Back to Journals » Cancer Management and Research » Volume 11
Management of mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL): focus on chlormethine gel
Authors Denis D, Beneton N, Laribi K, Maillard H
Received 19 August 2018
Accepted for publication 20 December 2018
Published 20 March 2019 Volume 2019:11 Pages 2241—2251
DOI https://doi.org/10.2147/CMAR.S138661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Video abstract presented by Daphné Denis.
Views: 1553
Daphné Denis,1 Nathalie Beneton,1 Kamel Laribi,2 Hervé Maillard1
1Dermatology Department, Centre Hospitalier Le Mans, Le Mans, France; 2Haematology Department, Centre Hospitalier Le Mans, Le Mans, France
Abstract: Mycosis fungoides (MF) is a low-grade cutaneous lymphoma accounting for more than half of primary cutaneous T-cell lymphomas (CTCLs). Due to the rarity of CTCL, randomized studies are lacking, and treatment is based mainly on the recent published European Organisation for Research and Treatment of Cancer guidelines. Basically, early-stage MF is treated with skin-directed treatments, whereas advanced-stage MF requires more aggressive therapies. Among the skin-directed therapies, nitrogen mustard has been used for more than 50 years. A gel formulation was developed recently, showing a slight decrease in efficacy, counterbalanced by better tolerance (essentially due to a decrease in delayed hypersensitivity reactions). This review aims to summarize the current management of MF and the role of chlormethine gel in the treatment of the disease.
Keywords: mycosis fungoides, nitrogen mustard, mechlorethamine, chlormethine, gel
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a group of extranodal non-Hodgkin lymphomas that account approximately for 2% of all lymphomas.1 Classification follows the 2016 revision of the World Health Organization (WHO) classification of cutaneous lymphomas.2 Mycosis fungoides (MF) is a low-grade cutaneous lymphoma accounting for more than half of all primary cutaneous lymphomas.1 Sézary syndrome (SS) is rarer, accounting for around 5% of cases.
Histology
MF is defined histologically by an initial skin infiltration with atypical cells having cerebriform nuclei, often located in the basal layer of the epidermis, which is called epidermotropism. These cells are clonally derived malignant CD4+CD45RO+-phenotype T lymphocytes lacking normal T-cell markers, such as CD7 and CD26.3 The diagnosis is often made after several aspecific biopsies.
Epidemiology
MF typically affects old adults with median age at diagnosis of 55–60 years, with a male-to-female ratio of 1.6–2:1.4 Incidence has been stable since 1995, at around 5.6 per million persons.5 It may also occur very rarely in children and adolescents.6
Clinical presentation
The clinical presentation of MF varies from patches or plaques in the early stages, often situated in sun-protected areas, to cutaneous tumors, sometimes with lymph node, visceral, or blood involvement4 (Figures 1 and 2). Some clinical variants, such as folliculotropic MF, pagetoid reticulosis, and granulomatous slack skin, were separated in the WHO–European Organization for Research and Treatment of Cancer (EORTC) classification,1,2 due to different clinicopathological features and biological response.
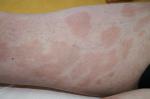  | Figure 1 Mycosis fungoides “patches” stage. |
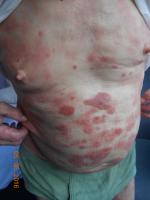  | Figure 2 Mycosis fungoides “plaques” stage |
Two different diseases
Most of the time, MF is an indolent disease, and the prognosis of patients with MF is extremely dependent on the tumor-node-metastasis-blood (TNMB) stage at diagnosis4,7 (Table 1). Recent guidelines have been published in 2017 by the EORTC.5
  | Table 2 ISCL/EORTC revision of mycosis fungoides and Sézary syndrome staging4 Note: aConsidered advanced-stage disease. Republished with permission of American Society of Hematology, from Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC), Olsen E, et al, 110, 6, 2007; permission conveyed through Copyright Clearance Center, Inc. Abbreviations: ISCL, International Society for Cutaneous Lymphomas; EORTC, European Organization for Research and Treatment of Cancer; |
In early-stage MF (IA–IIA), representing around 70% of patients, most patients can look forward to a normal life expectancy and the treatment aim is to prevent evolution to a more severe disease. Recommendations in theses stages are to use skin-directed treatments. Recently, a difference in prognosis has been highlighted between patches and plaques (T1/2a/b), with a poorer prognostic in the plaque diseases. (T1b or T2b).7 On the contrary, patients with advanced disease have a severe prognosis and have to be treated with chemotherapy, which often fails to offer durable remission, except for the highly selected subset of patients eligible for allogeneic stem-cell transplantation. New therapeutics are emerging, and clinical trials have to be proposed to patients if available.
