Back to Journals » Cancer Management and Research » Volume 10
Management of hemorrhage in gastrointestinal stromal tumors: a review
Authors Liu Q, Kong F, Zhou J , Dong M , Dong Q
Received 13 December 2017
Accepted for publication 31 January 2018
Published 12 April 2018 Volume 2018:10 Pages 735—743
DOI https://doi.org/10.2147/CMAR.S159689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leylah Drusbosky
Qi Liu,1 Fanmin Kong,1 Jianping Zhou,1 Ming Dong,1 Qi Dong2
1Department of Gastrointestinal Surgery, The First Hospital, China Medical University, Shenyang, China; 2Department of General Surgery, The People’s Hospital, China Medical University, Shenyang, China
Abstract: Gastrointestinal stromal tumors (GISTs) are relatively common mesenchymal tumors. They originate from the wall of hollow viscera and may be found in any part of the digestive tract. The prognosis of patients with stromal tumors depends on various risk factors, including size, location, presence of mitotic figures, and tumor rupture. Emergency surgery is often required for stromal tumors with hemorrhage. The current literature suggests that stromal tumor hemorrhage indicates poor prognosis. Although the optimal treatment options for hemorrhagic GISTs are based on surgical experience, there remains controversy with regard to optimum postoperative management as well as the classification of malignant potential. This article reviews the biological characteristics, diagnostic features, prognostic factors, treatment, and postoperative management of GISTs with hemorrhage.
Keywords: GIST, hemorrhage of digestive tract, prognosis, targeted therapy
Introduction
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors. They originate from the luminal wall and may occur in any part of the digestive tract. Most GISTs occur in the stomach (60%–70%) or the small intestine (25%–35%). The colon, rectum, appendix (5%), and esophagus (2%–3%) are relatively rare sites.1,2 They may even occur outside the digestive tract, including in the greater omentum, mesentery, and retroperitoneal sites.3 The size of most stromal tumors is about 5 cm at diagnosis.4 Approximately 70% of GIST patients are symptomatic. There are a wide variety of clinical manifestations, including rare presentations as part of the syndrome, known as Carneys triad: gastric stromal tumor, pulmonary chondroma, and paraganglioma. They are also seen in neurofibromatosis type 1.5,6 Stromal tumors of the small intestine often present as abdominal pain and hemorrhage of the digestive tract. Rectal stromal tumors may manifest as hematochezia and obstruction.7 Gastrointestinal bleeding is a relatively common presentation.8,9 Prognosis varies by location.
Because of the interference of various factors and limitations of the research samples, the classification of malignant potential of GISTs and postoperative management remains controversial. Age and gender may be the prognostic factors: it has been reported that the prognosis of women aged <50 years is better than that of women aged >50 years10 and that GISTs are more common in women aged 50–70 years.11 This last study also suggests that obesity may be a protective factor for patients with stromal tumors.12 The 2016.v2 National Comprehensive Cancer Network (NCCN)13 guidelines suggest that patients with high-risk factors for recurrence (tumor size >5 cm with large numbers of mitotic figures [>5/50 high-power field], tumor rupture, or recurrence risk >50%) should be treated with oral Gleevec postoperatively for least 36 months to reduce the risk of recurrence. However, there is controversy, as reported in Table 1, over the risk degrees for assessing malignant potential of GISTs.
Some investigators believe that gastrointestinal bleeding is caused by tumor invasion of the mucosa layer, resulting in ulceration,14 while tumor rupture occurs mostly in the serosa. Recently, many studies have reported that prognosis of GISTs with gastrointestinal bleeding is relatively poor compared with patients without bleeding. Recently, researchers have found that gastrointestinal bleeding may be an independent risk factor for recurrence.15–17 According to the 2012 edition of the Guidelines for the European Society of Oncology,18 GISTs of different malignant potential levels require different postoperative treatment and management. Although the emergence of targeted drugs such as imatinib has improved prognosis of stromal tumors more than that of other malignant tumors, there is also an incidence of adverse outcomes that lead to tumor recurrence and metastasis due to irregular treatment.
