Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Management of chronic hepatitis C virus infection in patients with end-stage renal disease: a review
Authors Valadez JM, Juárez IG, Pedrero RR, Torre A
Received 14 September 2014
Accepted for publication 28 October 2014
Published 27 February 2015 Volume 2015:11 Pages 329—338
DOI https://doi.org/10.2147/TCRM.S74282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Jonathan Aguirre Valadez,1 Ignacio García Juárez,1 Rodolfo Rincón Pedrero,2 Aldo Torre1
1Department of Gastroenterology, 2Department of Nephrology, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico City, Mexico
Abstract: Infection with hepatitis C virus (HCV) is highly prevalent in chronic kidney disease (CKD) patients, mainly in those on hemodialysis (HD). The seroprevalence of HCV in developing countries ranges between 7% and 40%. Risk factors for this infection in the CKD population include the number of blood transfusions, duration of end-stage renal disease (ESRD), and prevalence of HCV in HD. Chronic HCV infection in patients with ESRD is associated with an increase in morbidity and mortality in the pre and post kidney transplant periods. The increase in mortality is directly associated with liver complications and an elevated cardiovascular risk in HCV-infected patients on hemodialysis. Antiviral treatment may improve the prognosis of patients with HCV, and standard interferon remains the cornerstone of treatment. Treatment of HCV in patients with CKD is complex, but achieving a sustained viral response may decrease the frequency of complications after transplantation. It appears that HCV-infected patients who remain on maintenance dialysis are at increased risk of death compared with HCV patients undergoing renal transplantation.
Keywords: hepatitis C virus, chronic kidney disease, hemodialysis, interferon
Introduction
Hepatitis C virus (HCV) infection is highly prevalent in patients with chronic kidney disease (CKD) requiring renal replacement therapy, and is the most frequently recognized cause of liver injury in patients with CKD. The prevalence of anti-HCV in developing countries ranges between 7% and 40%.1–3 Risk factors for patients on hemodialysis (HD) for acquiring HCV infection include number of previous blood transfusions,4 duration of end-stage renal disease (ESRD),5 prevalence of HCV in HD units,2 a history of previous transplant6 and patient age.2,7
Chronic HCV infection in patients with ESRD leads to an increase in morbidity and mortality in the pre and post renal transplant period.8–11 The increase in mortality is directly associated with liver complications, although HCV-related liver disease tends to be mostly asymptomatic in patients on long-term dialysis.8,11 furthermore, patients undergoing kidney transplantation are at risk of developing graft nephropathy and diabetes mellitus secondary to HCV infection.12–15
Antiviral treatment may improve the prognosis of patients with HCV after kidney transplantation; however, despite the knowledge of the mechanisms of HCV transmission in HD units, experience in the treatment of HCV infection in patients with ESRD and in kidney transplant recipients is still limited.16
Transmission and prevalence
In most regions, HCV infection is significantly more common in people with kidney disease when compared with the general population.17 The estimated prevalence of HCV infection in HD patients is 3%–20% in the USA and Western Europe.17 In 2002, approximately 8% of patients on chronic HD in the USA were seropositive for HCV.18 Moreover, the seroprevalence in HD patients in other regions is significantly higher; for example, a study conducted in Egypt reported a higher prevalence (80%) in patients on chronic HD.19
Inappropriate infection control practices, such as incorrect parenteral drug delivery, poor equipment sterilization, or both, have been documented during some outbreaks.17 Therefore, guidelines exist for the prevention of HCV transmission in HD units, emphasizing infection control measures such as correct handling of parenteral medications, disinfection of HD machines and handwashing.20,21 HCV infection should be routinely sought in patients on chronic HD by determining anti-HCV antibody titers. In case of patients with negative results, the test should be repeated every 6–12 months.21
Natural history of HCV infection in patients on dialysis
HCV infection does not usually present with acute symptoms, and disease progression is a long-term process. Mostly, patients are diagnosed with HCV infection after blood screening; no symptoms or elevations of liver enzyme levels are specific of the disease. Spontaneous clearance of HCV RNA has been documented in 1% of untreated patients on HD.22
The impact of ESRD on the progression of liver injury secondary to HCV infection is difficult to assess due to the high mortality rate in patients in chronic HD units, and because assessing the degree of liver disease by biopsy is challenging due to the associated comorbidities, such as platelet disorders, prevailing in this population.
