, and their care is theoretically within the gambit of several disciplines, no specialty has consistently taken ownership. Consequently, much of the burden of caring for wounds, and especially complex and chronic wounds, has fallen upon independent nurse practitioners, frequently ill prepared and resourced to manage these patients effectively.
Plastic and reconstructive surgery is the logical lead for the wound care industry. While our understanding of the fundamentals of anatomy and physiology of wound healing has positioned us to play this role, it is our algorithmic approach to wound closure that should have secured it for us. Unfortunately, this opportunity has been neglected, partly due to our relatively small number, the inexorable trend toward subspecialization, and partly as a result of general lack of inclination. Setbacks have been witnessed in other fundamental areas of plastic surgery practice, such as head and neck reconstruction, oncoplastic breast surgery, hand surgery, and maxillofacial surgery, where other specialties threaten to usurp integral disciplines.1,2
Burn centers, where the wound is the sine qua non, are ideally placed to take up this challenge and address the proverbial vacuum in health care delivery internationally. With improvements in industrial, domestic, and recreational safety measures in developed countries, the incidence of major burn injuries is on the decline, and evolving may well contribute to burn unit self-preservation. As burn injuries will unfortunately never be completely eradicated, there will always be the need to maintain resources and expertise in regional centers.
Burn centers in North America are therefore frequently called upon to manage patients with a spectrum of wound pathologies including exfoliative skin disorders, major traumatic degloving wounds, necrotizing soft tissue infections, and complications from a variety of surgical interventions. Most of these burn centers with American Burn Association accreditation have the capacity to manage patients requiring prolonged hospital stays, mechanical ventilation, multiple operative visits (often with their own operative suite), as well as deep sedations in the unit to facilitate frequent and often extensive dressing changes. These units should also be well equipped with a variety of modern wound care dressings and adjuncts including negative-pressure wound therapy (NPWT) with instillation, versajet, and an allograft skin bank, and are staffed by individuals whose primary goal is to optimize wound care to reduce hospital stays and disability.
This paper alludes to some of the controversies and major challenges facing such units when addressing chronic wounds, which broadly include
- How best to utilize scarce resources such as operating time, nursing care, and hospital beds
- Which modern wound care adjuncts are required and how best to optimize the benefits they offer
- How to decipher contradictory, anecdotal, and industry-biased literature as it pertains to wound care products3
Principles of normal wound healing
Wound healing is generally a logical series of events, coordinated by the cellular release of various mediators and cytokines (Table 1). Traditionally, this process has been conceptualized as distinct inflammatory, proliferative, and maturation phases, but in reality, these are a continuum of overlapping priorities at each phase and may continue beyond a year. A comprehensive treatise on the fundamentals of wound healing is beyond the scope of this paper, but an understanding is critical to guide our interventions.
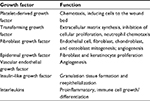  | Table 1 Important growth factors and their principle responsibilities |
After trauma and injury to blood vessels, intracellular calcium and tissue factor trigger the coagulation cascade, which then activates factor VII, a fibrin plug is formed, and vasoconstriction facilitates hemostasis. Platelets collect and release and activate proinflammatory adenosine diphosphate, tissue growth factor, and platelet-derived growth factors, which leads to the chemotaxis of neutrophils and fibroblasts. Polymorphonucleocytes then predominate, making use of inflammatory mediators and oxygen free radicals to suppress bacterial invasion. After the first day, circulating monocytes transition to macrophages in the wound, releasing interleukin 1 and fibroblast growth factor, further exacerbating inflammation, promoting angiogenesis, and stimulating further chemotaxis of fibroblasts, keratinocytes, and endothelial cells.4,5
During the proliferative phase, fibroblasts synthesize collagen and ground substance. They then cross-link and organize collagen molecules, contributing to wound tensile strength. Keratinocytes and endothelial cells expand during angiogenesis, as intact vessels generate buds in granulation tissue, thus supplying advancing fibroblasts with stimulatory growth factors. This granulation tissue is obvious in many wound evaluations, and is composed of ground substance, collagen, and capillaries. Epithelial cells then migrate in from the wound edge until the wound is “closed”. At this point, contact inhibition induces transformation of fibroblasts into myofibroblasts, which contain actin fibers, responsible for wound contraction. Collagen is remodeled in the maturation phase to increase wound tensile strength, until wound strength approaches 80% of its premorbid strength at about a year. Type 1 collagen continues to replace type III until the normal skin ratio of 4:1 is achieved.4,5 Figures 1 and 2 summarize the fundamental phases of wound healing.
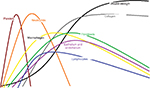  | Figure 1 Dominant cellular components in the healing process. Note: X-axis: time to 1 year; Y-axis: volume/quantity. |
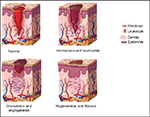  | Figure 2 Summary of prominent wound-healing processes, clockwise after “trauma”. |
Chronic wounds occur when this usually orderly process is impaired in some way. It is the wound practitioner’s role to make a thorough assessment of the wound, preferably to expedite acute wound healing, or to intervene to correct factors that threaten to undermine this.
