Back to Journals » Cancer Management and Research » Volume 11
Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression
Authors Li Y, Zhou J, Zhuo Q, Zhang J, Xie J, Han S, Zhao S
Received 30 March 2019
Accepted for publication 4 July 2019
Published 29 July 2019 Volume 2019:11 Pages 7123—7134
DOI https://doi.org/10.2147/CMAR.S210568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Yujuan Li,1,* Jing Zhou,1,* Qian Zhuo,2 Jingyun Zhang,1 Jingyan Xie,1 Suping Han,3 Shuli Zhao1,4
1Department of Obstetrics and Gynecology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Pathology, Xuzhou Medical College, Xuzhou, Jiangsu, People’s Republic of China; 3Department of Obstetrics and Gynecology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 4State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Background and purpose: To evade immune defense, cancer cells can employ extracellular vesicles (EVs) to inhibit the anti-tumor activity of lymphocytes in the tumor microenvironment. However, the mechanisms and key molecules that mediate the effects of EVs on lymphocytes are unclear.
Patients and methods: We used Quantibody® Human Cytokine Antibody Array 440 to determine the tumor immunity-related cytokine profile of peripheral blood lymphocytes (PBLs) stimulated with EVs derived from peritoneal washes or malignant ascites. We detected 21 upregulated and 27 downregulated proteins, including the immunosuppressive receptors Siglec-10, SLAM, PD-1, and TIM-3.
Results: Flow cytometry analysis of PBLs or ovarian cancer ascites suggested that Siglec-10 expression on CD3+ T cells was higher in ovarian cancer patients than in healthy controls and in the malignant ascites of ovarian cancer patients than in their blood. Moreover, the expression of CD24, the Siglec-10 ligand, was associated with tumor stage and cancer cell metastasis. Finally, compared to the benign peritoneal wash-derived EVs, the malignant EVs significantly upregulated Siglec-10 expression on Jurkat T cells, inhibited the protein kinase C activity induced by phorbol 12-myristate 13-acetate and ionomycin, and impaired the phosphorylation of the tyrosine kinase ZAP-70 activated by crosslinking with an anti-CD3 antibody.
Conclusion: The EVs secreted by malignant ovarian cells upregulated Siglec-10 expression on T cells and impaired T cell activation in the tumor microenvironment. We believe that a comprehensive understanding of the regulation of Siglec-10 and CD24 by malignant EVs has clinical importance, as it will aid in the development of better immunotherapeutic strategies for ovarian cancer.
Keywords: extracellular vesicles, Siglec-10, T cells, ovarian cancer, ascites
Introduction
Ovarian cancer remains the leading cause of death among gynecological malignancies; it is the fourth most common cause of cancer-related death among women.1 Continuous crosstalk between cancer cells and components of the immune system in the tumor microenvironment is required for cancer cell elimination and reinstatement of equilibrium, but also for tumor escape from immune surveillance.2,3 Although this dynamic interaction between the immune system and ovarian cancer has been widely recognized as important for cancer progression in recent years,4,5 we know little about the nature of the crosstalk between ovarian cancer cells and immune cells in the tumor microenvironment.
Accumulating evidence indicates that extracellular vehicles (EVs), novel vehicles for communication in the tumor microenvironment, play important roles in crosstalk; they directly stimulate target cells with their membrane molecules and facilitate the direct transfer of their contents, including proteins, lipids, and miRNA/mRNA/DNAs, between cells.6,7 Since many populations of lymphocytes, including T, B, and NK cells, can be important components of the tumor microenvironment,8,9 ascertaining the effects of the malignant EVs on lymphocytes will allow us to understand the underlying molecular mechanisms that govern anti-ovarian cancer immunity.10 Our previous results showed that exosomes (40- to 150-nm-in-diameter EVs) derived from ovarian cancer suppress lymphocyte-mediated anti-tumor immunity and enhance tumor invasion, angiogenesis, and metastasis via the interferon and NF-κB signaling pathways.11 However, there is currently little evidence of the specific molecules and mechanisms that mediate the effects of malignant exosomes on immune cells.