Transient elastography is a noninvasive tool designed to assess the severity of hepatic fibrosis in terms of organ stiffness; it has been evaluated in HCV-infected patients on HD and compared with liver biopsy. Transient elastography appeared to be superior to other noninvasive methods (aspartate aminotransferase/platelet ratio index), and could potentially decrease the need for staging liver biopsies in HD patients with HCV infection.23
Fabrizi et al established that the relative risk of death in patients with ESRD and HCV infection (all-cause mortality), was 1.35 (95% confidence interval [CI] 1.25–1.47); liver disease was the most frequent cause of death in this group of patients on HD.24
A study of the natural course of HVC infection in HD patients was conducted by Okuda and Yokosuka25 who compared 189 patients with chronic HVC infection on HD (cases) and patients with chronic HCV infection and no ESRD (controls); the patients were age-matched and followed for 4–23 years. No cases progressed to cirrhosis, while 25% of patients in the control group developed cirrhosis (P<0.0001). Although overall mortality increases with HCV infection in ESRD patients, disease progression and development of liver failure appear to be slower and/or less likely in uremic patients.26,27
A recent meta-analysis of 14 observational studies demonstrated an independent and significant impact of HCV on mortality among patients on long-term dialysis, with an adjusted relative risk of 1.35 (95% CI 1.25–1.47); cardiovascular mortality was 1.26 (95% CI 1.10–1.45) in HCV-positive, being a major cause of death.28
The course of the infection is not thoroughly understood, but some hypotheses argue that the viral load decreases in ESRD patients on HD when compared with nonuremic controls; however, not all the studies support this theory.29,30 Passage of viral particles into the dialysate fluid, trapping of the virus on the surface of the dialyzer membrane, and the significant amount of cytokines with antiviral properties indirectly produced by the host are the main areas of speculation in the tentative explanation of the effect of HD on HCV viremia.31
The evidence for a relationship between HCV and survival among patients on peritoneal dialysis is more limited, the incidence of liver disease-related mortality is higher in patients with HCV infection than in HCV-negative cases and may be related to impaired nutritional status.32
HCV infection in kidney transplant recipients
The management of kidney transplantation in HCV-positive patients remains a challenge because, aside from renal failure, liver disease must be taken into account. Various studies have revealed that, overall patient and graft survival are significantly shortened. Mortality resulting from liver disease as well as the risk of developing hepatocellular carcinoma are increased in HCV-positive kidney transplant recipients.33,34 Good survival rates have been obtained in these patients, especially if they have minimal or well-controlled liver disease.35 In patients undergoing renal transplantation in the setting of established cirrhosis and a hepatic portal venous gradient (HPVG) below 10 mmHg, a combined liver-kidney transplantation may be unnecessary and a kidney transplant alone may be safely performed.36,37
The impact of immunosuppression on the clinical course and progression of HCV infection in kidney transplant recipients is not completely elucidated; frequently, HCV RNA levels increase after transplantation, which may be related to a decrease in viral clearance.38 Further, the role of immunosupression on the progression of fibrosis in cases of HCV infection is uncertain in kidney transplant recipients. Several studies have found that the patterns of fibrosis progression are stable and may even improve after treatment;39,40 in fact, cyclosporine inhibits the replication of HCV in vitro.41
There are case reports of fibrosing cholestatic hepatitis after kidney transplant related to immunosuppression. This entity is difficult to treat and is associated with high morbidity and mortality rates, and its treatment is associated with risk of graft rejection.42,43 Survival of patients with fibrosing cholestatic hepatitis improves with early initiation of PEGylated interferon (IFN)-α2a and ribavirin therapy, strict monitoring by biopsy and HCV load determinations, and replacement of tacrolimus with cyclosporine.44 Aside from the liver complications associated with HCV infection, kidney transplant patients can develop immune complex glomerulonephritis and renal interstitial fibrosis and tubular atrophy (previously known as chronic allograft nephropathy).45
Despite the potential development of complications and progression of HCV infection in renal post-transplant patients, HCV infection is not considered a contraindication for kidney transplantation because survival after transplantation is markedly higher than that of HCV patients who remain on chronic HD.46,47
Treatment of HCV infection in CKD patients in different settings
More information on the treatment of HCV infection in patients with CKD has recently become available. As in most patients with hepatitis C, the decision to initiate therapy is largely based on the stage of the liver disease, the expectation of a sustained viral response (SVR) and associated comorbidities. HCV patients on HD who are kidney transplant candidates should be treated more aggressively since viral eradication before kidney transplantation fosters a decrease of both hepatic and renal complications.
Treatment of chronic HCV in patients with ESRD
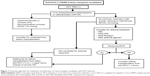
The risk of death in patients with HCV infection on dialysis is higher than in patients on dialysis without HCV.48 It is recommended that HCV-positive patients who are candidates for kidney transplantation be treated with antiviral therapies. Tested therapeutic modalities for this group of patients include IFN alone or in combination with ribavirin. Infections due to HCV genotypes 1 and 4 are less responsive to IFN-based therapy and require treatment for 48 weeks (Figure 1).
Monotherapy with standard IFN-α
There are reports demonstrating that use of IFN in patients awaiting kidney transplantation is associated with higher SVR rates as well as continuous biochemical improvement in the post-transplant period; also, HCV-infected patients who are renal transplant candidates and treated with IFN show lower rates of renal interstitial fibrosis and tubular atrophy.49 This may be the result of the lower viral loads described in these patients, or a result of the incomplete removal of IFN during HD.
IFN therapy on HD patients is not well tolerated so high dropout rates are seen. Neurological (21%) and gastrointestinal (18%) adverse effects are reported.