Sézary syndrome
SS is by definition an advanced disease, pathologically and clinically related to MF. It mostly presents with erythema, together with lymphadenopathy and blood involvement with Sézary cells. The prognosis is poor, with median survival of <3 years.8
Management of mycosis fungoides
Staging
As a first step, patients have to be investigated with a clinical and histological confrontation. Blood and sometimes blood-marrow investigations, as well as radiological tests, are to be staged following EORTC directives. Treatment will depend on the patient’s comorbidities and severity of disease based on this staging. For patients with clinical stage IA–IB and no palpable lymphadenopathy, no extensive staging evaluation is recommended. Suspicious lymph nodes, ie, >1.5 cm in diameter and/or firm, irregular, or clustered, have to be biopsied (core or excisional biopsy) for light-microscopy pathologic assessment, flow cytometry, and T-cell-receptor gene rearrangement.
Chest, abdomen, and pelvis computed tomography should be performed in patients with anything other than IA disease or with limited IB disease. In cases of potential lymphadenopathy, visceral involvement, or abnormal laboratory tests, fludeoxyglucose positron-emission tomography could be performed as well for further investigations. Bone-marrow biopsy is usually not required, unless there are unexplained hematologic abnormalities. These recommendations are outlined in Table 3.
  | Table 3 Recommended evaluation/initial staging of patients with mycosis fungoides/Sézary syndrome4 Note: Republished with permission of American Society of Hematology, from Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC), Olsen E, et al, 110, 6, 2007; permission conveyed through Copyright Clearance Center, Inc. Abbreviation: TCR, T-cell receptor. |
Treatment recommendations by disease stage
In view of the rarity of CTCL, randomized clinical studies are lacking, and treatment is based mainly on the recently published EORTC guidelines.5 This lack of evidence-based data leads to heterogeneity of treatment approaches, especially between US and non-US centers and between institutes.8 It is important to note that there is currently no curative treatment for MF (except for allogeneic stem-cell transplantation). The main treatment objective is to reach effective palliation with symptom improvement and/or enhance the patient’s quality of life.5 The risk of infection in patients undergoing immunosuppressive treatment is important, and patients should be carefully monitored.
Early-stage MF: IA–IIA
Frontline treatment
Frontline treatments for early stages are dominated by skin-directed therapies.
Topical corticosteroids
Only one controlled study has evaluated this treatment,9 with high potency compared to less potent topical steroids;5 however it is widely used. Clobetasol propionate is used mainly. It is a simple treatment for patients with a low number of patches or plaques.
Topical mechlorethamine
Mechlorethamine or nitrogen mustard (NM) has been used in MF in the US since 1949.5 This topical chemotherapy works as an alkylating agent by affecting rapidly dividing cells through DNA cross-linking, abnormal base pairing, or nucleic acid depurination. It may also alter the tumor-growth pattern and enhance immunogenic host potential.10 It was initially prepared in water, then in ointment form, and later a gel formulation was introduced.
Topical bexarotene
A 1% gel formulation was approved as a second-line treatment by the US Food and Drug Administration (FDA), based on a prospective phase III study reporting an overall response rate of 46%.13 In Europe, some practitioners use this treatment off-label in patients who have not tolerated other local therapies, as the toxicity is mild. Like every retinoid, bexarotene is teratogenic.