Molecular classifications and clinicopathological features of GIST
The average age of patients diagnosed with stromal tumors is 60 years. There are no significant epidemiological differences with respect to gender, ethnicity, or location.9,14,19 The prevailing hypothesis is that GISTs originate from the interstitial cells of Cajal in the muscularis propria and the myenteric plexus.20 Cell morphologies are characterized as spindle cell (70%), epithelioid cell (20%), and mixed cell (10%).21–23 KIT, DOG1, and CD34 have been identified as important immunohistochemical markers for making the diagnosis.24–26 In addition, CD34 and CD117 are likely to occur in GISTs.27 The expression of these markers plays an important role in regulating cell proliferation, differentiation, adhesion, and apoptosis. CD117 is currently recognized as one of the most significant markers of immune expression in stromal tumors. Hirota and other investigators have found that most GISTs produce genetic mutations, mostly in c-KIT. Approximately 90% of GISTs are associated with mutations in c-KIT,8 affecting the expression of exons 9, 11, 13, and 17.28 These mutations lead to continuous activation of tyrosine kinases, leading to cell proliferation and inhibition of apoptosis. These are the targets of tyrosinase inhibitors, such as imatinib. The distribution proportion of these mutations is approximately as follows: exon 11 (57%–71%); exon 9 (10%–18%); exon 13 (1%–4%); and exon 17 (1%–4%).24,25,29 The most frequent mutation in exon 11 is gene deletion, usually between codons 550 and 579, especially the codon 557–559.4 However, in c-KIT-negative GISTs, another tyrosine kinase receptor, called platelet-derived growth factor receptor alpha (PDGFRA), has also been identified.8,30 Most PDGFRA mutations affect exon 18 and less commonly exons 12 and 14.14,31–34 Overall, the PDGFRA gene mutation is estimated to account for 5%–10% of GISTs.21,32,35 It has been documented that 80% of PDGFRA mutations occur in the stomach and omental tissue.14
Approximately 10% of adult GISTs are not associated with KIT or PDGFRA mutations,22,36 while some stromal tumors do not harbor any known gene mutations. These are called “wild-type” tumors. These may arise from other pathogeneses, such as deficiency of succinate dehydrogenase37–39 or BRAF mutations.4
The pathological features of GISTs are closely related to prognosis. The Z90001 study by the American College of Surgeons Oncology Group found that the tumor size, location, and mitotic figure number are the most important factors affecting recurrence-free survival, as opposed to type of gene mutation.40 The classification of gene mutation often determines postoperative management and treatment, so it is important to improve gene analysis.
Common clinical manifestations and diagnostic features of GISTs
Clinical features
The clinical manifestations of GISTs are atypical and non-specific, depending on tumor size, location, and other factors. The most common clinical presentations are gastrointestinal bleeding and abdominal discomfort. Gastrointestinal bleeding accounts for about 30%–40%, abdominal pain 20%–50%, obstruction 10%–30%, and asymptomatic GIST patients account for 20%.8,9,41,42 Rare manifestations include biliary obstruction, dysphagia, intussusception, and hypoglycemia.