At least two meta-analyses have shown that standard IFN therapy is associated with the achievement of SVR, although there is no evidence that this impacts the survival of patients with ESRD on HD. In patients with ESRD infected with HCV, conventional treatment with IFN monotherapy at a dose of 1–6 million units daily or three times a week for 12–48 weeks leads to SVR rates of 39%–41%, with treatment-related dropout rates of 26%–27%.22,50
Predictive factors associated with the response to IFN include: dosage (3 million units or above), duration of therapy (at least 6 months), low pretreatment viral load and liver histology (moderate injury).22,51–53 Patients with decompensated liver disease are not candidates for treatment, the treatment should be stopped for those who do not achieve a negative viral load after 4–8 weeks of treatment, since the probability of SVR is remote.
In accordance with the 2008 KDIGO (Kidney Disease: Improving Global Outcomes) guidelines (Table 1),20 the standard IFN dosage should be adjusted if the glomerular filtration rate is below 15 mL/min/1.73 m2; recommended for the management of HCV patients who are kidney transplant candidates, those on HD, and those with stage 5 CKD.
  | Table 1 Summary by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative of KDIGO guidelines for the prevention, diagnosis, evaluation and treatment of hepatitis C in patients with chronic renal failure 200885 |
PEGylated IFN monotherapy
There is no reported difference in the clearance of PEGylated IFN monotherapy (PEG-IFN-α2a) between patients with normal renal function and those with significantly decreased renal function (glomerular filtration rate >100 mL/min versus 20–40 mL/min). PEG-IFN pharmacokinetics may vary during the HD process. The largest series of HCV patients treated with PEG-IFN reported SVR rate of 14.1% and a high rate of adverse events (83%).54 Recently, Wang et al prospectively evaluated the efficacy and tolerability of low doses of standard IFN-α2b (1.5×106 U three times per week) and PEG-IFN-α2a (67.5 μg once a week) in HCV-positive HD patients; SVR with PEG-IFN was obtained in 91.7% of cases, with a dropout rate of 8.3%. With standard IFN, SVR rate of 71.4% was achieved, with a dropout rate of 28.6%. Anemia was the most frequent side effect, observed in 55.5% of cases and erythropoietin therapy was required.55 Two meta-analyses showed SVR and treatment-related dropout rates of 31%–37% and 23%–28%, respectively, which is comparable with standard IFN therapy.22,56
A study directly comparing treatment with standard IFN vs PEG-IFN demonstrated superior efficacy and safety of PEG-IFN.57 Similarly, there were some predictors of SVR with PEG-IFN therapy, including low pretreatment levels of HCV RNA and a rapid virologic response.57 In HCV-infected patients with CKD stages 3, 4 and 5 awaiting dialysis therapy, monotherapy of PEG-IFN with doses adjusted according to kidney function is suggested in accordance with the 2008 KDIGO guidelines (Table 1).20
Combination therapy of standard IFN plus ribavirin
The elimination rate of ribavirin in patients with impaired renal function is decreased, and a small fraction of the drug is removed by HD. Ribavirin is contraindicated in patients with ESRD infected with HCV, because of the potential risk of life-threatening hemolytic anemia. However, some studies have shown that using a low dose of ribavirin (200–400 mg three times a week) in order to obtain serum levels of 10–15 μmol/L, combined with standard IFN plus high doses of erythropoietin, could be acceptable in these patients.
There is no information available on the appropriate ribavirin dosage or its adverse effects in patients with HCV who are on dialysis. There are some studies involving small numbers of patients treated with combination therapy (IFN + ribavirin) and different ribavirin doses, yielding different SVR (17%–66%) and discontinuation (0%–33%) rates.58–60
A meta-analysis by Fabrizi et al61 reported SVR rate of 56% (95% CI 28–85) and a dropout rate of 25% (95% CI 10–40). The most frequent side effects requiring interruption of treatment were anemia (26%) and heart failure (9%). Despite this evidence, ribavirin is not recommended for routine use in the management of HCV patients with a glomerular filtration rate below 50 mL/min unless a new large-scale study confirms its safety profile.