Wound assessment
The distinction between acute and chronic wounds is probably unrealistic and simplistic. Chronic wounds have traditionally been differentiated from acute wounds based on time: 1 month, 6 weeks, and 3 months are variably quoted. Not all problem wounds are chronic, however, and many acute wounds are complex and challenging in nature, meriting intense intervention from the outset. In addition, some wounds that meet criteria for “acute” from the point of view of time frame may have features in keeping with traditional descriptions of “chronic”. The relatively new concept and understanding of “biofilm”, for example, now characterize and influence chronic wound management, although acute wounds are also frequently afflicted.
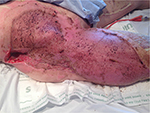
One might suggest that it is a failure of a burn unit to be managing chronic burn wounds, especially when these may have been acute wounds at the beginning of their care or on admission. However, it would be naïve to suggest that all deep burn wounds heal completely within a month, that burn surgeons debride and graft every deep partial-thickness burn, or even that they regraft all areas where there is graft loss. It has been estimated that 20% of patients optimally managed in a burn center are discharged from the burn unit with unhealed areas. Ninety percent of these go on to heal their wounds within 2 weeks of outpatient follow-up care. The remaining 10% (or 2% of all patients admitted to the burn center) have wound care requirements extending beyond this period and are managed on an ambulatory basis. These proportions are potentially larger in other groups of patients managed on the burn unit, those without burn injuries, although these groups have not been well studied and their heterogeneity does not permit comparison across regions and countries. These statistics also do not reflect those patients who have complete or >95% complete healing, and then develop, for instance, breakdown of contractures during therapy, or after reconstructive surgery, successful or otherwise. Figures 3–5 demonstrate three cases where grafting was performed in the acute setting, but where a sizeable area still required wound care. Patients may also develop the so-called chronic wounds while receiving care for another wound condition. Pressure ulcers are an example of such a complication, and have long been the “flag bearer” of the chronic wound in the paraplegic, the insensate, or immobilized patient, as much as the venous stasis ulcer is in the elderly patient with varicosities.
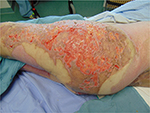  | Figure 3 Graft loss where regrafting was offered. |
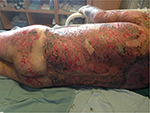  | Figure 4 Graft loss on the back after major burn injury. Note: Healing occurred subsequently using Prontosan gel and occasional silver nitrate applications to address overgranulation. |
Diabetes mellitus remains a prominent etiological factor in the development of lower limb “chronic wounds” but is also implicated in the aggravation of wound-healing difficulties in all categories of patients. Glycosylation in diabetes mellitus impairs neutrophil and macrophage phagocytosis of bacteria, prolonging the inflammatory phase. The proliferative phase is also protracted in the same disease as erythrocytes become less pliable and are less able to deliver oxygen to the wound for tissue metabolism and collagen synthesis. A substantial proportion of patients with burn injuries develop glucose intolerance, and antiglycemic agents are now routinely advocated for these individuals to optimize wound healing and recovery.6
Autograft loss is a common feature and complication of burn surgery. Up to 10% of loss may be deemed acceptable, especially in high-volume centers with limited operating time. The use of allograft to stage wound closure may reduce the likelihood of autograft loss.7 Excessive use is needlessly expensive, however, and commits patients to additional anesthetics and potentially longer hospital stays. Careful patient selection is necessary for the use of this valuable resource, and surgeons and nurses should constantly reassess the methods they employ intra- and perioperatively to optimize stable graft “take”. Standardized wound closure strategies may reduce the risk of graft loss, which may include methods of accurately assessing wound bed viability, hemostasis, securing and bolstering autografts, the optimal use of NPWT, and logical evidence-based immobilization principles (eg, splinting of extremities to prevent shear). Unfortunately, anecdotal evidence and personal preferences continue to dominate practice, as is the case with most areas of wound care.
Successful wound practitioners generally approach wounds systematically, addressing each of patient factors and wound factors sequentially. The evaluation begins with a thorough history and examination, followed by directed special investigative modalities. There is increasing awareness that genetics (and skin type) is the major factor determining the development of keloids, and to a lesser extent, hypertrophic scars. These problematic scars dominate the lives of predisposed individuals for years to come, and therapeutic modalities have, up to date, failed to offer substantial benefit. Keloids, prevalent in black populations especially, are often neither preventable nor treatable, while hypertrophic scars may be prevented by prompt wound closure by appropriate means (meticulous surgical technique if necessary), and attention to postoperative taping, silicone sheeting, and intralesional injections of steroid, or other modalities.8 Other hereditary conditions also predispose to delayed wound healing, notably abnormalities in collagen and elastin production which interfere with skin fragility and laxity. Fortunately, these conditions are uncommon, but they may require considerable resources to address effectively.