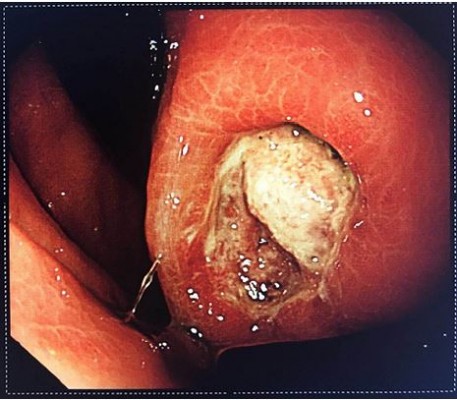
Gastrointestinal bleeding is the most common and the most dangerous complication, often necessitating emergency surgery. The risk of such surgery is significantly higher than that of elective surgery. GIST patients with chronic hemorrhage mainly present with anemia, emaciation, and melena. In cases of acute hemorrhage, the presentation may include peritonitis and shock. Spontaneous rupture of the tumor is rare in GISTs and often occurs in the gastrointestinal tract.43 Most hemorrhagic stromal tumors are associated with intact tunica serosa. Mucosal ulceration or tumor invasion of nutrient vessels leads to bleeding. In Figure 1, the tumor was ulcerated and bleeding was seen under endoscopy. Ulcers cause cancer-like umbilicated lesions. Some controversy remains as to whether these forms represent tumor rupture. Nevertheless, it has been mentioned in the literature that stromal tumors with hemorrhage may be independent risk factors for recurrence of stromal tumors.
  | Figure 1 Endoscopic manifestation of GIST with ulceration and active bleeding. Abbreviation: GIST, gastrointestinal stromal tumor. |
The causes of gastrointestinal bleeding in GIST
The causes of intraluminal hemorrhage of GISTs may be related to mucosal and submucosal destruction by tumor growth, invasion of nutrient vessels leading to vascular rupture, tumor necrosis, and the joint action of digestive juices, gastrointestinal peristalsis, and fecal transmission. GISTs are relatively fragile and more vascularized, compared with other common gastrointestinal tumors. In general, by the time symptoms of gastrointestinal bleeding appear, the tumor would already have attained a relatively large size.44 Studies have shown that the proportion of stromal tumor bleeding in the small intestine is much greater than in the stomach.16 This may be related to the size and space of the particular portion of the gastrointestinal tract.
Diagnostic features
In the acute hemorrhagic phase, digital subtraction angiography (DSA) is often positive and has hemostatic effects.45 However, DSA alone cannot distinguish benign from malignant tumors. Definitive diagnosis requires endoscopic ultrasonography (EUS) and pathological examination.46 Some stromal tumors may have “air sign” on plain film as shown in Figure 2, caused by internal bleeding, necrosis, or ulceration. There are few reported cases of bleeding outside the lumen in GISTs. Case reports suggest that trauma or external force may cause rupture and bleeding. The causes of GISTs bleeding are similar to those of other primary gastrointestinal malignant tumors; however, the proportion of GISTs that bleed is greater. Therefore, intestinal bleeding should raise the index of suspicion for GISTs. It is believed that the hemorrhage of stromal tumor is related to its pathological, immunohistochemical, or gene mutation type.
  | Figure 2 The enhanced-contrast CT image of a GIST with hemorrhage: the typical “air sign” is caused by hemorrhage and necrosis of the tumor. Abbreviation: GIST, gastrointestinal stromal tumor. |
Contrast-enhanced CT (ECT) is the most commonly used diagnostic modality for stromal tumors and is used as well for postoperative review and evaluation for recurrence and progression. EUS is also used to diagnose GISTs. By ultrasound, early tumor shows a hypoechoic mass. As the tumor grows, it replaces surrounding structures, forming cystic, necrotic, and bleeding areas. The sensitivity and specificity of EUS for diagnosis of stromal tumors in the stomach and colon are 98% and 64%, respectively.47 However, the diagnostic efficacy of endoscopic ultrasound-guided Trucut biopsy (EUS-TCB) and EUS-fine needle aspiration (EUS-FNA) are not very good. In addition, these procedures are complicated by pain, bleeding, and fever, among others. The complication rates are 3.3% for EUS-TCB and 8.1% for EUS-FNA.48 Early stromal tumors rarely metastasize to lymph nodes; however, these tumors tend to be large and they may infiltrate neighboring organs. Unlike other sarcomas, GIST does not metastasize to the bone and lungs. The most common target organs of distant metastasis are liver, peritoneum, and greater omentum.24,25,49 Therefore, TCB and FNA are not recommended if the patient is not being considered for conversion therapy, or if the clinician needs to