Combined antiviral therapy of PEG-IFN plus ribavirin
There is little information of the use of double therapy in HCV patients with CKD. In a prospective study of HCV patients on chronic HD (young patients awaiting kidney transplantation), cases were treated for 24–48 weeks depending on genotype; the SVR rate was 97.5%, 74% of patients required treatment with erythropoietin, and 31% required reduction of the ribavirin dosage. These patients also required higher erythropoietin doses (10,000–40,000 IU/week) to maintain an adequate dose of ribavirin during treatment to achieve adequate viral suppression. In this study, like in other settings, patients with HCV genotypes 2 and 3 had higher SVR rates compared with genotypes 1 and 4.62
Patients who relapsed after initial treatment with IFN monotherapy were evaluated in a study by Djordjeviƈ et al in which four patients relapsed after 12 weeks of conventional therapy were considered for a second treatment consisting of standard IFN administered for another 24 weeks, although all patients had viral suppression, none achieved SVR.63 In a second study conducted in 2009, 35 patients relapsing at week 24 were retreated with double standard therapy of IFN or PEG-IFN and ribavirin for 48 weeks (genotype 1) and 24 weeks (genotype 2), the average SVR was 60%, albeit higher in patients with genotype 2 (80% versus 52%), most patients required combined treatment with erythropoietin. Independent predictors of SVR were pretreatment viral load and rapid virologic response.64
Recently, 12 HCV-positive (kidney and liver) transplant recipients treated with PEG-IFN-α plus ribavirin were studied; no acute rejection was observed, renal function remained stable during and after discontinuing treatment, and there was no allograft dysfunction. Two patients had a partial virologic response and four had SVR; these data suggest that combination therapy does not increase the risk of acute kidney graft rejection after liver-kidney transplantation.65
Combined antiviral treatment with PEG-IFN and ribavirin is suggested in HCV-infected patients with CKD grades 1 or 2, as in the non CKD population. Ribavirin doses should be titrated according to patient tolerance and in accordance with the 2008 KDIGO guidelines (Table 1).20
Acute HCV therapy in patients with ESRD
HCV progresses to chronic infection in 90% of uremic patients. Monitoring aminotransferase levels in the HD population may facilitate the detection of acute viral infections. In terms of the management of acute HCV infection, some studies have evaluated therapy based on IFN-α for 12 weeks and reported that 67% of patients with acute hepatitis C achieved SVR compared with 0% of without treatment patients.66
A recent meta-analysis67 determined that virologic response after antiviral therapy was more common than spontaneous viral clearance in dialysis patients with acute hepatitis; also, IFN-based treatment of acute hepatitis C in dialysis populations yielded SVR in 50% of cases.
Treatment of chronic HCV in kidney transplant candidates
Evidence regarding standard IFN therapy in HD patients shows that 38% of patients achieved SVR; of these, 76% were transplanted and received immunosuppressive therapy with antithymocyte globulin, and viremia was absent in 100% of patients 22.5 months after transplantation.68
The use of IFN before renal transplantation may decrease the occurrence of de novo or recurrent glomerulonephritis. An additional benefit of pretransplant antiviral therapy is a decreased incidence of renal interstitial fibrosis and tubular atrophy, since HCV infection has been implicated in its pathogenesis. Antiviral therapy can also decrease the incidence of post-transplant diabetes mellitus in the graft recipient.
HPVG has recently been proposed as a parameter to determine whether well compensated cirrhotic patient can be considered for block transplantation or only kidney transplantation. The consensus suggests that patients with a HPVG <10 mmHg should be candidates only for renal transplant.34,69
A systematic review and meta-analysis suggest that HCV patients who remain on HD have 2.19 times greater risk of death when compared with those who undergo kidney transplantation; eight patients need to be transplanted to prevent one death, particularly in HCV patients aged 45 years or older.47
Patients with chronic hepatitis C should be treated with standard therapy or PEG-IFN. If there is no early viral response, treatment should be discontinued and patients should be referred to a specialist in hepatology for a second evaluation. Moreover, if an early viral response is obtained, waiting is recommended for at least 28 days after administration of IFN prior to kidney transplantation.20–34
Treatment of patients with HCV after kidney transplantation
It has been postulated that the immune stimulating effects of IFN can promote allograft rejection in patients who receive a kidney transplant. Hassan et al recently evaluated 12 HCV-positive liver-kidney transplant recipients treated with PEG-IFN-α plus ribavirin. No acute rejection was observed, renal function remained stable during and after discontinuing treatment, there was no allograft dysfunction, two patients had a partial viral response, and four had SVR. These data suggest that combination therapy did not foster a higher risk of acute kidney graft rejection after liver-kidney transplantation.70
Recently, Wei et al71 conducted an updated meta-analysis to evaluate IFN-based antiviral therapy in HCV infection after renal transplantation. The overall comparative SVR rates in PEG-IFN-based and standard IFN-based therapy were 40.6% and 20.9%, respectively. The most frequent side effect requiring discontinuation of treatment was graft dysfunction (occurring in 45% of cases), demonstrating the limited safety and efficacy of IFN-based antiviral therapy for HCV infection after kidney transplantation.
Based on the KDIGO guidelines (Table 1),20 IFN therapy should be considered in patients at high risk of graft loss, like fibrosing cholestatic hepatitis or threatening life vasculitis. If immunosuppressive agents are used with new antiviral drugs such as protease inhibitors, like telaprevir and boceprevir, the risk of drug toxicity is increased.72
Kidney donor with positive serology for HCV
Several studies have established that HCV-positive transplant recipients receiving organs from HCV-positive donors suffer from higher rates of liver disease but not lower survival rates when compared with patients who receive organs from HCV-negative donors.73 HCV can be transmitted from an infected donor to the recipient, and there are factors that influence the transmission of HCV infection, such as viral load.