Age is perhaps the single most important irreversible systemic factor determining wound healing. The elderly have, over and above their predisposition for other independent factors that delay wound healing (eg, ischemia and infection), impaired production of growth factors, matrix molecules, and collagen, and increased susceptibility to hypoxia. Collagen fibril cross-linking requires oxygen to hydroxylate proline and lysine. Hypoxia also compromises oxidative phosphorylation, critical to bacterial killing. Certain correctable factors should be addressed if time permits. Smoking, for instance, causes vasoconstriction and increases platelet adherence. Angioplasty or arterial bypass grafting may be required, or medical management for cardiac failure or hypertension. Venous stasis or lymphatic insufficiency may be improved with compressive garments. Other common and important systemic comorbidities that require consideration include immunosuppression (eg, HIV, chemotherapy, and steroid use). Local effects of radiation may also be particularly severe on attempts to heal wounds locally, sometimes warranting fat grafting, for instance, to optimize wound healing.
Malnutrition results in diminished fibroblast proliferation, impaired neovascularization, and decreased immunity, and replacement is generally protocolized in a burn unit setting, often finding its way onto standard admission proformas even before a thorough dietician’s assessment (which should also occur as standard practice). Burn wounds impose considerable metabolic demands, particularly within granulation tissue. Amino acids are essential for normal cell function and the repair of cutaneous wounds. Fatty acids are substrates for inflammatory mediators such as eicosanoids and constitute cell membranes. Vitamin C, copper, and iron are required for the hydroxylation of the amino acids lysine and proline, which cross-link and stabilize collagen. Vitamins A and E are also fundamental to skin healing.
Our supplementation strategy/antioxidant protocol is represented in Figure 6. Besides premorbid deficiencies associated with westernized dietary habits, alcohol, and drug abuse, a major challenge in burn centers is the underfeeding that can result when patients are kept nil per os for prolonged periods while awaiting surgical interventions or procedures. It is our policy for patients undergoing surgery, with an endotracheal tube in situ, not to have feeds withheld. The majority of critically ill patients will also have a nasojejenal fine bore feeding tube placed under radiological guidance. Our nutrition team will assess each patient and make a recommendation in terms of volume, appropriate route, and type of feed and our nurses will institute a volume-based feeding protocol. This method aims to compensate for periods when feed was withheld, as is often the case in the operating room, and/or when the patient is positioned prone, for instance.9
  | Figure 6 The antioxidant proforma for patients with complex wounds and burns. |
A local wound examination should complete a thorough evaluation and relates to wound characteristics themselves (size, shape, location, extent, structures exposed or injured), the presence or absence of infection or inflammation, the leading wound edge if there is one, the moisture in the wound, and an evaluation of the depth of the wound (Figure 7). In addition to a vascular assessment, a neurological examination should also be performed, if necessary by a consultant specialist.
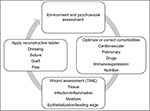  | Figure 7 Outline of structured approach to wound assessment and management. |
Standard laboratory studies that are warranted include a full blood count including differential, renal function tests including electrolytes, urea, and creatinine, evaluation of random and fasting glucose and hemoglobin A1C, liver function tests including both albumin and prealbumin, and evaluation of inflammatory markers including erythrocyte sedimentation rate and c-reactive protein. Chest radiography, echo- and electrocardiograms, and pulmonary function tests are also all justified and may assist in optimizing the patient medically. Other investigative diagnostic modalities may be required, including other plain films, ultrasound, computed tomography, magnetic resonance imaging and angiograms, Doppler studies, ankle brachial indices, transcutaneous PO2, and quantitative cultures and biopsies.
Evaluating wound exudate
Attentive wound practitioners will routinely evaluate a wound’s exudate prior to each new dressing application. Significant changes in volume, color, or consistency of the exudate may herald a change in the wound environment as occurs with infection. Increases in volume may also be a result of prolonged leg dependence or absent compression bandaging. Well known to burn surgeons is the characteristic fishy odor and green exudate of Pseudomonas aeruginosa colonization, which may encourage one to use a more desiccating antiseptic soak (eg, acetic acid 0.5% for a short period). Many dressings handle fluid by absorbing it and/or by allowing it to evaporate. Certain dressings will also sequestrate or retain fluid. Cottons, foams, and sponges hold fluid within their structure. Hydrocolloids are more interactive, taking up fluid and forming a gel. Certain dressings manage infection by deposition of the antimicrobial or antiseptic substance onto the wounds, whereas others “wick” fluid away and address the bacteria within the dressing.