Ultraviolet phototherapy
Psoralen plus ultraviolet A (PUVA), as well as UVB are both recommended in MF. A recent review highlighted the lack of evidence in terms of long-term efficacy and safety, because of the lack of standardization between studies. It aimed at providing guidelines for clinicians and investigators.14 This treatment offers the advantage of rapid relief in pruritus and lesion size. The main limitations are accessibility of the treatment and the potential risk of secondary skin cancer. This was studied in psoriasis, but not in MF,15,16 and proven only with PUVA. This treatment has also been reported as a safe and effective option in childhood MF.17
Localized radiotherapy
Especially indicated in individual or localized lesions, alone or in combination with other therapies, radiotherapy may even induce long-term remission. The treatment can also be effective in pagetoid reticulosis.18
Photodynamic therapy with methylaminolevulinic acid
A recent prospective French study evaluated this treatment in 20 patients with paucilesional forms, and reported an ORR of 75%, with good tolerance.19 A recent Italian review confirmed this effectiveness.20
Second-line treatment
In second-line treatment, systemic therapies are most commonly used.
Total-skin electron-beam therapy
In this therapy, a linear accelerator generates attenuated electrons that penetrate the skin to a limited depth, thus avoiding internal organ toxicity. There is a debate between conventional doses or low-dose therapy.21,22
Retinoids (including bexarotene)
Among these molecules, bexarotene is the only one that was developed and approved specifically for the treatment of CTCL. It is indicated in patients with MF refractory to at least one prior therapy.23 The overall response rate is 31%–51%, depending on the study end-point definition.23 Acitretin and isotretinoin are also commonly used.24 The main adverse events (AEs) behind the teratogenic effect are drying of the skin and mucosa, hyperlipidemia, and central hypothyroidism.24
Interferon-α
Recombinant IFNα is the most widely used drug, but with varying treatment schedules. EORTC guidelines recommend starting with 3 million units three times a week. The overall response rate ranges from 0 to 80%.25 Given its inhibitory effects on eosinophil chemotaxis and activation,8 it is very useful in patients with eosinophilia.
Low-dose methotrexate
This cytotoxic treatment is commonly used alone26 or in association with IFN27 in refractory MF. The recommended dose is 10–25 mg once weekly.5
Combinations of these treatments
Combinations, mostly of PUVA with retinoids, or less classically with IFNα, and of retinoids (acitretin) and IFNα, are used.28 Methotrexate (Mtx) has also been combined with IFNα, phototherapy, and radiotherapy.29 These systemic therapies are also often combined with skin-directed therapies.5
Advanced-stage MF
In addition to the EORTC guidelines, a recent retrospective study from the Cutaneous Lymphoma International Consortium reported the most common approaches by stage,30 and a recent review also focused on this advanced-stage disease.8
First-line treatment
Stage IIB: patients with cutaneous tumors
When patients show MF with tumors, the same systemic treatments as in second-line treatment of early-stage MF are recommended,5 with bexarotene being the most commonly used,30 followed by localized radiotherapy, total-skin electron-beam therapy, and gemcitabine.5 Low-dose Mtx, IFNα, and PEGylated liposomal doxorubicin can also be used.
Stage III: patients with erythematous MF or SS
In stage IIIA, Mtx is the most commonly used treatment. The dose in this regimen is often 25 mg weekly.31 In stage IIIB, the same treatments are recommended, alone or in combination with extracorporeal photochemotherapy,5 where blood is exposed to photoactivated 8-methoxypsoralen.32 This treatment is well tolerated and efficient. The main difficulty is accessibility. It is often associated with IFN, bexarotene, retinoids. The reported response rate is 20%–63%.8
Stage IV: patients with high-grade lymph-node involvement and/or blood involvement with or without SS
Patients are treated with radiotherapy and/or chemotherapy, except for highly selected patients who can undergo allogeneic stem-cell transplantation. This is the only curative treatment for MF. Patient selection must be very careful, given the high morbidity rate of this treatment (immunomediated graft-versus-host disease effect).33 In stage IVA, photopheresis is the most common first-line treatment, followed by IFNα and chlorambucil.30
In aggressive forms with blood involvement (IVB), polychemotherapy is used as first-line treatment, with the cyclophosphamide–hydroxydaunorubicin–oncovin–prednisolone regimen being most widely used, but other combinations are available.5 In SS, chlorambucil–prednisone is used. It is worthy of mention that there are specific recommendations for SS published by the United States Cutaneous Lymphoma Consortium that insist on several principles, such as preserving host immunity by using immunomodulatory therapy before chemotherapy, unless burden of disease or failure of prior treatment warrants otherwise.34
Second-line treatment
Mono- or polychemotherapy are the standard of care, together with (in some highly selected patients) allogeneic stem-cell transplantation as a rescue. The FDA recently approved the histone deacetylase inhibitors romidepsin and vorinostat as second-line treatments in advanced CTCL. The ORR has varied with the studies: from 30% with vorinostat35 to 38% with romidepsin.36 The main AEs are gastrointestinal and asthenic. These treatments are available in France, with temporary authorization of use.
