Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Management of Suspected Life-Threatening Perioperative Anaphylaxis and Risk Factors for Near-Fatal and Fatal Outcomes: A Retrospective Study in China
Authors Cai H , Liu X , Wang D, Li W, Ma H, Zhao J
Received 21 February 2023
Accepted for publication 28 April 2023
Published 10 May 2023 Volume 2023:19 Pages 383—394
DOI https://doi.org/10.2147/TCRM.S406515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Huamei Cai,1,2,* Xiaowen Liu,1,* Dingyi Wang,3,4 Weixia Li,1 Hongli Ma,1,2 Jing Zhao1,2
1Department of Anesthesiology, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 2Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 3National Center for Respiratory Medicine & National Clinical Research Center for Respiratory Diseases, Institute of Respiratory Medicine & Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Department of Clinical Research and Data Management Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Zhao, Department of Anesthesiology, China- Japan Friendship Hospital, No. 2 Yinghua East Road, Beijing, 100029, People’s Republic of China, Tel +86 010 8420 5876, Email [email protected]
Purpose: Perioperative anaphylaxis (POA) is an acute severe systemic hypersensitivity reaction characterized by life-threatening respiratory and circulatory collapse. In our previous study, we reported the epidemiology of suspected POA in China. In the present study, we aimed to elucidate the management and outcomes of these cases and further verify the risk factors for near-fatal and fatal outcomes.
Patients and Methods: This was a retrospective study of 447 cases of suspected life-threatening POA encountered at 112 tertiary hospitals in mainland China between September 2018 and August 2019. Patient characteristics, symptoms, duration of hypotension, treatments, and clinical outcomes were documented. Bivariate logistic regression was used to identify risk factors for near-fatal and fatal outcomes.
Results: Most cases of suspected POA (89.9%) were recognized and treated within 5 min. Epinephrine was administered as the initial treatment in 232 (51.9%) cases. Corticosteroids (26.6%), other vasoactive drugs (18.3%), and bronchodilators (1.6%) were also administered as the initial treatment instead of epinephrine. The initial dosage of epinephrine (median, 35 μg) was insufficient according to the anaphylaxis guidelines. On multivariable analysis, age ≥ 65 years (odds ratio [OR] 7.48; 95% confidence interval [CI]: 1.33– 41.87, P=0.022), ASA physical status IV (OR 17.68; 95% CI: 4.53-68.94; P< 0.001), and hypotension duration ≥ 15 min (OR 3.63; 95% CI: 1.11– 11.87; P=0.033) were risk factors for fatal and near-fatal outcomes.
Conclusion: Most cases in this study were managed in a timely manner, but the epinephrine application should be optimized according to the guidelines. Age ≥ 65 years, ASA physical status IV, and long-term hypotension were risk factors for near-fatal and fatal outcomes.
Keywords: perioperative anaphylaxis, epinephrine, risk factors, perioperative outcomes
Introduction
Life-threatening perioperative anaphylaxis (POA) is a severe, systemic hypersensitivity reaction that may lead to death during anesthesia.1 Clinical signs of life-threatening POA primarily affect the cardiovascular system (severe hypotension, tachycardia, or cardiac arrest) and respiratory system (bronchospasm, or increased airway pressure in ventilated patients); these symptoms may or may not be accompanied by skin changes.1 The reported incidence of POA varies between countries and ranges from one in 10,000 to one in 20,000.2 According to an epidemiological study, the incidence rate of life-threatening POA in China was 1 in 11,360 which varied significantly by region.3 The mortality rate of severe POA was 2%.3 Many anesthesiologists lack experience in diagnosing and managing life-threatening POA because of the low incidence of severe POA. The National Audit Project (NAP6) study in the UK investigated the management and outcomes of life-threatening POA, leading to an increased focus on this subject.4 Several studies have identified the need to improve the diagnosis of POA, epinephrine administration, and fluid resuscitation.4–8 The management of severe POA is a challenge for anesthesiologists owing to its low incidence and high mortality rate.