Sialic acid-binding immunoglobulin-type lectins (Siglecs), a group of cell surface transmembrane receptors expressed on immune cells, can regulate immune balance in autoimmune diseases and cancer.12,13 Siglec-10 is an inhibitory receptor that is broadly expressed on B cells, T cells, dendritic cells, and macrophages; it inhibits the anti-tumor activity of T cells by impairing the formation of major histocompatibility complex class I–peptide complexes and the phosphorylation of the T cell receptor-associated kinases Lck and ZAP-70.13–15 However, there has been no report about the expression levels and regulated mechanisms of Siglec-10 on immune cells in the tumor microenvironment.
In this study, we investigated the different molecules mediated by EVs derived from the peritoneal washes of benign ovarian cyst patients or the malignant ascites of ovarian cancer patients using Quantibody® Human Cytokine Antibody Array 440, and determined that EVs secreted by malignant ovarian cells upregulated Siglec-10 expression on T cells and impaired T cell activation in the tumor microenvironment, which will aid in the development of better immunotherapeutic strategies for ovarian cancer.
Materials and methods
EV purification and characterization
We extracted EVs from the malignant ascites of epithelial ovarian cancer (EOC) patients or peritoneal washes from benign ovarian cyst patients by differential centrifugation at 4 °C based on previously described methods with slight modifications.16,17 In brief, the ascites (50 mL) or peritoneal washes were subjected to centrifugation at 2,000×g for 30 min, then 10,000×g for 30 min to remove debris and apoptotic bodies, followed by centrifugation at 100,000×g for 2 h (Optima™ XE-90, Beckman Coulter, Brea, CA, USA). Subsequently, the EV pellets were re-suspended in sterilized phosphate-buffered saline (PBS) and washed by centrifugation at 100,000×g for 2 hrs. Finally, we re-suspended the EV pellets in PBS (500 μL) to measure their size distribution with a Malvern Zetasizer Nano ZS90 and quantify their total protein concentrations using a Bradford assay (Pierce™, Thermo Fisher Scientific, Waltham, MA, USA). We stored the samples at −80 °C until use. The EV pellets were observed under a transmission electron microscope (TEM; JEM-1010, Jeol, Tokyo, Japan) at 80.0 kV. Images were captured with a digital camera.
Co-incubation of lymphocytes with EVs
PBLs were extracted from the whole blood of 4 healthy female volunteers by a density gradient centrifugation method using Ficoll-Paque (TBD Science, Tianjing, China) according to the manufacturer’s instructions.11 Subsequently, lymphocytes were sorted with an anti-CD45-PE antibody, then re-suspended in Gibco® CTS™ AIM-V™ serum-free medium (Thermo Fisher Scientific). The purified lymphocytes from each volunteer were randomly assigned to 7 groups and separately plated into 6-well plates (2×106 cells/well). Lymphocytes were then separately treated with PBS control (n=1) or malignant or benign isolated EVs (n=3 for each EV group, 1×109 EV particles/mL) for 48 h at 37 °C. Finally, the lymphocytes were collected, total protein was extracted in cell lysis buffer containing an optimized mixture of protease inhibitors, and the total protein contents of the 4 samples in each group were pooled to eliminate individual differences and analyzed using the Quantibody® Human Cytokine Antibody Array 440 Kit (Catalog number: QAH-CAA-440, RayBiotech, Norcross, GA, USA).