Recently, targeted therapies have been developed and have shown promising results as second-line treatments in these aggressive forms:
- Brentuximab vedotin was recently approved by the European Medicines Agency (EMA) for the treatment of CD30-expressing CTCL as a second-line treatment, according to the ALCANZA phase III study, showing superiority to Mtx and bexarotene.37,38 The overall response rate in this study was 67% with brentuximab vedotin vs 20% with treatment of physician’s choice. This drug consists of the combination of an anti-CD30 IgG1 antibody attached to a microtubule-disrupting agent, which after internalization induces cell-cycle arrest. The main limiting AE is neurosensitive peripheral neuropathy.
- Mogamulizumab is a humanized monoclonal antibody that targets the CC chemokine receptor 4, modified by glycoengineering to enhance its antibody-dependent cell-mediated cytotoxicity. It recently showed significant improvement in ORR and progression-free survival over vorinostat in 372 patients with advanced-stage MF as second-line treatment in the MAVORIC study,39 with median progression-free survival of 7.7 vs 3.1 months and improved ORR of 28% vs 4.4%. It is especially interesting in patients with blood involvement. This treatment is approved in Japan and in the US, but not available yet in Europe. AEs include flu-like symptoms, rash (two cases of Stevens–Johnson syndrome), and infusion reactions.8
- Alemtuzumab is an anti-CD52 human IgG-derived monoclonal antibody. Although it is commercialized to treat multiple sclerosis, it is available through a special-access program for the treatment of lymphoid neoplasms. A phase II study showed an overall response rate of 55%,40 with a preferential use in erythematous MF/SS. A recent study has shown long-term remission in this subgroup of patients.41 The main AEs are opportunistic infections, including cytomegalovirus replication, due to profound induced immunosuppression.
Table 4 summarizes first- and second-line treatments by disease stage, keeping in mind that no clear algorithm can be recommended.
Folliculotropic mycosis fungoides
This clinical and histological subtype of the disease has been reported for many years to have a worse prognosis. However, the Dutch Cutaneous Lymphoma Group recently published a clinical staging system and consequently new treatment recommendations in this disease.42,43
Maintenance therapy
When remission is achieved, maintenance therapy is recommended, keeping in mind the high importance of maintaining the quality of life of patients suffering from this chronic disease. Treatment agents that have shown some efficacy with tolerable side effects for this purpose are extracorporeal photochemotherapy, IFNα, low-dose Mtx, mechlorethamine, PUVA and UVB, retinoids, and topical corticosteroids.5 One of the important aspects to take into account, because of its important impact on quality of life, is pruritus, which is often improved by the treatment of MF, but sometimes requires a specific regimen. Mirtazapine and gabapentin have shown efficacy in this indication.44
What place for chlormethine gel?