Epinephrine is the first-line treatment for anaphylaxis.9,10 Epinephrine, as an alpha-receptor and beta-receptor agonist, reverses peripheral vasodilation, reduces tissue edema, and dilates the bronchial airways instantly by inhibiting the activation of mast cells and suppressing histamine and leukotriene release.11,12 The guidelines recommend early use of intramuscular epinephrine for anaphylaxis.9,10,13 However, in some special circumstances like Grade III or IV POA reaction, a minimum initial dosage of 50 µg intravenous epinephrine is strongly recommended.14–16 Delayed administration of epinephrine in anaphylaxis can aggravate the instability of circulatory and respiratory systems.6,8 The NAP6 research identified that most anesthesiologists referred to the AAGBI guideline for the management of POA, but the management was not satisfactory in terms of epinephrine treatment, cardiac compressions, and fluid resuscitation.17 Similarly, there is considerable variability in China with regard to the recognition of suspected POA, the diagnostic criteria used, and the management owing to the use of different guidelines, such as the Expert Consensus on the Diagnosis and Treatment of Perioperative Anaphylaxis in China, the Resuscitation Council anaphylaxis guideline, AAGBI anaphylaxis guideline, and Advanced Life Support (ALS) cardiac arrest protocol for the diagnosis and management of suspected POA.18 Moreover, no studies have characterized the diagnosis and management of suspected anaphylaxis in China. Thus, we sought to explore the clinical profiles and outcomes of POA in China.
The understanding of fatalities from POA has improved in recent years.19 General anesthetic agents and antibiotics are the most common causative agent in fatal drug anaphylaxis. Risk factors for fatal POA include increased age, high ASA physical status, morbid obesity, asthma, β-blocker and angiotensin-converting enzyme inhibitor use, and cardiac procedure.19–23 The incidence of fatal POA and risk factors for POA in China remain unknown. This nationwide study aimed to research the incidence and risk factors of near-fatal and fatal suspected POA.
Materials and Methods
Study Design, Approval, and Data Source
We conducted a retrospective analysis of data from the nationwide suspected life-threatening POA database that was established by us for our previous epidemiological study. The reference period for the present study was from September 2018 to August 2019. This database includes written and electronic case report forms and anesthesia record forms from 112 tertiary hospitals in mainland China. The study was approved by the Institutional Review Board of China-Japan Friendship Hospital and registered at the Chinese Clinical Trials Registry on September 15, 2019 (ChiCTR1900025956). The requirement for informed consent in the retrospective study was waived by Institutional Review Board of China-Japan Friendship Hospital. All data in the case report forms and anesthesia record forms for this retrospective study are anonymous, and therefore informed consent was unnecessary. The study was conducted in compliance with the Declaration of Helsinki and funded by National Centre for Medical Service Administration, National Health and Family Planning Commission, China (Bureau of Medical Administration [2019] No. 049). STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist for cross-sectional studies was followed for the conduct and reporting of this study.
Definition of Terms
Diagnosis of life-threatening anaphylaxis corresponded to grade III and IV reactions of Ring and Messmer scale,24 which was recommended in several guidelines for classifying the immediate allergic reaction severity. Anaphylaxis was defined using published guidelines. The diagnosis was based on the presence of at least one of the following clinical signs: (1) life-threatening cardiovascular changes, defined as severe hypotension, tachycardia, or cardiac arrest; (2) respiratory symptoms including bronchospasm and airway obstruction in awake patients or increased airway pressures in ventilated patients; and (3) integumentary symptoms such as erythema, urticaria, mucosal changes of angioedema, or edema. Based on the published research and the database used for the present study, we defined long duration of hypotension as absolute systolic blood pressure <80 mmHg for a minimum duration of 15 min,25,26 which was related to the elevated risks of end-organ injury.
Measures
Drug administration and patient characteristics including age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, allergy history, comorbidity, and procedure type were collected from the written and electronic case report forms. The duration of hypotension was also assessed from the anesthesia record forms. Near-fatal and fatal cases were identified and compared with the remaining cases. Near-fatal cases were defined as patients with grade IV reaction of the Ring and Messmer scale (cardiac arrest or respiratory arrest) who did not expire during their hospitalization. Fatal cases included deaths occurring during the hospitalization period.
Statistical Analysis
The EpiData 3.1 (Odense, Denmark) statistical software package was used for database design and data entry. Two data entry clerks who were blinded to the study objective performed data entry. Accuracy was checked by visual inspection of the database and examination of data for unexplained outliers.