differentiate GISTs from other tumors, for the following reasons: 1) stromal tumors are generally relatively easy to remove, 2) tumor rupture should be prevented in case of implantation metastasis, and 3) because of the presence of tumor capsule, it is difficult to get sufficient tissue to make a definite diagnosis using these modalities.23 An MRI is useful mainly to analyze invasion by pelvic lesions and to evaluate for liver metastases. For other locations, enhanced CT is preferred. Compared with other tumors of digestive tract, PET is not useful in the evaluation of GISTs. It may be only helpful for the case of GISTs with liver metastasis.50 However, the sensitivity and positive predictive value of PET-CT using 18 F-fluorodeoxyglucose in stromal tumors are 86% and 98%, respectively. These rates are better than those of CT for the recurrence and metastasis of stromal tumors.51
Treatments for GIST with gastrointestinal bleeding
Surgery is the treatment of choice for stromal tumors, whether there are symptoms of gastrointestinal bleeding. Some investigators believe that surgery is indicated when GIST size is >2 cm, otherwise it can be managed expectantly.1,52 However, if chronic blood loss is confirmed by fecal occult blood and ECT, surgery should be performed immediately.
Increasing number of small stromal tumors are being treated with endoscopic techniques. Endoscopy presents a number of advantages: short-term efficacy is acceptable, but the risk of long-term recurrence remains uncertain. However, endoscopic treatment has some limitations, related to the size and location of the tumor. In addition, the R0 resection rate is not as good as that of traditional surgery and carries a risk of GI tract perforation. Therefore, endoscopic treatment can increase the risk of tumor bleeding and rupture.23 Therefore, larger tumors should be treated with surgery to ensure maintenance of tumor integrity.
At present, laparoscopic techniques are developing rapidly, but the indications for surgical treatment of stromal tumors remain controversial. Some studies have shown that laparoscopic surgery can reduce recent complications and have no effect on the long-term prognosis of the tumor.53,54 For stomach tumors with a diameter <5 cm, laparoscopic wedge resection is recommended.2 Endoscopic retrieval bag is used to collect and remove the specimen, and forceps are contraindicated because of the risk of tumor rupture.
Traditional surgical methods continue to account for most treatments for stromal tumors, owing to the ease of the operation, and the higher R0 resection rate. Primary GISTs tend to transfer to, rather than invade adjacent structures. Therefore, the conventional wedge resection or local resection can be performed without routine lymph node dissection.55 The purpose of the surgery is to achieve negative margins (R0 resection). R1 resection or positive margins are not recommended for reoperation. Nevertheless, there is no evidence that the prognosis for R1 resections is worse than R0 resections.56,57 McCarter et al divided tumors into 3 groups: R0 (grossly and histologically negative margin), R1 (grossly negative but histologically positive margins), and R2 (grossly positive margins). The risks of recurrence were assessed without imatinib. No significant differences in outcome were found.58 Pathological specimens should be immediately evaluated for capsule rupture. It is recommended to collect fresh or frozen tissues, as new molecular pathology assessments and gene analysis can be performed soon after surgery.4
For patients with large tumors and chronic bleeding, oral imatinib is recommended. The reduction of tumor volume is expected to achieve optimal surgical outcome and reduce postoperative recurrence. If acute hemorrhage is not controlled, emergency surgical treatment becomes necessary. Approximately 15%–47% of patients have distant metastases at diagnosis, with common sites being the liver, peritoneum, and omentum.31,59,60 If this is the case, targeted therapy should be performed first. NCCN recommends imatinib prior to surgery as neoadjuvant treatment, including for patients with incomplete resection or high risk of recurrence after resection.13 It is not recommended that patients with D842V PDGFRA mutations use imatinib because of well-known resistance of these tumors to drugs and TKIs.