Mechlorethamine or NM have been used in MF in the US since 1949.5 This topical chemotherapy works as an alkylating agent by affecting rapidly dividing cells, altering the tumor-growth pattern, and enhancing immunogenic host potential.10 NM treatment has evolved over the last few years with regard to application site, vehicle method, and duration. Before 1980, topical NM was prepared in water, and thus, owing to its instability, had to be applied immediately to the skin. The main AE of this aqueous preparation is hypersensitivity. In the early 1980s, an ointment preparation of topical NM was introduced in the US. Several advantages were claimed: decreased risk of sensitization, decreased cost, ease of application, and an emollient effect.11 In Europe, a mechlorethamine solution (Caryolysine) was approved by the EMA in 1946, but since 2006 supply difficulties have made its access impossible. A gel preparation was introduced and approved by the FDA in 2013,45 and since 2014 individual and then collective (2017) special authorization have allowed practitioners to prescribe this treatment in France.12
From aqueous preparation to gel
Several studies have reported efficacy data on treatment with mechlorethamine aqueous solutions. A recent review highlighted the difficulty in comparing them, as the frequency and duration of treatment varied, together with the use of additional therapies.46 However, the complete response (CR) rate reported was 51%–84% in T1 patients and 34%–62.2% in T2 patients.46 In most of these studies, topical NM was not used alone, being associated with intralesional NM in tumors,47,48 localized radiotherapy,10,49 electron-beam therapy,50 or phototherapy.51
As reported earlier, the ointment preparation was introduced in the 1980s in the US. Colleagues from the Stanford University clinic reported their experience using this ointment in three main studies.11,49,50 In 1983,50 they prospectively evaluated ointment-based mechlorethamine (0.01%) in 43 patients with MF stage I–III, for a majority of patients after total-skin electron-beam therapy. The primary end point was to observe the development of allergic contact dermatitis (ACD) with this preparation. However, they documented a CR in 17.6% for stage IA and 27.3% for stage IB patients. This decrease in efficacy with the ointment was explained by the authors as due to the variation in concentration (0.001%–0.02%). In 1987, they retrospectively reviewed 123 patients treated with NM therapy (aqueous and ointment 0.01%–0.02%):49 they did not note any difference in efficacy between ointment and aqueous preparations (overall or relapse-free survival). However, 66% patients developed delayed hypersensitivity reactions (DHRs) with the aqueous preparation vs <5% with the ointment. In 2003, they updated the long-term results, reviewing 203 patients treated with aqueous and ointment-based preparation at the same concentration (0.02%):11 the CR rate was 65% in T1 and 34% in T2 patients. Similarly, no difference in terms of efficacy was observed between the two preparations. However, the ointment was not used in all the countries, especially not in France, because there was no guarantee on stability.52
In 2013, Lessin et al53 demonstrated in a randomized multicenter blinded study the noninferiority of the gel preparation in comparison with an ointment preparation (0.02%) as second-line treatment in stage IA–IIA MF patients. Following this study, the treatment was approved by the FDA in 2013, in Israel in 2016, and in France in September 2017, after temporary individual and then cohort authorization of use since 2014. It offers the advantage of better adherence, as the greasiness of the ointment was often highlighted.46
Efficacy of mechlorethamine gel
In Lessin et al,53 a total of 260 patients randomly received either the gel or the ointment form once daily for up to 12 months. The CR rate was 13.8% in the gel arm and 11.5% in the ointment arm, and the overall response rate (partial response [PR] + CR) was 58.5% in the gel arm and 47.7% in the ointment arm. The gel arm had a time to 50% response of 26 weeks, while this was 42 weeks in the ointment arm. CR rates were lower than in previous studies with the aqueous solution and even with the ointment, probably because the use of concomitant therapies was not allowed in this study; 85.5% of patients in the gel arm had maintained response at 12 months. Eight patients with MF variants (folliculotropic, syringotropic, large-cell transformation) were included, and four of six who ended the study showed a response. Fifteen patients in the gel arm had progressive disease during follow-up. Among them, seven patients continued the treatment and achieved a confirmed response later.
Kim et al conducted an extension trial with 0.04% mechlorethamine gel for patients who had not achieved CR with the 0.02% formulation after 12 months.54 A total of 98 patients were enrolled to apply the 0.04% gel once daily for 7 months, and 26 (26.5%) achieved a response (CR six, PR 20). No severe AEs were noted. In France, Mathieu et al55 reported retrospectively the data of 107 patients treated during the “temporary authorization of use” from December 2014 to December 2015. All patients were treated with chlormethine gel. Ninety-three percent of the patients were early-grade MF (50% IA, 37% IB, 6% IIA). For the majority of patients (85%), the gel was applied three times weekly and as a second-line treatment. Topical corticosteroids were used as concomitant treatment in 77% of patients. The overall response rate was 57% (CR 7%, PR 50%).