All statistical analyses were performed using SPSS Statistics 26.0 (IBM SPSS, Armonk, NY, USA). The normality of distribution of continuous variables was assessed using the Kolmogorov–Smirnov test. Continuous data with a normal distribution were expressed as mean ± standard deviation (SD), and those with a nonnormal distribution were expressed as median (interquartile range, IQR). Dichotomous variables were described as frequency (percentage). Between-group differences with respect to continuous variables were assessed using the independent sample t-test and those with respect to dichotomous variables were assessed using the Pearson chi-square test or Fisher’s exact test. Variables that were statistically different between the near-fatal plus fatal group and the non-near-fatal plus non-fatal group were included in the univariate logistic regression to derive odds ratio (OR) and 95% confidence intervals (95% CI) and multivariable stepwise logistic regression to identify risk factors associated with near-fatal and fatal suspected anaphylaxis. A stepwise framework was adopted in the multivariable regression model with an entry level of 0.10 and a stay level of 0.05 odds ratios and 95% confidence intervals were used to quantify the magnitude and precision of associations. P values less than 0.05 were considered indicative of statistical significance.
Results
Recognition of Suspected Life-Threatening Perioperative Anaphylaxis
A total of 447 cases of suspected life-threatening POA corresponding to Ring and Messmer grades III and IV were enrolled in the study. The clinical suspicion of anaphylaxis was based on a series of suggestive symptoms and the correlation of their onset with potential triggers. In our preceding study,3 hypotension and tachycardia were the most common presenting signs (n=424; 94.9%), while respiratory symptoms manifested in 155 (34.7%) patients and skin symptoms manifested in 306 (68.4%) cases. The time between the recognition of a critical clinical symptom and the initial management for suspected anaphylaxis was within 5 min in 402 (89.9%) cases and within 15 min in 443 (99.1%) cases (Figure 1). Only four patients were administered treatment after 15 min as they all showed skin and mucosal signs with moderate hypotension at first, which aggravated subsequently.
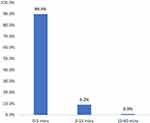 |
Figure 1 Proportion (%) of cases (y-axis) and the time (min) between the diagnosis of perioperative anaphylaxis and initial treatment (x-axis). |
Pharmacological Treatments
Once the patient was suspected to have a perioperative allergic reaction, resuscitation was performed by anesthesiologists in all cases. The first medication administered for relieving the circulatory and respiratory symptoms is shown in Figure 2. Specifically, epinephrine was administered as the first treatment after diagnosis of POA in 232 (51.9%) cases and was administered in 373 (83.4%) cases at any time during resuscitation. Corticosteroids including hydrocortisone, dexamethasone, and methylprednisolone were the first choice in 119 (26.6%) cases and were administered in 398 (89.0%) cases during resuscitation. Other vasoactive drugs such as norepinephrine were administered in 82 (18.3%) cases as the initial treatment. The number of patients who received norepinephrine, phenylephrine, ephedrine, metaraminol, dopamine, and methoxamine as the first treatment was 27 (32.9%), 26 (31.7%), 15 (18.3%), 6 (7.3%), 4 (4.9%), and 4 (4.9%), respectively. Only seven cases (1.6%) were administered bronchodilators as the initial treatment. Other types of vasoactive drugs administered for treatment of suspected POA are shown in Figure 3. In this nationwide study, apart from epinephrine, norepinephrine ranked as the most common vasoactive drug administered to the patients (n=151; 33.8%) during resuscitation, and ephedrine ranked the second (n=60; 13.4%), followed by phenylephrine (n=55; 12.3%), metaraminol (n=20; 4.5%), dopamine (n=14; 3.1%), and methoxamine (n=14; 3.1%). Vasopressor was administered to two patients during cardiac compression and one patient after the onset of persistent severe hypotension. Atropine was administered to 33 (7.4%) patients due to bradycardia.
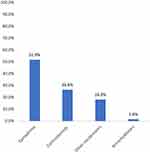 |
Figure 2 Proportion (%) of cases receiving different medicines as the first treatment. |
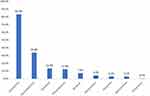 |
Figure 3 Proportion (%) of cases receiving vasoactive drugs. |
Administration of Epinephrine
In the 373 patients receiving epinephrine during resuscitation, the median first dosage and median total dosage of epinephrine were 0.035 mg (IQR 0.010–0.100 mg) and 0.370 mg (IQR 0.068–1.010 mg), respectively. The number of patients who received epinephrine as initial treatment by IV, subcutaneous, IM, and inhalation route was 361 (96.8%), 9 (2.4%), 2 (0.5%), and 1 (0.3%), respectively. Five of the nine patients (55.6%) who were administered subcutaneous epinephrine subsequently received IV epinephrine. Patients who received epinephrine through the inhalation route also subsequently received IV epinephrine. However, the two cases who received IM epinephrine did not require an additional epinephrine dose. We collected 321 cases for whom clear records of the duration of hypotension were available and divided them into two groups: the short-term hypotension group (hypotension duration <15 min) and the long-term hypotension group (hypotension duration ≥15 min). The association between epinephrine administration and hypotension duration is shown in Table 1. There was a significant difference between the two groups with respect to epinephrine administration (121 patients, 90.3% vs 179 patients, 96.8%, P=0.016). The median first dosage of epinephrine was 20 µg and 50 µg, respectively. The median total dosage of epinephrine administered during resuscitation in the long-term hypotension group (506 µg) was notably higher than that in the short-term hypotension group (151 µg). The rate of epinephrine administered as the first-line treatment in the short-term hypotension group was higher than that in the long-term hypotension group (72 patients [53.3%] vs 87 patients [46.8%]), although the difference was not statistically significant (P=0.246). In addition, epinephrine was given timely in the short-term hypotension compared to the long-term hypotension group. The median time to the first dose of epinephrine from the onset of clinical symptoms was 2 min and 4 min in the short-term and long-term hypotension group, respectively (P=0.052).