The surgery should involve as little disruption of healthy tissue as possible so as to reduce other surgical complications and to improve postoperative quality of life. The target drugs should be discontinued 5–7 days prior to surgery and restarted 2 weeks after surgery. Multidisciplinary team (MDT) methods include endoscopy and pathology. It is necessary for the consultants from departments of radiology, oncology, and surgery to develop individualized treatment regimens.
Small GISTs without gastrointestinal bleeding do not require any particular treatment; however, conventional follow-up remains important. If bleeding occurs, immediate treatment becomes necessary. Many studies indicate that small stromal tumors can be managed expectantly, but when bleeding occurs, there is a high probability of tumor necrosis and ulceration, suggesting that the tumor is developing rapidly, leading to poor prognosis.16,17
Postoperative management of GIST
Genetic testing should be performed routinely to guide the medication regimen. The most recent European consensus proposed that the KIT and PDGFRA gene mutation can be used to analyze and confirm the diagnosis of GISTs, especially in cases with negative CD117.61 CT is the most effective modality for follow-up. Here, we recommend enhanced abdominal and pelvic CT. In low-risk groups, CT can be performed yearly during the first 5 years. For the middle- and high-risk groups, it is recommended every 6 months. If medication is discontinued, it is recommended that CT examination be performed every 3–4 months during the first 2 years and every 6–12 months in the following 10 years.62 We suggest that patients with interstitial tumors with hemorrhage be managed similar to the high-risk group.
According to NCCN guidelines, patients in the moderate- and high-risk groups should be treated with imatinib for 1–3 years to reduce the possibility of recurrence. The benefits and tolerability of imatinib for 5 years are currently under study. However, there is not enough scientific evidence to support the imatinib adjuvant therapy in patients with moderate risk.4 In Table 2, many studies on imatinib and other targeted drugs have gradually changed the postoperative management of GISTs. The usual dose of imatinib is 400 mg/day. The average duration of resistance to imatinib followed by progression is 2–2.5 years. For patients who use imatinib 400 mg/day, if progression occurs, the dose can be increased to 800 mg/day.63–65 The higher dose may improve the patients’ progression-free survival and overall survival.66 KIT exon 9 mutation requires oral administration of 600–800 mg/day, although the dosage has never been prospectively confirmed.23 The study by Blanke et al67 suggested that higher doses of imatinib do not improve the survival time due to side effects. However, in GIST patients with exon 9 mutations, the relative risk decreased by 61%.68 If the higher dose of imatinib is not tolerated, or the disease progresses, sunitinib can provide significant, persistent clinical benefits in patients with imatinib resistance or intolerance.69 Those who use imatinib preoperatively for neoadjuvant therapy should have a total course of 3 years.55 But 26% of the patients stopped using imatinib for a variety of reasons.65 A number of studies have shown that, compared with patients who continued treatment, patients who took imatinib for only 1 year had significantly higher progression rates 3 years later.70,71
Mutations detected during treatment are often resistant to TKIs and are known as secondary mutations.35,36 Secondary resistance occurs initially in response to imatinib and progresses to the clonal expansion, making the tumors drug resistant.72 If the increased dose of the disease continues to progress, second-line treatment of sunitinib can be considered. George et al73 showed that sunitinib dose at 37.5 mg/day remained effective, and tolerance was better. Regorafenib, sorafenib, ponatinib, and other multi-targeted TKIs can be selected according to the individual conditions. Multidisciplinary approaches with individualized treatment plans are essential.
Prognostic factors
Risk factors for GIST recurrence include location, size, numbers of mitotic figures, and tumor rupture.1,74 Among these, number of mitotic figures is the most important. Whether KIT, PDGFRA mutations, and gastrointestinal bleeding should be added to the risk stratification scheme remains controversial.