From August 2014 to February 2017, data of 187 patients treated in France with mechlorethamine gel were reported, with an overall response rate of 79.2%. In the study by Lessin et al,53 patients who used NM as maintenance therapy had a longer response than those who did not.53 This effect of maintenance therapy was also reported in previous studies with NM.11,51 However, the use of NM as maintenance therapy is not mentioned by the FDA or Haute Autorité de Santé.
Safety
The tolerance profile is not very different from the mechlorethamine solution used for many years. During the trial by Lessin et al,53 no drug-related severe AE was reported. Data of the patients treated with mechlorethamine gel from October 2014 to February 2017 in France were also published by the Haute Autorité de Santé.12 During this period, 478 patients received NM as second-line treatment, 93% of them to treat early-stage MF (IA–IIA). A total of 194 patients stopped the treatment, 135 (69.6%) for AEs, progression, or inefficiency. There were in total 182 pharmacovigilance reports concerning 450 AEs, of which 81 were considered severe. The FDA also published periodic reports of pharmacovigilance from the US, Rwanda, Israel, and France: 2,071 AEs concerning 1,750 patients were reported, 188 (8.9%) of which were severe. These AEs were related to hypersensitivity and skin side effects.12
Hypersensitivity reactions
These include DHRs developed in skin side effects and immediate hypersensitivity, ie, urticaria. Anaphylactic-type reactions following urticaria are of concern, and represent the most common reason to stop the treatment and the only contraindication.46
Skin side effects
The most common side effects reported with mechlorethamine gel are ACD, DHRs, skin irritation, pruritus, erythema, and hyperpigmentation. Hyperpigmentation is reversible in most patients. Skin irritation is often managed by reducing the frequency of application. In Lessin et al,53 it was higher in the gel arm of the study, and 62% of patients reported a skin-related AE. Patch testing was not systematically required, but ACD was estimated to affect around 16.4% of the patients. This proportion of ACD was significantly lower than the reported DHR incidence with the aqueous preparation. In the French study, ACD was reported in around 20% of patients.55
Secondary malignancies
In Lessin et al, 20 nonmelanoma skin cancers were detected in the 24-month follow-up period, of which only six were in treatment areas.53 Most appeared in sun-exposed areas and in patients with a history of skin cancer. This supports the results from a previous Danish study reporting no significantly increased risk of nonmelanoma skin cancers in patients treated with NM.56
Systemic toxicity
Systemic absorption was not detected in a phase II study53 evaluating 16 patients who received the gel formulation, despite high-performance liquid chromatography serum assays. Moreover, no change in hematology values was noted.
Future directions
Further studies will be needed to assess the efficacy of mechlorethamine gel in association with other treatments. There are no efficacy and safety data for mechlorethamine gel in children.
Conclusion
NM has been used for >50 years in MF. The mechlorethamine gel formulation is safe and has shown to be effective in early-stage MF refractory to first-line treatment. The main AEs involve the skin, and reducing the frequency of application and using concomitant topical steroids can reduce the majority of them. Moreover, the gel formulation is responsible for fewer DHRs than the aqueous solution. The gel formulation allows treatment on an outpatient basis. This local treatment can also be associated with systemic therapies in advanced-stage MF, due to the absence of systemic absorption of the product, although associations have to be evaluated. This treatment is readily available in the US and Israel, with temporary authorization in France and in discussion in other European countries (especially Italy).
Disclosure
The authors report no conflicts of interest in this work.