Only 29% of HCV-positive recipients are transplanted with HCV-positive kidneys. The kidneys are discarded 2.5 times more often due to the sense that HCV-positive kidneys may adversely compromise recipient liver function. Despite the slightly increased risk, a national study has suggested that there is likely to be a survival benefit in most HCV-positive patients transplanted from an HCV-positive kidney compared with waiting for an HCV-negative organ.74 Recently, Kucirka et al analyzed 6,250 patients with HCV who had undergone a kidney transplant and were captured in the United Network for Organ Sharing (UNOS) database. They recorded the liver-related outcomes and found that 1% of the HCV-positive recipients eventually enter to the liver transplant waiting list over a 13-year study period. Those who received HCV-positive kidneys had a 2.6-fold higher hazards ratio of enrolling in the liver transplant list (P≤0.0001). They concluded that transplantation of an HCV-positive kidney may decrease the recipient’s time on the list by over a year, which is a better option than waiting for an HCV-negative kidney, due to the high risk of kidney-related mortality while awaiting transplantation.75
Morales et al76 compared the outcomes in kidney transplant recipients (HCV-positive) who received a graft from an HCV-positive donor with those of patients (HCV positive) who received a kidney graft from an HCV-negative donor. They found no significant difference in patient survival at 5 and 10 years (84.8% at 5 years and 72.7% at 10 years versus 86.6% and 76.7%, respectively). Decompensated liver disease rates were also not significantly different between the two groups.
Transplanting kidneys from HCV-positive organ donors into HCV-positive/RNA-negative recipients leads to greater viral reactivation than in those HCV-positive/RNA-positive recipient.77 Thus, many transplant centers have adopted the policy of transplanting HCV-positive kidneys into HCV-positive/RNA-positive patients or those with active viremia.78 The type of HCV genotype might not have a significant impact on survival in patients with ESRD, since survival in patients with mixed genotypes was similar to that of patients with a single genotype.79
New therapies for HCV in patients with ESRD?
In May 2011, the US Food and Drug Administration (FDA) approved the NS3/4A protease inhibitors, boceprevir and telaprevir, for the treatment of HCV genotype 1, marking the beginning of an era of direct-acting antiviral agents. Protease inhibitors such as boceprevir and telaprevir in combination with IFN and ribavirin (triple therapy) have become another new management strategy for HCV genotype 1 infection, whereby up to 75% of previously untreated patients with HCV genotype 1 have achieved SVR. However, these new drugs have not been studied in patients with renal impairment. The protease inhibitor simeprevir has recently been licensed, others including faldaprevir, asunaprevir, vaniprevir, and ritonavir-boosted ABT-450 are currently in phase II or phase III studies. Boceprevir, telaprevir, and simeprevir are all metabolized in the liver, and renal clearance contributes minimally to the elimination of these drugs. Therefore, it is not expected that renal impairment will have an important influence on the pharmacokinetics of HCV protease inhibitors. No clinically significant difference in the pharmacokinetic parameters of boceprevir was observed between patients with ESRD and healthy subjects, so there appears to be no dose adjustment required.80
Recently, Durmortier et al reported four ESRD patients with HCV (most commonly genotype 1b) who did not respond to a prior course of PEG-IFN and ribavirin; while awaiting kidney transplantation, they received a second-line antiviral regimen of PEG-IFN, ribavirin, and telaprevir. After 12 weeks of therapy, tolerance was acceptable and HCV-RNA became undetectable in three of the four patients. The dose of ribavirin ranged from 200 mg three times per week to 200 mg/day, and the severity of liver fibrosis ranged from grade 1 to grade 3.81
The pharmacokinetic parameters for simeprevir were also not influenced by creatinine clearance, and no dose adjustments were necessary in patients with mild, moderate, or severe renal impairment, but there is no clear evidence on its safety and efficacy in patients with ESRD or in those on HD.82
Oral nucleotide inhibitors of the HCV nonstructural protein 5B, such as sofosbuvir, have proven activity against all HCV genotypes. Sofosbuvir was approved by the FDA in 2013 for use in combination with ribavirin for the treatment of HCV genotypes 2 and 3 or in combination in PEG-IFN and ribavirin for infection in genotypes 1 and 4.82 Sofosbuvir is eliminated by the kidneys, not require dose adjustment in early grades of CKD; however, there is no current dosing recommendation for patients with ESRD.83
No adjustment of boceprevir dosage is required for patients with impaired renal function, but despite this observation, a paucity of studies evaluating standard combination therapy suggests that routine use of this combination should not be applied in the population with advanced renal failure.84
With the development of sofosbuvir and the more recent drugs, there will be promising IFN-free and ribavirin-free therapy regimens, but unfortunately there are no clinical trials studying patients with HCV and associated CKD. However, we believe that, in the near future, regimens with higher success rates and less severe adverse effects, especially in this particular population of patients, will be available. To date, there are no studies on the efficacy and safety of these new agents in organ recipients, including kidney transplant recipients.