In other malignant tumors of the digestive system, hematogenous spread is an important mode of metastasis. Prognosis becomes poor once hematogenous metastasis occurs. Tumor spread occurs easily following rupture and bleeding. Malignant tumor bleeding is often accompanied by infection or perforation, complicating clinical treatment. The opportunity to perform surgery is often lost when the disease is discovered in late stage or when the patient cannot tolerate surgery. Most tumor bleeding during the perioperative period requires transfusion. One study suggests that perioperative blood transfusion may be related to low immune function, thereby increasing the possibility of tumor recurrence.75 Intraoperative blood loss also impacts prognosis. The less blood loss at surgery, the better the prognosis. This may be due to the fact that bleeding is often related to vascular invasion or serosal involvement.76 Blood from tumors may induce mesothelial cells to release large amounts of cellulose into the peritoneum, giving rise to favorable conditions for tumor implantation and growth.77,78 The 5-year survival rate of gastrointestinal tumors with bleeding and perforation is only 24%, possibly due to peritoneal dissemination.79 However, this mechanism needs further study and confirmation.
Stromal tumor bleeding may be caused by the enhancement of tumor invasion to mucous membrane after gene mutation, leading to hemorrhage caused by destruction of the mucosal layer. Recently, a number of studies have highlighted the ability of GISTs to produce angiogenic factors that not only enhance feeding of tumors but also mediate tumor infiltration. The endothelial cell markers CD31, CD34, and FVIII-Ragd are commonly used to mark neovascularization and tumor growth.80 Although expression of CDll7 and CD34 in GIST plays an important role in the diagnosis, the relationship of these markers with bleeding and prognosis is not clear.81
Conclusion
In the latest version of the NCCN guidelines, tumor size, location, number of mitotic figures, and tumor rupture are noted as risk factors for grading purposes. Although the mechanism of bleeding in GISTs is not yet fully understood, existing studies suggest that GISTs associated with bleeding tends to indicate poor prognosis. The risk stratification of GIST recurrence is closely related to prognosis, which determines subsequent treatment. We believe that recurrence risk classification of GISTs will improve through future studies. With the increasing popularity of imaging and EUS, the diagnostic success rate of GISTs is increasing. There is a rapid increase in basic research on GISTs. The pathological and biological characteristics are becoming clearer. With the continued appearance of tyrosinase inhibitors, such as imatinib, the prognosis of GISTs has improved significantly. Some late-stage cases that cannot be treated by surgery, as well as patients who cannot use the standard medications, may benefit from MDT and translational medicine. GIST requires multidisciplinary management, which improves both prognosis and quality of life.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgment
This work was supported by Scientific Research of Special-Term Professor from the Educational Department of Liaoning Province, China (Liao Cai Zhi Jiao No. 2012-512).
Disclosure
The authors report no conflicts of interest in this work.