A greater understanding of the balance of pro- and anti-inflammatory mediators, autologous cytokines, and enzymes in the exudate, and how to influence them, may prove increasingly fruitful. Matrix metalloproteinases appear to play a regulatory role involving growth factors and are increased in chronic ulcers. Widgerow et al, for instance, proposed that considerable information may be obtained from examining the cellular, protein, and mediator composition of exudates, which has the potential to impact on our understanding of the wound mileau and consequently decisions about the timing of surgery, and how to facilitate healing and ameliorate scarring.10
Wound infection, prevention, and control
Fortunately, most acute wounds, despite being colonized by bacteria, are able to resist invasive infection, and durable functional integrity is restored within a few weeks. When patients are at risk, or if consistent logical management is not adhered to, they are more likely to manifest wound infection (defined as a concentration of 105 organisms per gram of tissue), which remains the most significant cause of mortality and morbidity in the context of acute burns, and is also the single most important local reason for the development of a chronic wound.
Cellulitis, for instance, commonly witnessed in the context of the unexcised deep partial-thickness acute burn wound, prolongs the inflammatory phase by maintaining high levels of proinflammatory cytokines and tissue proteases, which degrade granulation tissue and delay collagen deposition. Debridement is the most efficacious means of addressing infection and can be achieved mechanically (with surgery) or chemically (with selected dressings). Debridement removes devitalized tissue, a source of endotoxins that inhibit migration of fibroblasts and keratinocytes into the wound. The organisms infecting acute wounds tend to be more susceptible to therapy, both surgical and medical, despite their potential impact on efforts to close wounds with grafts or flaps. Chronic wounds are often colonized by more resistant organisms, and surgeons justifiably tend to be more hesitant to attempt surgical wound closure. One approach we often employ is to “convert a chronic wound to an acute wound” by surgical excision, or to apply NPWT (often with instillation of antiseptics shortly after it), before staging closure with allograft, and then autograft.
Staphylococcus aureus remains a leading culprit of soft tissue infections, and its methicillin-resistant strains are becoming more prevalent, placing a tremendous strain on health care resources. In the context of the burn unit, several other organisms including Gram-negative bacteria such as P. aeruginosa may also be at play and are frequently implicated in the development of biofilm-related infections. Biofilm is a complex community or network (extracellular polymeric slime) created by bacteria following attachment to a surface. Its development has been shown to protect bacteria from antibiotics, the immune system, and topical antiseptics. It also enables bacterial species to maintain an inflammatory state, which improves supply of nutrients, and also permits multiple species to coexist, share genetic information, as well as to disseminate and colonize new surfaces by seeding off planktonic bacteria. Biofilm-related infections are difficult to confirm using traditional techniques and require multipronged strategies to address.11
Intuitively, we should resist the use of systemic antimicrobials where topical therapy is possible. Topical agents target the therapy more precisely and avoid some of the problems with resistance. Antimicrobial products may be applied to the full spectrum of dressing modalities (films, gauze, hydrogel, hydrocolloids, hydrofibers, and foams), therefore allowing us to adjust this according to the comprehensive wound assessment.
Topical antiseptics
Antiseptic soaks are a subgroup of dressings that inhibit or destroy the growth of microorganisms and are in practice usually a daily or a twice-daily solution applied to a gauze medium and then to the wound, often with a nonadherent interface dressing. All of these are then covered by a secondary absorbent layer and secured in place. With the advent of negative-pressure dressings, these antiseptic soaks can now be applied to the wound via the device, without regularly changing the interface or filling medium. The most commonly used agents are summarized in Table 2.
  | Table 2 Agents in common use at the RTBC Note: aBiocompatibility index for selected antiseptic substances after 30-minute contact in minimal essential medium cell culture in the presence of 10% fetal bovine serum (L929 cells/Staphylococcus aureus).13 N/A indicates those not tested in referenced study. Data from Hirsch et al.15 Abbreviations: RTBC, Ross Tilley Burn Centre; MRSA, methicillin-resistant S. aureus; N/A, not available; -, not applicable. |
The main rationale for using antiseptics is to increase the rate of the healing process. Microbial pathogens delay wound healing by producing inflammatory mediators and metabolic toxins and by maintaining an activated local and systemic neutrophil population, which in turn produce cytolytic enzymes and oxygen free radicals. This prolonged inflammatory response contributes to further host injury. Bacteria also compete with host cells for nutrients and oxygen. Wound infection may contribute to tissue hypoxia, render the granulation tissue hemorrhagic and fragile, reduce fibroblast number and collagen production, and impair reepithelization. Clearly addressing infection, as well as bacterial load, is critical and as important as facilitating healing by other means.
It is important to be aware of the toxic effects of these antiseptic dressings and soaks on normal and healing cells, as it is to know about their antibacterial efficacy. Alexander Fleming noted a century ago that “Antiseptics will only exercise a beneficial effect in a septic wound if they possess the property of stimulating or conserving the natural defensive mechanism of the body against infection”.12 A recent quality improvement initiative at our burn center has reduced the concentration of pharmacy-supplied sodium hypochlorite from 0.5% (Dakin’s solution) to 0.05% for this reason, and while this is potentially an important step, it is recognized that even 0.005% is likely as effective an antiseptic while being still less cytotoxic.13,14 Frequently, the concentration of the agent is all that requires adaptation, as was demonstrated with sodium hypochlorite.