Conclusion
Despite the screening of blood products, nosocomial HCV transmission continues to occur in HD units. HCV infection decreases the survival of patients and grafts. Treatment for HCV in patients with CKD is complicated, but achieving SVR can decrease post-transplant complications. Kidney transplantation alone must be considered in patients with compensated HCV-positive with cirrhosis and a HPVG <10 mmHg. Patients with ESRD who remain on HD are at higher risk of death when compared with those who receive a kidney graft. Kidneys obtained from HCV-positive donors and transplanted into HCV-positive recipients may be useful in expanding the donor pool by increasing the rate of utilization of these kidneys. Antiviral treatment can improve renal function in patients with HCV-associated glomerulopathy. New antiviral therapies needs to be evaluated to confirm the role of treatment in the ESRD HCV-positive population.
Disclosure
The authors report no conflicts of interest in this work.
References
Finelli L, Miller J, Tokars J, Arduimo M. National surveillance of dialysis-associated diseases in the United States. Semin Dial. 2005;18:52–61. | ||
Fissell RB, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335–2342. | ||
Jadoul M, Poignet JL, Geddes C, et al. The changing epidemiology of hepatitis C virus (HCV) infection in haemodialysis: European multicentre study. Nephrol Dial Transplant. 2004;19:904–909. | ||
Wreghitt T. Blood–borne virus infections in dialysis units – a review. Rev Med Virol. 1999;9:101–109. | ||
Amiri Z, Shakib A, Toorchi M. Seroprevalence of hepatitis C and risk factors in haemodialysis patients in Guilan, Islamic Republic of Iran. East Mediterr Health J. 2005;11:372–376. | ||
Sypsa V, Psichogiou M, Katsoulidou A, et al. Incidence and patterns of hepatitis C virus seroconversion in a cohort of hemodialysis patients. Am J Kidney Dis. 2005;45:334–343. | ||
Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. | ||
Nakayama E, Akiba T, Marumo F, Sato C. Prognosis of anti-hepatitis C virus antibody-positive patients on regular hemodialysis therapy. J Am Soc Nephrol. 2000;11:1896–1902. | ||
Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther. 2004;20:1271–1277. | ||
Legendre C, Garrigue V, Le Bihan C, et al. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65:667–670. | ||
Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–263. | ||
Fabrizi F, Poordad F, Martin P. Hepatitis C infection and the patient with end stage renal disease. Hepatology. 2002;36:3–10. | ||
Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008;49:613–624. | ||
Fabrizi F, Lampertico P, Lunghi G, Mangano S, Aucella F, Martin P. Review article: hepatitis C virus infection and type-2 diabetes mellitus in renal diseases and transplantation. Aliment Pharmacol Ther. 2005;21:623–632. | ||
Sezer S, Ozdemir F, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant. 2004;18:619–623. | ||
Al-Freah M, Zeino Z, Heneghan M. Management of hepatitis C in patients with chronic kidney disease. Curr Gastroenterol Rep. 2012;14:78–86. | ||
Patel P, Thompson N, Kallen A, Arduino M. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371–378. | ||
Tokars J, Frank M, Alter M, Arduino M. National surveillance of dialysis-associated diseases in the United States, 2000. Semin Dial. 2002;15:162–171. | ||
Gohar S, Khalil R, Elaish N, Khedr E, Ahmed M. Prevalence of antibodies to hepatitis C virus in hemodialysis patients and renal transplant recipients. J Egypt Public Health Assoc. 1995;70:465–484. | ||
Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;Suppl 109:S1–S99. | ||
[No authors listed]. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. | ||
Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment and efficacy and harms. Am J Kidney Dis. 2008;51:263–277. | ||
Liu CH, Liang CC, Huang KW, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol. 2011;6:1057–1065. | ||
Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. | ||
Okuda K, Yokosuka O. Natural history of chronic hepatitis C in patients on hemodialysis: case control study with 4–23 years of follow up. World J Gastroenterol. 2004;10:2209–2212. | ||
Trevizoli JE, de Paula Menezes R, Ribeiro Velasco LF, et al. Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol. 2008;3:1385–1390. | ||
Furusyo N, Hayashi J, Ariyama I, et al. Maintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infection. Am J Gastroenterol. 2009;95:490–496. | ||
Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19:601–607. | ||
Azevedo HA, Villela-Nogueira CA, Perez RM, et al. Similar HCV viral load levels and genotype distribution among end-stage renal disease patients on hemodialysis and HCV infected with normal renal function. J Nephrol. 2007;20:609–616. | ||