References
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. | ||
Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol. 2017;8:135–144. | ||
Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(Suppl 5):S39–S51. | ||
Poveda A, García Del Muro X, López-Guerrero JA, et al. GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107–119. | ||
Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543–552. | ||
Takazawa Y, Sakurai S, Sakuma Y, et al. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen’s disease). Am J Surg Pathol. 2005;29:755–763. | ||
Jiang ZX, Zhang SJ, Peng WJ, Yu BH. Rectal gastrointestinal stromal tumors: imaging features with clinical and pathological correlation. World J Gastroenterol. 2013;19:3108–3116. | ||
Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: a review. World J Gastrointest Oncol. 2013;5:102–112. | ||
Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer. 2005;103:821–829. | ||
Kramer K, Knippschild U, Mayer B, et al. Impact of age and gender on tumor related prognosis in gastrointestinal stromal tumors (GIST). BMC Cancer. 2015;15:57. | ||
Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol. 1995;2:26–31. | ||
Stiles ZE, Rist TM, Dickson PV, et al. Impact of body mass index on the short-term outcomes of resected gastrointestinal stromal tumors. J Surg Res. 2017;217:123–130. | ||
von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 22016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(6):758–786. | ||
Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S1–S41; quiz S42–S44. | ||
Lv A, Li Z, Tian X, et al. SKP2 high expression, KIT exon 11 deletions, and gastrointestinal bleeding as predictors of poor prognosis in primary gastrointestinal stromal tumors. PLoS One. 2013;8:e62951. | ||
Liu Q, Li Y, Dong M, Kong F, Dong Q. Gastrointestinal bleeding is an independent risk factor for poor prognosis in GIST patients. Biomed Res Int. 2017;2017:7152406. | ||
Yin Z, Gao J, Liu W, et al. Clinicopathological and prognostic analysis of primary gastrointestinal stromal tumor presenting with gastrointestinal bleeding: a 10-year retrospective study. J Gastrointest Surg. 2017;21:792–800. | ||
ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii49–vii55. | ||
Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–184. | ||
Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–389. | ||
Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. | ||
Pantaleo MA, Astolfi A, Indio V, et al. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:983–987. | ||
Lim KT, Tan KY. Current research and treatment for gastrointestinal stromal tumors. World J Gastroenterol. 2017;23:4856–4866. | ||
Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. | ||
Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. | ||
Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. | ||
Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–734. | ||
Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. | ||
DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422–429. | ||
Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. | ||
Ho MY, Blanke CD. Gastrointestinal stromal tumors: disease and treatment update. Gastroenterology. 2011;140:1372.e2–1376.e2. | ||
Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–266. | ||
Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. | ||
Isozaki K, Hirota S. Gain-of-function mutations of receptor tyrosine kinases in gastrointestinal stromal tumors. Curr Genomics. 2006;7:469–475. | ||
Reichardt P, Hogendoorn PC, Tamborini E, et al. Gastrointestinal stromal tumors I: pathology, pathobiology, primary therapy, and surgical issues. Semin Oncol. 2009;36:290–301. | ||
Martín-Broto J, Rubio L, Alemany R, López-Guerrero JA. Clinical implications of KIT and PDGFRA genotyping in GIST. Clin Transl Oncol. 2010;12:670–676. | ||
Ben-Ami E, Barysauskas CM, von Mehren M, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol. 2016;27:1794–1799. | ||
Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30:2377–2383. | ||
Demetri GD, Casali PG, Blay JY, et al. A phase I study of single-agent nilotinib or in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:5910–5916. | ||
Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–1570. | ||
Sleijfer S, Seynaeve C, Wiemer E, Verweij J. Practical aspects of managing gastrointestinal stromal tumors. Clin Colorectal Cancer. 2006;6(Suppl 1):S18–S23. | ||
Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269–280. | ||
Ajduk M, Mikulić D, Sebecić B, et al. Spontaneously ruptured gastrointestinal stromal tumor (GIST) of the jejunum mimicking acute appendicitis. Coll Antropol. 2004;28:937–941. | ||
Caterino S, Lorenzon L, Petrucciani N, et al. Gastrointestinal stromal tumors: correlation between symptoms at presentation, tumor location and prognostic factors in 47 consecutive patients. World J Surg Oncol. 2011;9:13. | ||