This concept is referred to as biocompatibility (also referred to as bio-“tolerability”), and an index (biocompatibility index) is obtained from the quotient of LC50, the molar concentration at which 50% of the cells subjected to cytotoxicity test are no longer vital, and the molar concentration that in the quantitative suspension test against bacterial test microorganisms results in a reduction of at least 3 log steps. A value of >1 describes good tolerability, while a value <1, poor microbial killing combined with high cytotoxicity.15 Hirsch et al studied commonly used topical antiseptics, to determine their relative efficacy in terms of minimal inhibitory concentrations and their tissue tolerability. Prontosan (B. Braun, Melsungen, Germany) (discussed later), a polyhexamethylene biguanide (PHMB) product available commercially, for instance, demonstrated excellent efficacy at all concentrations of the “generic” tested (1%–20%), while others required higher concentrations to completely inhibit growth. Povidone iodine products required concentrations in the region of 7.5%–10% to similarly combat P. aeruginosa, and somewhat lower concentrations against Enterococcus faecalis and S. aureus.15,16
Langer et al have demonstrated that cell microcirculation is also impaired by many of these antiseptic dressings and solutions, resulting in disordered blood vessel leakage, capillary functional densities, and red cell velocity, which may again have profound impact on wound healing.17 Literature is not widely discussed nor evaluated in the wound practice environment, as much appears contradictory and confusing, particularly with respect to some of the newer wound care products (eg, silver and cadexemer iodine-based products). These, while effectively addressing or preventing wound infection and inflammation, have less clear data on relative cytotoxicity. This has obviously been influenced by the strong financial incentives of industry in question. For instance, povidone iodine has been approved by the US Food and Drug Administration for short-term treatment of superficial and acute wounds but is noted in the literature to both promote and inhibit wound healing. Many wound care practitioners maintain stringent bias against certain products but are quite happy to utilize others, oftentimes agents with aggravated cytotoxic effects. Diluting some of the products may not actually be possible, or leave them less efficacious against bacteria, hence requiring more care in their use, and perhaps using them more sparingly than is currently the case.
Prontosan
Prontosan (B. Braun) wound irrigation solution and wound gel are composed of purified water, PHMB 0.1%, and Betaine 0.1%. The PHMB is similar to naturally occurring broad-spectrum antimicrobial peptides able to compromise the integrity of the lipopolysaccharide layer of the bacterial cell wall, with minimal effect on the neutral lipids in human cell membranes. Betaine is a surfactant, which lowers surface tension, with both hydrophilic and hydrophobic heads. This enables Prontosan (B. Braun) to be an effective irrigating fluid by being partly in solution, while the hydrophobic end out of solution adheres to necrotic tissue, foreign material, and debris, allowing them to be flushed away. Betaine has also been shown to effectively influence the communication within biofilm colonies (referred to as quorum sensing), by interfering with homoserine lactone manufacture.18,19
Several studies have demonstrated Prontosan’s (B. Braun) in vitro and in vivo wound healing and antiseptic efficacy; to date, resistance is not described, and it is distinguished from other antiseptics in having a very favorable biocompatibility index. It also has a low risk of contact sensitivity, and is sterile and odorless, and both the solution (for initial wound cleansing or as a soak) and gel (as a daily hydrogel dressing) are very versatile, easy to apply, and can be used with other dressing modalities. The gel can also be utilized for prolonged periods with minimal pain on application or removal. All of these characteristics make it a suitably benign yet efficacious antiseptic agent for any wound, although its impact on biofilm makes it the ideal agent for facilitating moist wound healing in chronic wounds. Of note, this agent has become the first-line instillation solution we use with NPWT.18,19
Further experimental and clinical data are required to support the discontinuation of other antiseptics altogether, though. One concern with doing this is of course the theoretical development of resistance to an agent used exclusively. At the present time, for an established infection, it is logical to alternate suitable agents wherever possible but to use the lowest effective concentration, where they are known to be cytotoxic. Such a strategy is outlined in the protocol (Table 3).