References
Willemze R. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. | ||
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. | ||
Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978–1988. | ||
Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722. | ||
Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer. 2017;77:57–74. | ||
Wu M, Chang MD. Pediatric Mycosis Fungoides. NEJM J Watch. 2014;2014. | ||
Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739. | ||
Photiou L, van der Weyden C, Mccormack C, Miles Prince H. Systemic Treatment Options for Advanced-Stage Mycosis Fungoides and Sézary Syndrome. Curr Oncol Rep. 2018;20(4):32. | ||
Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. 1998;134(8):949–954. | ||
Ramsay DL, Halperin PS, Zeleniuch-Jacquotte A. Topical mechlorethamine therapy for early stage mycosis fungoides. J Am Acad Dermatol. 1988;19(4):684–691. | ||
Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. 2003;139(2):165–173. | ||
LEDAGA. brief summary of the transparency committee opinion. 2017. https://www.has-sante.fr/portail/upload/docs/application/pdf/2018-03/ledaga_summary_ct16175.pdf. Accessed June 10, 2018. | ||
Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–815. | ||
Olsen EA, Hodak E, Anderson T, et al. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2016;74(1):27–58. | ||
Stern RS, PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553–562. | ||
Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(Suppl 3):22–31. | ||
Laws PM, Shear NH, Pope E. Childhood mycosis fungoides: experience of 28 patients and response to phototherapy. Pediatr Dermatol. 2014;31(4):459–464. | ||
Specht L, Dabaja B, Illidge T, Wilson LD, Hoppe RT, International Lymphoma Radiation Oncology Group. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92(1):32–39. | ||
Quéreux G, Brocard A, Saint-Jean M, et al. Photodynamic therapy with methyl-aminolevulinic acid for paucilesional mycosis fungoides: a prospective open study and review of the literature. J Am Acad Dermatol. 2013;69(6):890–897. | ||
Seyed Jafari SM, Cazzaniga S, Hunger RE. Photodynamic therapy as an alternative treatment for mycosis fungoides: a systemic review and meta-analysis. G Ital Dermatol Venereol. 2018;153(6):827–832. | ||
Jones GW, Kacinski BM, Wilson LD, et al. Total skin electron radiation in the management of mycosis fungoides: Consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47(3):364–370. | ||
Elsayad K, Kriz J, Moustakis C. Total Skin Electron Beam for Primary Cutaneous T-cell Lymphoma. Int J Radiat Oncol. 2015; 1;93(5):1077–1086. | ||
European Medicines Agency - Find medicine - Targretin. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000326/human_med_001078.jsp&mid=WC0b01ac058001d124. Accessed April 23, 2018. | ||
Huen AO, Kim EJ. The role of systemic retinoids in the treatment of cutaneous T-Cell lymphoma. Dermatol Clin. 2015;33(4):715–729. | ||
Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16(4):311–321. | ||
Zackheim HS, Kashani-Sabet M, Mcmillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol. 2003;49(5):873–878. | ||
Aviles A, Neri N, Fernandez-Diez J, Silva L, Nambo MJ. Interferon and low doses of methotrexate versus interferon and retinoids in the treatment of refractory/relapsed cutaneous T-cell lymphoma. Hematology. 2015;20(9):538–542. | ||
Trautinger F. Phototherapy of mycosis fungoides. G Ital Dermatol E Venereol. 2017;152(6):597–606. | ||
Wood GS, Wu J. Methotrexate and Pralatrexate. Dermatol Clin. 2015;33(4):747–755. | ||
Quaglino P, Maule M, Prince HM, et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol. 2017;28(10):2517–2525. | ||
Zackheim HS, Kashani-Sabet M, Hwang ST. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. J Am Acad Dermatol. 1996;34(4):626–631. | ||
Trautinger F, Just U, Knobler R. Photopheresis (extracorporeal photochemotherapy). Photochem Photobiol Sci. 2013;12(1):22–28. | ||
Virmani P, Zain J, Rosen ST, Myskowski PL, Querfeld C. Hematopoietic stem cell transplant for mycosis fungoides and sézary syndrome. Dermatol Clin. 2015;33(4):807–818. | ||
Olsen EA, Rook AH, Zic J, et al. Sézary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol. 2011;64(2):352–404. | ||
Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–3115. | ||
Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(29):4485–4491. | ||
European Commission Approves ADCETRIS (brentuximab vedotin) for CD30-Positive Cutaneous T-Cell Lymphoma after One Prior Systemic Therapy– Providing an Innovative Treatment Option to Patients. Available from: https://www.takeda.com/newsroom/newsreleases/2018/european-commission-approves-adcetris-brentuximab-vedotin-for-cd30-positive-cutaneous-t-cell-lymphoma-after-one-prior-systemic-therapy-providing-an-innovative-treatment-option-to-patients/. Accessed April 23, 2018. | ||
Kim YH, Whittaker S, Horwitz SM. Brentuximab vedotin demonstrates significantly superior clinical outcomes in patients with CD30-expressing cutaneous T cell lymphoma versus physician’s choice (Methotrexate or Bexarotene): the phase 3 Alcanza Study. Blood. 2016;128(22):182–182. | ||
Kim YH, Bagot M, Pinter-Brown L. Anti-CCR4 monoclonal antibody, mogamulizumab, demonstrates significant improvement in PFS compared to vorinostat in patients with previously treated cutaneous T-cell lymphoma (CTCL): results from the phase III MAVORIC Study. Blood. 2017;130(Suppl 1):817–817. | ||
Lundin J. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267–4272. | ||
de Masson A, Guitera P, Brice P, et al. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014;170(3):720–724. | ||
van Santen S, Roach RE, van Doorn R, et al. Clinical Staging and Prognostic Factors in Folliculotropic Mycosis Fungoides. JAMA Dermatol. 2016;152(9):992–1000. | ||
van Santen S, van Doorn R, Neelis KJ, et al. Recommendations for treatment in folliculotropic mycosis fungoides: report of the Dutch Cutaneous Lymphoma Group. Br J Dermatol. 2017;177(1):223–228. | ||
Demierre MF, Taverna J. Mirtazapine and gabapentin for reducing pruritus in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2006;55(3):543–544. | ||
VALCHLOR™ (mechlorethamine) [package insert]. Malvern, PA: Ceptaris Therapeutics, Inc.; 2013. | ||
Liner K, Brown C, Mcgirt L. Clinical potential of mechlorethamine gel for the topical treatment of mycosis fungoides-type cutaneous T-cell lymphoma: a review on current efficacy and safety data. Drug Des Devel Ther. 2018;12:241–254. | ||
van Scott EJ, Kalmanson JD. Complete remissions of mycosis fungoides lymphoma induced by topical nitrogen mustard (HN2). Control of delayed hypersensitivity to HN2 by desensitization and by induction of specific immunologic tolerance. Cancer. 1973;32(1):18–30. | ||
van Scott EJ, Winters PL. Responses of mycosis fungoides to intensive external treatment with nitrogen mustard. Arch Dermatol. 1970;102(5):507–514. | ||
Hoppe RT, Abel EA, Deneau DG, Price NM. Mycosis fungoides: management with topical nitrogen mustard. J Clin Oncol. 1987;5(11):1796–1803. | ||
Price NM, Hoppe RT, Deneau DG. Ointment-based mechlorethamine treatment for mycosis fungoides. Cancer. 1983;52(12):2214–2219. | ||
Zachariae H, Thestrup-Pedersen K, Søgaard H. Topical nitrogen mustard in early mycosis fungoides. A 12-year experience. Acta Derm Venereol. 1985;65(1):53–58. | ||
Beylot-Barry M, Dereure O, Vergier B, et al. Prise en charge des lymphomes T cutanés: recommandations du Groupe français d’étude des lymphomes cutanés [Management of cutaneous T-cell lymphomas: Recommendations of the French Cutaneous Lymphoma Group]. Ann Dermatol Vénéréologie. 2010;137(10):611–621. French. | ||
Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149(1):25–32. | ||
Kim YH, Duvic M, Guitart J, et al. Tolerability and efficacy of Mechlorethamine 0.04% gel in CTCL (Mycosis Fungoides) after initial treatment with topical Mechlorethamine, 0.02% gel. Presented at the T-cell Lymphoma Forum. 23-25 January 2014; San Francisco, CA, USA. Poster number TS14-1. | ||
Mathieu S, Ram-Wolff C, Baylot-Barry M, D’Incan M, Bagot M. L’expérience française de l’usage du gel de chlorméthine pour le traitement du mycosis fongoïde: une série rétrospective de 107 cas du GFELC [The French experience of the use of chlormethine gel for the treatment of mycosis fungoides: a retrospective series of 107 cases of GFELC]. Ann Dermatol Vénéréologie. 2016;143(12):S155. French. | ||
Lindahl LM, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170(3):699–704. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.