Ba MC, Qing SH, Huang XC, Wen Y, Li GX, Yu J. Diagnosis and treatment of small intestinal bleeding: retrospective analysis of 76 cases. World J Gastroenterol. 2006;12:7371–7374. | ||
Blanchard DK, Budde JM, Hatch GF, et al. Tumors of the small intestine. World J Surg. 2000;24:421–429. | ||
Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–208. | ||
Na HK, Lee JH, Park YS, et al. Yields and utility of endoscopic ultrasonography-guided 19-gauge trucut biopsy versus 22-gauge fine needle aspiration for diagnosing gastric subepithelial tumors. Clin Endosc. 2015;48:152–157. | ||
Kobayashi K, Gupta S, Trent JC, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006;107:2833–2841. | ||
Van den Abbeele AD. The lessons of GIST–PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13. | ||
Gayed I, Vu T, Iyer R, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004,45(1):17–21. | ||
Tan Y, Tan L, Lu J, et al. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017;2:115. | ||
Piessen G, Lefèvre JH, Cabau M, et al. Laparoscopic versus open surgery for gastric gastrointestinal stromal tumors: what is the impact on postoperative outcome and oncologic results? Ann Surg. 2015;262:831–839; discussion 829–840. | ||
Novitsky YW, Kercher KW, Sing RF. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738–745; discussion 745–747. | ||
Keung EZ, Raut CP. Management of gastrointestinal stromal tumors. Surg Clin North Am. 2017;97:437–452. | ||
Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. | ||
Zhi X, Jiang B, Yu J, et al. Prognostic role of microscopically positive margins for primary gastrointestinal stromal tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:21541. | ||
McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215:53–59; discussion 59–60. | ||
Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. 2009;23:69–78. | ||
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. | ||
ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii21–iii26. | ||
Joensuu H, Martin-Broto J, Nishida T, Reichardt P, Schöffski P, Maki RG. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611–1617. | ||
Keung EZ, Fairweather M, Raut CP. The role of surgery in metastatic gastrointestinal stromal tumors. Curr Treat Options Oncol. 2016;17:8. | ||
Vadakara J, von Mehren M. Gastrointestinal stromal tumors: management of metastatic disease and emerging therapies. Hematol Oncol Clin North Am. 2013;27:905–920. | ||
von Mehren M. Gastrointestinal stromal tumors. J Clin Oncol. 2018;36:136–143. | ||
Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35:1713–1720. | ||
Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. | ||
Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006,42(8):1093–1103. | ||
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. | ||
Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–1113. | ||
Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–949. | ||
Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol. 2007;14:526–532. | ||
George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–1968. | ||
Rubin BP, Blanke CD, Demetri GD, et al. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med. 2010;134:165–170. | ||
Jagoditsch M, Pozgainer P, Klingler A, Tschmelitsch J. Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum. 2006;49:1116–1130. | ||
Dhar DK, Kubota H, Tachibana M, et al. Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg. 2000;24:588–593; discussion 593–594. | ||
Fernandez PM, Patierno SR, Rickles FR. Tissue factor and fibrin in tumor angiogenesis. Semin Thromb Hemost. 2004;30:31–44. | ||
Aoyagi K, Kouhuji K, Yano S, et al. VEGF significance in peritoneal recurrence from gastric cancer. Gastric Cancer. 2005;8:155–163. | ||
Steigen SE, Bjerkehagen B, Haugland HK, et al. Diagnostic and prognostic markers for gastrointestinal stromal tumors in Norway. Mod Pathol. 2008;21:46–53. | ||
Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O. Organ-specific endoglin (CD105) expression in the angiogenesis of human cancers. Pathol Int. 2006;56:717–723. | ||
Park SS, Ryu JS, Oh SY, Kim WB, Lee JH, Chae YS. Surgical outcomes and immunohistochemical features for gastrointestinal stromal tumors (GISTS) of the stomach: with special reference to prognostic factors. Hepatogastroenterology. 2007;54:1454–1457. | ||
Majer IM, Gelderblom H, van den Hout WB, et al. Cost-effectiveness of 3-year vs 1-year adjuvant therapy with imatinib in patients with high risk of gastrointestinal stromal tumour recurrence in the Netherlands; a modelling study alongside the SSGXVIII/AIO trial. J Med Econ. 2013;16:1106–1119. | ||
Patrikidou A. Key messages from the BFR14 trial of the French Sarcoma Group. Future Oncol. 2017;13:273–284. | ||
Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013;14:1175–1182. | ||
Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33:4276–4283. | ||
Miettinen M, Sobin LH. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. | ||
Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