  | Table 3 Summary of protocol for the use of antiseptic soaks for infected wounds at Ross Tilley Burn Centre Abbreviation: BI, biocompatibility index. |
Silver
Silver has a long history in the context of wound care and especially burns. Silver sulfadiazine, for instance, has been in use for >50 years. It deposits a substantial quantity of silver onto the wound surface at once, and while in favor internationally due to its low cost, care is compromised by the formation of a pseudoeschar and the need for twice-daily applications. Silver nitrate is now sparingly used as a solution due to concerns around cytotoxicity, but silver nitrate sticks are efficacious and are in common use for suppressing overgranulation to facilitate more rapid epithelialization. Most silver products have been shown to be cytotoxic in vitro.20,21
Nevertheless, there are umpteen commercially available silver dressings, with representatives from each dressing category. The most popular silver dressing in our facility is currently Acticoat (Smith and Nephew, Hull, UK), which is advertised to have both antimicrobial and anti-inflammatory properties. It has the added capacity to release nanocrystalline silver over a prolonged period of time, with either 3- or 7-day versions available. This delayed deposition contributes to the dressing’s efficacy, as Ag readily binds to Cl ions present in exudate to form AgCl, thus deactivating it; it may also contribute to it being somewhat less cytotoxic than more traditional silver dressings. Silver exhibits three oxidation states (Ag+, Ag2+, and Ag3+), the Ag+ being the only species stable enough for use as an antibiotic (the others are highly reactive and short living). Few cases of resistance have been described, probably on account of the multipronged mechanisms of action, including:
- Inhibition of electron transport system/respiratory chain in bacteria
- Interaction and rupture of the cell membrane and cell wall
- Interference with bacterial cell DNA
- Silver free radical production, using all the mechanisms mentioned20,21
Acticoat (Smith and Nephew) is principally used with prophylactic indications in this burn center, considerably reducing the number of dressings while awaiting wound evolution, or over nonadherent dressings after skin grafting. Clinicians generally do not consider its use to demonstrably compromise wound healing. In light of some isolated reports of silver resistance, however, particularly in the context of Pseudomonas and polymicrobial infections, it is probably sensible to review our indications and unrestrained use of silver, as well as to more accurately evaluate its biocompatibility, and the optimal silver elution by parts per million (analogous to concentration when comparing it to antiseptic solutions).
Negative-pressure wound therapy
NPWT has been in use for over 30 years, probably first described in the Russian literature in the 1970s, characterized by Chariker in 1989 and popularized (and novel components patented) by Argenta and Morykwas in the 1990s. NPWT’s popularity (and the accompanying anecdotal evidence) has been so overwhelming that it has exceeded our ability to produce lock-step quality randomized trials to evaluate its efficacy; some investigators consider it now unethical to randomize patients to a control group.3,22
NPWT has really come into its own in its ability to prepare a wound bed for grafts or flaps or delayed primary or even secondary closure, by stimulating granulation tissue, applying mechanical forces both via micro- and macrostrain, by maintaining a closed environment, and by managing moisture and exudate. For many years, practitioners have injected antiseptics into their negative-pressure devices, and now, this is also available commercially so that dressing changes are performed less frequently, thus reducing operative visits, pain, and undue tissue trauma, exposure, and desiccation. NPWT is also an excellent bolster over skin grafts, both split and full-thickness, where the processes of plasmatic imbibition and inosculation are reliant on the absence of shear, absent underlying fluid collection, and constant uniform pressure to initiate the process of graft take.
Negative pressure has also shown considerable promise on closed incisions and suture lines. In the context of a chronic or a so-called “at risk” incision or wound that has required surgical closure, application of this technique may contribute to durable wound closure by stabilizing the suture line until maximal strength is achieved. There is also some evidence for reductions in pain and swelling with this approach (DA Hudson, personal communication, January, 2014).
The dressing in direct contact with the wound is referred to as the interface, and nonadherent dressings are often preferred, which may have antimicrobial properties. The interface may also be the filler. Most studies favor either gauze or foam as the filler. There has been unequivocal bias when manufacturers are involved.3 Experimental studies indicate that pressure is equally well distributed through foam as it is through gauze and both effectively drain harmful metalloproteinases and proteolytic enzymes from the wound. Placing an interface beneath sponge may reduce the ingrowth of granulation into the foam and also the pain experienced when sponges are removed. Malmsjo et al have demonstrated work showing that both fillers, gauze (often Kerlix AMD; Covidien, Dublin, Ireland), and foam, cause a mechanical effect on the wound, as well as stimulating granulation. Many in clinical practice have noticed that foam stimulates considerably more granulation (and consequently more hypertrophic scarring) than gauze-based filler systems (which might in reality be denser).23,24
Gauze probably conforms better to undulating or irregular cavity type wounds, thus obtaining greater contact to all areas where the NPWT effect is desired. It also does not adhere as much to the wound surface, and ingrowth may be reduced. We have also found it useful to apply a rim of gauze, which is hydrophilic, to dependent parts of a challenging foam NPWT (for instance, when a circumferential pelvic or trunk NPWT is applied), to manage any exudate that may drain and disturb the adherence of the film seal, on application. Gauze may also be applied to complement foam fillers, which is inherently less conformable. In addition, we have found that using several layers of Kerlix AMD (Covidien) (a roll of antimicrobial gauze) is extremely useful around hands or feet, especially after sheet grafting. This NPWT doubles as a splint, as the hand can be placed in a more precise functional position (a slightly extended wrist, extended interphalangeal joints, and flexed metacarpophalangeal joints) on application of the pressure, especially on the small hands of children.25