 |
Table 1 Epinephrine Administration in the Short-Term Hypotension Group and the Long-Term Hypotension Group |
Characteristics of Patients with Fatal and Near-Fatal Suspected Perioperative Anaphylaxis
The characteristics of 447 patients who were categorized into two groups according to fatal or near-fatal outcome are summarized in Table 2. The proportion of elderly patients (≥65 years) in the fatal and the near-fatal suspected POA group was significantly higher than that in the non-fatal and non-near-fatal suspected POA group (77 [18.6%] vs 12 [37.5%], P=0.032). The proportion of patients with ASA physical status III and IV was higher (98 [23.6%] vs 9 [28.1%]; 10 [2.4%] vs 7 [21.9%]; respectively, P<0.001). There was a significant difference between the two groups in terms of the history of drug allergy (58 [14.0%] vs 9 [28.1%], P=0.040), neurological diseases (46 [11.3%] vs 8 [25.0%], P=0.043), cardiovascular diseases (118 [28.6%] vs 15 [46.9%], P=0.030), cardiac procedure (23 [5.5%] vs 5 [15.6%], P=0.041), and long-term hypotension (168 [56.2%] vs 18 [81.8%], P=0.019). The fatal and the near-fatal patients were more likely to receive epinephrine as the first-line treatment compared with the non-fatal and non-near-fatal patients (22 [68.8%] vs 180 [43.4%], P=0.005). In the two groups, either the first dosage (median 200 µg vs 30 µg, P<0.001) or the total dosage (median 3100 µg vs 215 µg, P<0.001) of epinephrine during the resuscitation was higher in the fatal and the near-fatal patients.
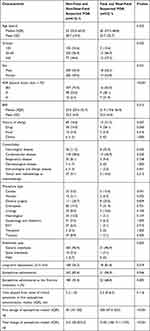 |
Table 2 Characteristics of Patients with Fatal and Near-Fatal Suspected Perioperative Anaphylaxis |
In this cohort, surgeries were performed in 382 (85.5%) patients after the resolution of anaphylaxis but were canceled in 62 patients (13.9%). Although only one patient died in the operation room after resuscitating for nearly 6 h, eight patients died after admission to ICU. The remaining 23 patients were near-fatal cases that corresponded to grade IV reaction of the Ring and Messmer scale (patients with cardiac arrest or respiratory arrest who did not expire during hospitalization). In 8 (25%) of 32 patients, cardiac arrest occurred within 5 min of administration of the suspected culprit drug. The remaining patients experienced cardiac arrest, which was followed by prolonged hypotension and two patients manifested dyspnea at first before anesthesia. Cardiac compressions were performed in 27 of 32 patients. The median time of CPR was 7.5 min (IQR, 2–38.5 min).
Risk Factors for Fatal and Near-Fatal Suspected Perioperative Anaphylaxis
Variables that showed a significant association with a worse outcome on univariate analysis were included in the multivariable analysis (Table 3). Age ≥65 years (OR 7.48; 95% CI: 1.33–41.87, P=0.022), ASA physical status IV (OR 17.68; 95% CI: 4.53-68.74; P<0.001), and long-term hypotension (OR 3.63; 95% CI: 1.11–11.87; P=0.033) showed an increased risk of fatal and near-fatal suspected POA based on the multivariable analysis. Comorbid neurological or cardiovascular disease, undertaking cardiac procedure, and history of drug allergy were associated with higher odds of fatal and near-fatal suspected POA on univariate analysis, but they were not included in the multivariable analysis.