Fabrizi F, Messa P, Martin P. Impact of hemodialysis therapy on hepatitis C virus infection: a deeper insight. Int J Artif Organs. 2009;32:1–11. | ||
Rostaing L, Peres C, Tkaczuk J, et al. Ex vivo flow cytometry determination of intracytoplasmic expression of IL-2, IL-6, IFN-gamma and TNF alpha in monocytes and T-lymphocytes in chronic hemodialysis patients. Am J Nephrol. 2000;20:18–26. | ||
Wang SM, Liu H, Chou C, Huang C, Shih C, Chen W. Mortality in hepatitis C-positive patients treated with peritoneal dialysis. Perit Dial Int. 2008;28:183–187. | ||
Bloom RD, Lake JR. Emerging issues in hepatitis C virus positive liver and kidney transplant recipients. Am J Transplant. 2006;6:2232–2237. | ||
Morales JM, Aguado JM. Hepatitis C and renal transplantation. Curr Opin Organ Transplant. 2012;17:609–615. | ||
Roth D, Reddy KR, Kupin W. Long-term impact of HCV on clinical outcomes and liver histology in kidney recipients. Am J Transplant. 2004;A478:289. | ||
Paramesh AS, Davis JY, Mallikarjun C, et al. Kidney transplantation alone in ESRD patients with hepatitis C cirrhosis. Transplantation. 2012;94:250–254. | ||
Campos S, Parsikia A, Zaki RF, Ortiz JA. Kidney transplantation alone in ESRD patients with hepatitis C cirrhosis. Transplantation. 2012;94:65–66. | ||
Justa S, Mintz R, Mintz M, et al. Serial measurements of hepatitis C viral load by real-time polymerase chain reaction among recipients of living-donor renal transplants: a short-term follow-up study from a single center. Transplant Proc. 2010;42:3568–3573. | ||
Roth D, Gaynor J, Reddy K, et al. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152–1160. | ||
Alric L, Di-Martino V, Selves J, et al. Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology. 2002;123:1494–1499. | ||
Manuel O, Baid-Agrawal S, Moradpour D, Pascual M. Immunosuppression in hepatitis C virus-infected patients after kidney transplantation. Contrib Nephrol. 2012;76:97–107. | ||
Toth C, Pascual M, Chung R, et al. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation. 1998;66:1254–1258. | ||
Siddiqui AR, Abbas Z, Luck NH, et al. Experience of fibrosing cholestatic hepatitis with hepatitis C virus in kidney transplant recipients. Transplant Proc. 2012;44:721–724. | ||
Cimsit B, Assis D, Caldwell C, et al. Successful treatment of fibrosing cholestatic hepatitis after liver transplantation. Transplant Proc. 2011;43:905–908. | ||
Stokes M. Immune complex glomerulonephritis in patients with hepatitis C. Saudi J Kidney Dis Transpl. 2000;11:396–404. | ||
Vallet-Pichard A, Fontaine H, Mallet V, Pol S. Viral hepatitis in solid organ transplantation other than liver. J Hepatol. 2011;55:474–482. | ||
Ingsathit A. Kamanamool N, Thakkinstian A, Sumethkul V. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta analysis. Transplantation. 2013;95:943–948. | ||
Espinosa M, Martin-Malo A, Alvarez de Lara MA, et al. Risk of death and liver cirrhosis in anti-HCV-positive long term hemodialysis patients. Nephrol Dial Transplant. 2001;16:1669–1674. | ||
Solez K, Colvin RB, Racusen LC, et al. Banff´05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant. 2007;7:518–526. | ||
Fabrizi F, Dixit V, Messa P, Martin P. Interferon monotherapy of chronic hepatitis C in patients: meta-analysis of clinical trials. J Viral Hepat. 2008;15:79–88. | ||
Chan T, Wu P, Lau J, Lok A, Lai C, Cheng I. Interferon treatment for hepatitis C virus infection in patients on haemodialysis. Nephrol Dial Transplant. 1997;12:1414–1419. | ||
Liu C, Liang C, Lin J. Pegylated interferon alpha 2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: a randomised study. Gut. 2008;57:525–530. | ||
Gordon C, Uhlig K, Lau J, Schmid C, Levey A, Wong J. Interferon for hepatitis C virus in hemodialysis – an individual patient meta-analysis of factors associated with sustained virological response. Clin J Am Soc Nephrol. 2009;4:1449–1458. | ||
Covic A, Maftei I, Mardare N, et al. Analysis of safety and efficacy of PEGylated interferon alpha 2a in hepatitis C virus positive hemodialysis patients: results from a large, multicenter audit. J Nephrol. 2006;19:794–801. | ||
Wang KL, Xing HQ, Zhao H, et al. Efficacy and tolerability of low dose interferon-α in hemodialysis patients with chronic hepatitis C virus infection. World J Gastroenterol. 2014;20:4071–4075. | ||
Fabrizi F, Dixit V, Messa P, Martin P. Interferon monotherapy of chronic hepatitis C in patients: meta-analysis of clinical trials. J Viral Hepat. 2008;15:79–88. | ||
Liu C, Liang C, Lin J. Pegylated interferon alpha-2a versus standard interferon alpha-2a for treatment naive dialysis patients with chronic hepatitis C: a randomised study. Gut. 2008;57:525–530. | ||
Bruchfeld A, Stahle L, Andersson J, Schvarez R. Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection – a pilot study. J Viral Hepat. 2001;8:287–292. | ||