Kairinos et al demonstrated that NPWT always applies a positive pressure to the wound bed. The exact pressure experienced depends on the negative pressure delivered, the nature of the wound, and its application (whether circumferential, a cavity, or a surface). Clinical judgment should therefore dictate the pressure settings applied, as certain patients’ circulation may be impaired. Circumferential negative-pressure dressings should therefore be regarded as relatively contraindicated in at-risk patients.26–32
Morykwas and Argenta’s interpretation of laser Doppler (and the law of continuity) suggested that NPWT created an increase in perfusion in the wound, thus stimulating healing. Thermography and other studies have refuted this argument, showing that in fact, due to the positive pressure applied to the wound surface, perfusion is actually reduced. This initial relative ischemia, when the pressure is applied, stimulates the release of growth factors and other vasoactive agents, hence resulting in increased granulation routinely seen clinically.25–31 This is most evident with the use of intermittent or variable settings, where demonstrably, more granulation is stimulated than that which occurs with the continuous setting.23 Laser Doppler is flawed as a measure of perfusion because perfusion is dependent on both velocity and blood vessel diameter. While their theory would have been true if the vessel had remained constant in diameter, we know that positive pressure applied to a vessel will reduce its diameter and therefore increase its velocity, falsely presenting (to laser Doppler) as an increase in perfusion. This flaw is further exposed when attempting to use laser Doppler to test “perfusion” to skin when a weight is applied to it. Intuition will tell you that perfusion should be reduced to skin under a weight, but the laser Doppler will suggest an increase in perfusion.26–32
Why was –125 mmHg chosen as the proposed ideal setting? This was likely the most negative pressure applied to a wound to give the highest velocity before the underlying vessels were actually occluded by positive pressure. This is similar to what is observed when you spray water from a hose pipe and apply your thumb to the end with progressively greater force until you completely occlude the end: you will note that the velocity increases until it suddenly stops altogether.26–32
These truths have clinical relevance, as manufacturers recommend reducing the NPWT in response to the occasional clinical occurrence of bleeding under the NPWT. In the face of bleeding in other clinical scenarios (eg, a laceration), we would always apply positive, not negative pressure. We would advocate that a more logical response to obtain hemostasis might therefore be to increase the NPWT setting, to instill a dilute vasoconstricting solution, or to remove the dressing completely if the bleeding is excessive. If the output is frank blood in significant volume (as against a mixture with exudate or tumescence as is often the case in the context of the major burn), we would prefer to remove the dressing in order to address the source of bleeding.
Growth factors and stem cell applications
Plastic surgeons have long been injecting fat grafts, often derived from liposuction aspirates, for lipo-filling, to augment or contour defects, for both reconstructive and esthetic indications. The recognition that lipoaspirate contains stem cells was made in 2001, now referred to as adipose-derived stem cells (ADSCs). This holds considerable promise in the context of chronic wounds and aims to improve the durability, contour, and pliability of wound closures, including wounds closed by secondary intention, by grafts, flaps, or sutures. Various clinical trials have since also shown the regenerative capability of ADSCs in other clinical spheres.33–35
It is believed that providing injured tissues and wounds with multipotent stem cells augments the secretion of numerous growth factors and cytokines, thereby exerting an influence over wound healing and scarring. Some of these factors include epidermal growth factor, tumor necrosis factor-α, fibroblast growth factor, keratinocyte growth factor, transforming growth factor-β1, vascular endothelial growth factor, interleukins, and platelet-derived growth factor.33–35 A recent evaluation using a pig model showed significant benefit with the addition of epidermal growth factor to the antiseptic topical agents commonly used in the acute treatment of burn injuries.36
We need to determine the optimal timing, origin, volume, and vehicle required to realize the potential of ADSCs. At present, though, it appears that injection of lipoaspirates into a wound or scar is an appropriate approach, although it does need to be performed serially because as much as 50% of each injection will be resorbed. It is our preference at this time to allow separation of the fractions of lipoaspirate by standing, rather than by centrifugation, to derive the ADSC-rich stromal vascular fraction. Expansion of ADSC populations in culture has the capacity to yield hundreds of times more progenitor cells than isolation from bone marrow, and owing to its availability in many plastic surgical procedures, will likely remain the gold standard for years to come.33–35
Conclusion
Wound healing is usually a logical physiological process, which may be facilitated or impaired by patient factors and our interventions. A thorough patient and wound assessment usually allows us to identify and address the etiology of the chronic wound. Understanding the modalities at our disposal is critical to implementation of a successful strategy; these include the logical and timely use of biocompatible topical antiseptic agents, NPWT, and carefully executed surgical interventions, making use of the reconstructive ladder as required. Clinicians engaged in the practice of wound care require clarity on the optimal timing, source, volume, and medium to apply growth factor and stem cell therapies to facilitate rapid and durable wound closure without hypertrophy and contracture. Burn centers, with the advantages of a multidisciplinary involvement, critical care resources, access to surgery, and modern dressings, are well placed to take ownership of the relatively neglected area of chronic and complex wound care.
Disclosure
The authors report no conflicts of interest in this work.