 |
Table 3 Predictors of Fatal and Near-Fatal Anaphylaxis (n=32) |
Discussion
To investigate the management and outcomes of patients with suspected POA, we performed a multicenter retrospective study enrolling 447 cases from 112 tertiary hospitals in mainland China from September 2018 to August 2019. In this study, we described the pharmacological treatment, especially the administration of epinephrine in the management of POA, and analyzed the risk factors for near-fatal and fatal suspected POA. We found that the initial dosage of epinephrine (median dosage: 35 µg) was insufficient according to the guidelines. Compared with non-near-fatal and non-fatal cases, elderly age, ASA physical status IV, and long-term hypotension were risk factors for fatal and near-fatal outcomes. To the best of our knowledge, this is the first nationwide study of the management and outcomes of suspected life-threatening POA in China.
In our study, most cases of suspected POA were recognized and treated within 5 min. Time to initial administration was more than 5 min only in a small proportion of cases (n=45, 10.1%). Difficulties in diagnosis and lack of experience with POA have been shown to be potential reasons for delayed treatment.10,16 Firstly, the reasons for perioperative hypotension may include hemorrhagic shock, vasodilation after neuraxial blockade, and overdose of anesthetic agents. Furthermore, vasodilatory asthma, airway hyperreactivity, and insufficient anesthesia as well as POA can lead to increased airway pressure. Last but not least, since the patients are covered with sterile drapes, it is difficult to identify the erythema and angioedema promptly. Therefore, in patients with isolated skin and mucosal symptoms or moderate hypotension as the first symptom, an allergic reaction is less likely to be considered by anesthesiologists leading to delayed pharmacological treatment. In this study, we also sent out a questionnaire to the anesthesiologists of 112 study centers in this study to assess their awareness of and familiarity with the management guidelines for suspected POA and the administration of epinephrine. To our surprise, 45 (40.1%) anesthesiologists claimed that they were aware but were not familiar with the management of POA. Lack of knowledge and the rare occurrence of POA may result in difficulties in diagnosis and treatment.
Most anesthesiologists managed the critical incident based on the Expert Consensus of Perioperative Anaphylaxis Diagnosis and Treatment in China, 2011, of which the diagnosis criteria of POA are similar to the Ring and Messmer scale.27 Epinephrine and IV fluids are recommended as the first-line treatment in the consensus. The recommended dosage of epinephrine is 30–50 µg IV, repeated every 5–10 min and IV phenylephrine, norepinephrine, vasopressor, and glucagon, when necessary. Bronchodilators, corticosteroids, and antihistamines are also advised in the early stage. In our study, the median first dosage of epinephrine was 35 µg (IQR 10–100 µg), which corresponded to the guideline. However, compared with the suggested first dosage of 50 µg IV epinephrine for life-threatening anaphylaxis in an international consensus,9 the initial dosage of epinephrine administered in this study was lesser. Although most anesthesiologists were aware that epinephrine is the first-line treatment for anaphylaxis, they were reluctant to use epinephrine, which is similar to that observed in a previous study.8 This may be attributable to their lack of experience with applying epinephrine outside the cardiac arrest condition and their worries about the adverse effects of the drug. Adverse effects of epinephrine including cardiac arrhythmias, myocardial infarction, and acute respiratory distress syndrome (ARDS) were often related to an excessive dose.28,29 Notably, ARDS could happen in patients with supra-therapeutic doses of epinephrine due to increased pulmonary arterial pressure and pulmonary edema.30,31 Five out of nine subcutaneous epinephrine cases and one inhaled epinephrine case was subsequently administered IV epinephrine. As the maximum plasma epinephrine concentration rose slowly in these cases,32 the clinical symptoms were not relieved promptly. In addition, two patients received IM epinephrine as the initial treatment that effectively relieved the allergic reaction. The emergency treatment consensus for anaphylaxis recommends IM epinephrine as the first-line treatment even if intravenous access is available since it poses minimal risk to an individual.9,10,13 However, with IV access and monitoring, IV epinephrine is recommended for the treatment of life-threatening POA.14 Corticosteroids, other vasoactive drugs, and antihistamines instead of epinephrine were administered as the initial treatment, but these drugs have not been found to be effective in severe anaphylaxis.33 Corticosteroids play a role in anti-inflammatory reactions by modulating the gene expression to reduce the release of inflammatory mediators, which requires 4–6 h to manifest. Therefore, these drugs are not recommended as a first-line treatment for severe symptoms. Other vasoactive drugs such as norepinephrine and ephedrine were administered to reverse the circulatory collapse; however, these drugs are not beta-receptor agonists and lack the ability to reverse peripheral vasodilation, reduce tissue edema, and dilate the bronchial airways like epinephrine.