Tan A, Brouwer J, Glue P. Safety of interferon and ribavirin therapy in haemodialysis patients with chronic hepatitis C: results of a pilot study. Nephrol Dial Transplant. 2001;16:193–195. | ||
Mousa D, Abdalla A, Al-Shoail G, Al-Sulaiman M, Al-Hawas F, Al-Khader A. Alpha interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplant Proc. 2004;36:1831–1834. | ||
Fabrizi F, Dixit V, Martin P, Messa P. Combined antiviral therapy of hepatitis C virus in dialysis patients: meta analysis of clinical trials. J Viral Hepat. 2011;18:e253–e269. | ||
Rendina M, Schena A, Castellaneta N. The treatment of chronic hepatitis C with peginterferon alfa 2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768–774. | ||
Djordjević V, Kostić S, Stefanović V. Treatment of chronic hepatitis C with interferon alpha in patients on maintenance hemodialysis. Nephron. 1998;79:229–231. | ||
Liu C, Liang C, Liu C. Pegylated interferon alfa 2 plus low dose ribavirin for the retreatment of dialysis chronic hepatitis C patients who relapsed from prior interferon monotherapy. Gut. 2009;58:314–316. | ||
Hassan Q, Roche B, Buffet C, et al. Liver-kidney recipient with chronic viral hepatitis C treated with interferon-alpha. Transpl Int. 2012;25:941–947. | ||
Al-Harbi A, Malik G, Subaity Y, Mansy H, Abutaleb N. Treatment of acute hepatitis C virus infection with alpha interferon in patients with hemodialysis. Saudi J Kidney Dis Transpl. 2005;16:293–297. | ||
Fabrizi F, Dixit V, Messa P, Martin P. Interferon therapy of acute hepatitis C in dialysis patients: meta-analysis. J Viral Hepat. 2012;19:784–791. | ||
Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D. Evidence that clearance of hepatitis C virus RNA after interferon therapy in dialysis patients in sustained after renal transplantation. J Am Soc Nephrol. 2003;12:2092–2098. | ||
Roth D, Bloom R. Selection and management of hepatitis C-virus infected patients for the kidney transplant waiting list. Contrib Nephrol. 2012;176:66–76. | ||
Hassan Q, Roche B, Buffet C, Sessade T, Samuel D, Charpentier B, Durrbach A. Liver-kidney recipient with chronic viral hepatitis C treated with interferon-alpha. Transpl Int. 2012;25:941–947. | ||
Wei F, Liu F, Hu H, Ren H, Hu P. Interferon based antiviral therapy for hepatitis C virus infection after renal transplantation: an update meta-analysis. PLoS One. 2014;9:1–11. | ||
Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. The effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20–27. | ||
Woodside KJ, Idshihara K, Theisen JE, et al. Use of kidneys from hepatitis C seropositive donors shortens waitlist time but does not alter one-yr outcome. Clin Transplant. 2003;17:433–437. | ||
Kucirka LM, Singer AL, Ros RA, et al. Underutilization of hepatitis C-positive kidneys for hepatitis C positive recipients. Am J Transplant. 2010;10:1238–1246. | ||
Kucirka LM, Peters TG, Segev DL. Impact of donor hepatitis C virus infection status on death and need for liver transplant in hepatitis C virus-positive kidney transplant recipients. Am J Kidney Dis. 2012;60:112–120. | ||
Morales JM, Campistol JM, Domínguez-Gil B, et al. Long term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10:2453–2462. | ||
Morales JM, Campistol JM, Castellano G, et al. Transplantation of kidneys from donors with hepatitis C antibody into recipients with pre-transplantation anti-HCV. Kidney Int. 1995;47:236–240. | ||
Veroux P, Veroux M, Sparacino V, et al. Kidney transplantation from donors with viral B and C hepatitis. Transplant Proc. 2006;38:996–998. | ||
Natov SN, Lau JY, Ruthazer R, Schmid CH, Levey AS, Pereira BJ. Hepatitis C virus genotype does not affect patient survival among renal transplant candidates. The New England Organ Bank Hepatitis C Study Group. Kidney Int. 1999;56:700–706. | ||
Treitel M, Marbury T, Preston RA, et al. Single-dose pharmacokinetics of boceprevir in subjects with impaired hepatic or renal function. Clin Pharmacokinet. 2012;51:619–628. | ||
Durmortier J, Guillaud O, Gagnieu MC, et al Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: is it feasible? J Clin Virol. 2013;56:146–149. | ||
US Food and Drug Administration. Sovaldi prescribing information. 2013. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/205123Orig1s000ClinPharmR.pdf. Accessed July 16, 2014. | ||
Sovaldi (sofosvubir) [package insert]. Foster City, CA, USA: Gilead Sciences, Inc; 2013. | ||
Ghany M, Nelson D, Strader D, Thomas D, Seeff L. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guidelines by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. | ||
Gordon CE, Balk EM, Becker BN, et al. KDOQI US Commentary on the KDIGO clinical practice guideline for the prevention diagnosis, evaluation, and treatment of hepatitis C in CKD. Am J Kidney Dis. 2008;52:815–825. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.