References
Rogers AD. Comments regarding ‘The inadequacy of wound management training for medical professionals’. Wound Healing Southern Africa. 2013;6(2):98. | ||
Rogers AD, dos Passos G, Hudson DA. The scope of plastic surgery. S Afr J Surg. 2013;51(3):106–109 | ||
Kairinos N, Pillay K, Solomons M, Hudson DA, Kahn D. The influence manufacturers have on negative-pressure wound therapy research. Plast Reconstr Surg. 2014;133(5):1178–1183. | ||
Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2014;133(2):199e–207e. | ||
Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012;39(1):85–97. | ||
Jeschke MG. Clinical review: glucose control in severely burned patients. Crit Care. 2013;17(4):232. | ||
Rogers AD. Cadaver skin use for burns and complex wounds. Wound Healing Southern Africa. 2013;6(2):54–55. | ||
Gauglitz G. Management of keloids and hypertrophic scars: current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–114. | ||
Hall KL, Shahrokhi S, Jeschke MG. Enteral nutrition support in burn care: a review of current recommendations as instituted in the Ross Tilley Burn Centre. Nutrients. 2012;4(11):1554–1565. | ||
Widgerow AD, King K, Tocco-Tussardi I, et al. The burn wound exudate-an under-utilized resource. Burns. 2015;41(1):11–17. | ||
Rogers AD, Hudson DA. Ten principles of preventing biofilm in surgical practice. S Afr Med J. 2013;103(10):701. | ||
Fleming A. The action of physiological antiseptics in a septic wound. Br J Surg. 1919;7:99–129. | ||
Coetzee E, Rode H, Kahn D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg. 2013;51(2):50–53. | ||
Coetzee E, Whitelaw A, Kahn D, Rode H. The use of topical, un-buffered sodium hypochlorite in the management of burn wound infections. Burns. 2012;38(4):529–533. | ||
Hirsch T, Scipp H-M, Jacobsen F, et al. Antiseptics in surgery. ePlasty. 2010;10:e39. | ||
Paddle-Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg. 2006;117(7 Suppl):110S–118S; discussion 119S–120S. | ||
Langer S, Sedigh Salekdeh M, Goertz O, et al. The impact of topical antiseptics on skin microcirculation. Eur J Med Res. 2004;9:449–454. | ||
Eberlein T, Assadian O. Clinical use of polyhexanide on acute and chronic wounds for antisepsis and decontamination. Skin Pharmacol Physiol. 2010;23(Suppl 1):45–51. | ||
Bradbury S, Fletcher J. Prontosan made easy. Wounds Int. 2011;2(2):1–6. | ||
Burd A, Kwok CH, Hung SC, et al. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen. 2007;15(1):94–104. | ||
Hartmann CA, Rode H, Kramer B. Acticoat stimulates inflammation, but does not delay healing, in acute full-thickness excisional wounds. Int Wound J. 2015. doi:10.1111/iwj.12525. | ||
Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563–576; discussion 577. | ||
Malmsjö M, Gustafsson L, Lindstedt S, Gesslein B, Ingemansson R. The effects of variable, intermittent, and continuous negative pressure wound therapy, using foam or gauze, on wound contraction, granulation tissue formation, and ingrowth into the wound filler. ePlasty. 2012;12:e5. | ||
Birke-Sorensen H, Malmsjo M, Rome P, et al. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)–steps towards an international consensus. J Plast Reconstr Aesthet Surg. 2011;64 Suppl:S1–S16. | ||
Kairinos N, Hudson DA. The ‘vacsplint’ for hands. J Plast Reconstr Aesthet Surg. 2010;63(4):e425. | ||
Kairinos N, McKune A, Solomons M, Hudson DA, Kahn D. The flaws of laser Doppler in negative-pressure wound therapy research. Wound Repair Regen. 2014;22(3):424–429. | ||
Kairinos N, Holmes WJ, Solomons M, Hudson DA, Kahn D. Does a zone of increased perfusion exist around negative-pressure dressings? Plast Reconstr Surg. 2013;132(4):978–987. | ||
Kairinos N, Hudson DA, Solomons M. The influence of different sizes and types of wound fillers on wound contraction and tissue pressure during negative pressure wound therapy. Int Wound J. 2011;8(6):656–657. | ||
Kairinos N, Hudson D, Solomons M. Depth of penetration of negative pressure wound therapy into underlying tissues. Wound Repair Regen. 2009;17(3):456. | ||
Kairinos N, Voogd AM, Botha PH, et al. Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg. 2009;123(2):601–612. | ||
Kairinos N, Solomons M, Hudson DA. Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg. 2009;123(2):589–598; discussion 599–600. | ||
Kairinos N, Solomons M, Hudson DA. The paradox of negative pressure wound therapy–in vitro studies. J Plast Reconstr Aesthet Surg. 2010;63(1):174–179. | ||
Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2008;35(2):171–180. | ||
Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121(3):860–877. | ||
Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25(1):73. | ||
Theunissen D, Seymour B, Forder M, Cox SG, Rode H. Measurements in wound healing with observations on the effects of topical agents on full thickness dermal incised wounds. Burns. 2016. doi:10.1016/j.burns.2015.09.014. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.