In our study, we also analyzed the association between the administration of epinephrine and the duration of hypotension. Although there was no significant difference between the two groups with respect to the time to first epinephrine administration from the onset of clinical symptoms, patients in the long-term hypotension group received epinephrine late (median time 2 min vs 4 min) and were given more epinephrine either on the first dosage (median dosage 20 µg vs 50 µg) or the total dosage (median dosage 151 µg vs 506 µg). We suspect that the delayed epinephrine treatment resulted in longer duration of hypotension and higher dosage of epinephrine IV was subsequently administered to maintain the blood pressure. Studies have shown that prolonged hypotension can impair end-organ perfusion, which may lead to acute kidney ischemia (AKI), myocardial injury, stroke, and death.34–38 A systematic review reported that any exposure to intraoperative MAP < 50–55 mmHg was associated with moderately or highly elevated risks of adverse outcomes including mortality, AKI, MI, ischemic stroke, and delirium.26
One of the focus areas of this study was to identify risk factors for near-fatal and fatal outcomes of POA. The results showed that deaths by POA occurred more frequently in elders, which is consistent with previous studies in which higher age was found to be associated with higher rate of severe anaphylaxis.20,23,39 Elderly patients have significantly lower ability to compensate for hypotension, hypoxia, and peripheral vasodilation because of the high prevalence of multiple cardiac or respiratory comorbidities. Furthermore, the cardiovascular effects of medications taken by elders (such as the ß-blockers and angiotensin-converting enzyme inhibitors) influence anaphylaxis.21,39 In our study, higher ASA physical status class was associated with higher risk of fatal and near-fatal POA.40 Patients with severe systemic disease are more vulnerable to the physiological stress induced by anaphylaxis. Moreover, the long-term hypotension (≥15 min) was an independent risk factor for poor outcomes. The longer insufficient perfusion time of important organs might be responsible for the long hypotension duration. Ruetzler et al found that intraoperative hypotension was associated with myocardial injury after noncardiac surgery (MINS), usually asymptomatic and silent, which was noticed only by routine troponin monitoring and was strongly associated with mortality.34 In addition, compared with standard management strategy of treating SBP less than 80 mmHg, individualized blood pressure management strategy aiming at target SBP within 10% of the reference value reduced the risk of postoperative organ dysfunction.25 Occurrence of hypotension, hypoxia, and even cardiac arrest during POA jeopardizes the already dysfunctional system. Therefore, maintaining steady intraoperative blood pressure is crucial to maintain end-organ perfusion. Thus, prevention of prolonged hypotension caused by delayed diagnosis and resuscitation in POA is a key imperative. In our cohort, neurological disease (including cerebral hemorrhage and stroke) and cardiovascular diseases (hypertension, coronary artery disease, heart failure) were also associated with worse outcome.23 Although cardiac procedure was not associated with fatal and near-fatal POA in the multivariable analysis, it was found to be a risk factor in the univariate analysis.22 Administration of blood products, protamine, heparin, and other drugs, which have a higher potential for inducing anaphylaxis are administered more frequently in cardiac surgeries.41 Furthermore, with cardiovascular or respiratory system dysfunction, patients undergoing cardiac procedure are more vulnerable to adverse outcomes of POA, which resulted in a worse outcome. History of drug allergy was notably associated with near-fatal and fatal POA. In a prior study, a previously uninvestigated perioperative hypersensitivity reaction was found to be the main risk factor for anaphylaxis. The existence of antigen-specific IgE has been regarded as a risk factor for anaphylaxis because it rapidly binds to the allergen on the next exposure.1 Transfer of specific IgE to the mice also showed the same result.42 A comprehensive review of the clinical and perioperative history is essential before any procedure.
Some limitations of this study should be considered. Firstly, this study enrolled cases with suspected POA. Diagnosis of POA was not confirmed by skin tests or in vitro diagnostic tests; thus, the culprit agents were not identified. Nevertheless, further research will be performed in the follow-up study. Secondly, there was a risk of overfitting the multivariate analysis because of the relatively small sample size even though 112 hospitals in mainland China were involved.
Conclusions
In this retrospective study, we characterized the management of suspected life-threatening POA and identified the risk factors for near-fatal and fatal POA in China. Firstly, most cases of suspected anaphylaxis were recognized and treated promptly. Secondly, although epinephrine was administered in most cases, the dosage of epinephrine for treating life-threatening anaphylaxis was insufficient, which may lead to prolonged duration of hypotension. Finally, age ≥65 years, ASA PS IV, and long-term hypotension (≥15 min) were independent risk factors for near-fatal and fatal suspected POA.
Data Sharing Statement
The database which includes case report forms of suspected life-threatening perioperative anaphylaxis from 112 tertiary hospitals in mainland China are not publicly available due to privacy concerns but are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the Institutional Review Board of China-Japan Friendship Hospital and registered at the Chinese Clinical Trials Registry on September 15, 2019 (ChiCTR1900025956). The study was conducted in compliance with the Declaration of Helsinki and funded by National Centre for Medical Service Administration, National Health and Family Planning Commission, China (Bureau of Medical Administration [2019] No. 049).
Author Contributions
All authors made significant contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Scientific Research Fund of China-Japan Friendship Hospital (No. 2017-RC-3).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Ebo DG, Clarke RC, Mertes PM, Platt PR, Sabato V, Sadleir PHM. Molecular mechanisms and pathophysiology of perioperative hypersensitivity and anaphylaxis: a narrative review. Br J Anaesth. 2019;123(1):e38–e49. doi:10.1016/j.bja.2019.01.031
2. Mertes PM, Ebo DG, Garcez T, et al. Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth. 2019;123(1):e16–e28. doi:10.1016/j.bja.2019.01.027
3. Zhang P, Liu X, Li W, et al. Epidemiology of suspected life-threatening perioperative anaphylaxis: a cross-sectional multicentre study in China. Br J Anaesth. 2022;128(1):45–54. doi:10.1016/j.bja.2021.09.020
4. Marinho S, Kemp H, Cook TM, et al. Cross-sectional study of perioperative drug and allergen exposure in UK practice in 2016: the 6th National Audit Project (NAP6) allergen survey. Br J Anaesth. 2018;121(1):146–158. doi:10.1016/j.bja.2018.04.016
5. Ebo DG, Van Gasse AL, Decuyper II, et al. Acute management, diagnosis, and follow-up of suspected perioperative hypersensitivity reactions in Flanders 2001–2018. J Allergy Clin Immunol Pract. 2019;7(7):2194–2204 e7. doi:10.1016/j.jaip.2019.02.031
6. Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–1150. doi:10.1046/j.1365-2222.2000.00864.x
7. Au EYL, Lau CS, Lam K, Chan E. Perioperative anaphylaxis and investigations: a local study in Hong Kong. Singapore Med J. 2020;61(4):200–205. doi:10.11622/smedj.2019156
8. Garvey LH, Belhage B, Kroigaard M, Husum B, Malling HJ, Mosbech H. Treatment with epinephrine (Adrenaline) in suspected anaphylaxis during anesthesia in Denmark. Anesthesiology. 2011;115(1):111–116. doi:10.1097/ALN.0b013e318218119d
9. UK RC. Emergency Treatment of Anaphylaxis Guidelines for Healthcare Providers. UK RC; 2021.
10. Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022;77(2):357–377. doi:10.1111/all.15032
11. Abe N, Toyama H, Ejima Y, et al. alpha (1)-adrenergic receptor blockade by prazosin synergistically stabilizes rat peritoneal mast cells. Biomed Res Int. 2020;2020:3214186. doi:10.1155/2020/3214186
12. Chong LK, Morice AH, Yeo WW, Schleimer RP, Peachell PT. Functional desensitization of beta agonist responses in human lung mast cells. Am J Respir Cell Mol Biol. 1995;13(5):540–546. doi:10.1165/ajrcmb.13.5.7576689
13. Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127(3):587–93e1–22. doi:10.1016/j.jaci.2011.01.038
14. Li X, Ma Q, Yin J, et al. A clinical practice guideline for the emergency management of anaphylaxis (2020). Front Pharmacol. 2022;13:845689. doi:10.3389/fphar.2022.845689
15. Harper NJ, Dixon T, Dugue P, et al. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64(2):199–211. doi:10.1111/j.1365-2044.2008.05733.x
16. Garvey LH, Dewachter P, Hepner DL, et al. Management of suspected immediate perioperative allergic reactions: an international overview and consensus recommendations. Br J Anaesth. 2019;123(1):e50–e64. doi:10.1016/j.bja.2019.04.044
17. Harper NJN, Cook TM, Garcez T, et al. Anaesthesia, surgery, and life-threatening allergic reactions: management and outcomes in the 6th National Audit Project (NAP6). Br J Anaesth. 2018;121(1):172–188. doi:10.1016/j.bja.2018.04.015
18. Che L, Li X, Zhang X, et al. The nature and reported incidence of suspected perioperative allergic reactions: a cross-sectional survey. J Clin Anesth. 2021;74:110404. doi:10.1016/j.jclinane.2021.110404
19. Weiler CR, Schrijvers R, Golden DBK. Anaphylaxis: advances in the past 10 years. J Allergy Clin Immunol Pract. 2023;11(1):51–62. doi:10.1016/j.jaip.2022.09.019
20. Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169–1178. doi:10.1016/j.jaip.2017.06.031
21. Mirone C, Preziosi D, Mascheri A, et al. Identification of risk factors of severe hypersensitivity reactions in general anaesthesia. Clin Mol Allergy. 2015;13(1):11. doi:10.1186/s12948-015-0017-9
22. Gonzalez-Estrada A, Campbell RL, Carrillo-Martin I, Renew JR, Rank MA, Volcheck GW. Incidence and risk factors for near-fatal and fatal outcomes after perioperative and periprocedural anaphylaxis in the USA, 2005–2014. Br J Anaesth. 2021;127(6):890–896. doi:10.1016/j.bja.2021.06.036
23. Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Risk factors for severe anaphylaxis in the United States. Ann Allergy Asthma Immunol. 2017;119(4):356–361 e2. doi:10.1016/j.anai.2017.07.014
24. Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1(8009):466–469.
25. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. doi:10.1001/jama.2017.14172
26. Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721. doi:10.1016/j.bja.2018.04.036
27. Wu XM, Xue ZG, Wang JK, Ye TH, Zhao J. Expert consensus on diagnosis and prevention of perioperative anaphylaxis. China Continuing Medical Education; 2011.
28. Sheikh A, Shehata YA, Brown SG, Simons FE. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev. 2008;2008(4):CD006312. doi:10.1002/14651858.CD006312.pub2
29. Cardona V, Ferre-Ybarz L, Guilarte M, et al. Safety of adrenaline use in anaphylaxis: a multicentre register. Int Arch Allergy Immunol. 2017;173(3):171–177. doi:10.1159/000477566
30. Fernando UPM, Dharmawardhane MP, Subramaniam N, Nimalan S, Gunathilake KUIS, Munasinghe BM. Acute respiratory distress syndrome following anaphylactic shock—“A Deadly Duel”—case report and literature review. Open J Anesthesiol. 2021;11(2):33–38. doi:10.4236/ojanes.2021.112004
31. Munasinghe BM, Perera RN, Kumara S. Two lives at stake: obstetric anaphylactic shock resulting in acute respiratory distress syndrome - A case report. SAGE Open Med Case Rep. 2022;10:2050313X221084843. doi:10.1177/2050313X221084843
32. Grabenhenrich LB, Dolle S, Rueff F, et al. Epinephrine in severe allergic reactions: the European Anaphylaxis Register. J Allergy Clin Immunol Pract. 2018;6(6):1898–1906 e1. doi:10.1016/j.jaip.2018.02.026
33. Liyanage CK, Galappatthy P, Seneviratne SL. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur Ann Allergy Clin Immunol. 2017;49(5):196–207. doi:10.23822/EurAnnACI.1764-1489.15
34. Ruetzler K, Khanna AK, Sessler DI. Myocardial injury after noncardiac surgery: preoperative, intraoperative, and postoperative aspects, implications, and directions. Anesth Analg. 2020;131(1):173–186. doi:10.1213/ANE.0000000000004567
35. Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44(6):811–822. doi:10.1007/s00134-018-5224-7
36. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013
37. Mathis MR, Naik BI, Freundlich RE, et al. Preoperative risk and the association between hypotension and postoperative acute kidney injury. Anesthesiology. 2020;132(3):461–475. doi:10.1097/ALN.0000000000003063
38. Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
39. Worm M, Francuzik W, Renaudin JM, et al. Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy. 2018;73(6):1322–1330. doi:10.1111/all.13380
40. Francuzik W, Dolle-Bierke S, Knop M, et al. Refractory anaphylaxis: data from the European Anaphylaxis Registry. Front Immunol. 2019;10:2482. doi:10.3389/fimmu.2019.02482
41. Levy JH, Adkinson NF
42. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335–348. doi:10.1016/j.jaci.2017.06.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