Human cytokine microarray analysis
To evaluate the effects of malignant EVs on lymphocytes from healthy donors, we assayed the levels of 440 tumor immunity-related cytokines in the total protein extracts of lymphocytes using the Quantibody® Human Cytokine Antibody Array Q440 (RayBiotech).18 In each lysed lymphocyte preparation, we quantified the concentration of total proteins using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific), which we adjusted to 500 μg/mL by dilution with buffer, then performed the experimental procedure in accordance with the manufacturer’s instructions. Briefly, the completely air-dried, pre-coated antibody array glass slides were blocked with coating buffer for 30 min. Then, standard cytokine solution (100 μL) or sample (500 μg/mL total protein) was added to each well; the plates were incubated overnight at 4 °C. After incubation, we washed 5 times (5 min/wash) with Wash Buffer I with gentle rocking at room temperature, then incubated the membranes with the detection antibody cocktail at room temperature for 2 h. After washing 5 times (5 min/wash) with Wash Buffer I, then twice with Wash Buffer II with gentle rocking at room temperature, we added Cy3-equivalent dye-conjugated streptavidin (80 μL) to each well and incubated the plate at room temperature for 1 h in a dark room. Finally, after completely washing the plate, we visualized the signals with an Axon GenePix® laser scanner equipped with a Cy3 wavelength filter (green channel). We performed densitometric analyses of the spots with microarray analysis software, including GenePix, ScanArray Express, ArrayVision, and MicroVigene.
Patient recruitment
Patients diagnosed with primary EOC or benign ovarian cysts were recruited from June 2016 to October 2017 at Nanjing First Hospital (Nanjing, China), Jiangsu Cancer Hospital (Nanjing, China), and Jiangsu Provincial Hospital of Traditional Chinese Medicine (Nanjing, China). All patients with EOC were diagnosed according to the recommended criteria in the classification proposed by the World Health Organization in 2004. We recruited 18 benign ovarian cyst patients and 72 primary EOC patients who had undergone debulking surgery. None of the patients had been treated with preoperative chemotherapy or radiation. We collected samples of surgical tissue, ascites, and blood from the patients and blood samples from 30 healthy, female volunteers of a similar age. All volunteers with immune-related diseases or current clinical symptoms of inflammatory diseases were excluded. According to the criteria specified by the Nanjing Medical University, we obtained written informed consent from the patients and the healthy volunteers prior to recruitment. This study was approved by the Human Research Ethics Committee.
Cell preparation and flow cytometric analysis
We assessed the levels of biomarkers on the surfaces of the immune cells from the blood and ascites samples by flow cytometry.19 Briefly, each specimen underwent erythrocyte lysis with red blood cell lysis buffer, then washing with PBS. Then, we incubated the specimens (100 μL) with either anti-CD3-FITC, anti-CD19-percp-cy5.5, anti-Siglec-10-APC (Catalog number: 130-103-731, clone: 5G6, Miltenyi Biotec, Bergisch Gladbach, Germany), and anti-CD150-PE (eBioscience, San Diego, CA, USA) or anti-PD-1-PE (eBioscience) and anti-TIM-3-APC (eBioscience) at working concentration. Then, the specimens were incubated in the dark at 4 °C for 20 min, re-suspended, and washed twice in PBS. Flow cytometry was performed using a FACSCanto™ II flow cytometer (BD Biosciences, San Diego, CA, USA). The data were analyzed with FlowJo software (Treestar, San Carlos, CA, USA). Antigen expression is depicted as fluorescence intensity on dual-parameter scattergrams.
Immunohistochemistry
We determined the expression of CD24, the Siglec-10 ligand, in paraffin-embedded tissues from 37 EOC and 28 benign ovarian cyst tissues by immunohistochemistry. Briefly, 5-μm sections of tissue were prepared by extracting the paraffin in xylene, then re-hydrating, and inactivating endogenous peroxidase with 3% H2O2 in PBS for 15 min. Then, we treated the sections with citrate solution (10 mM, pH 6.0; Maixin-Bio, Fujian, China) to retrieve the antigens, incubated with 2% bovine serum albumin to block nonspecific reactivity, and incubated with anti-human CD24 monoclonal antibody (15 µg/mL; catalog number: MAB5248, R&D Systems, Minneapolis, MN, USA) overnight at 4 °C. After washing 3 times with PBS, we incubated the sections with the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (Catalog number: CTS002, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Subsequently, the sections were counterstained with hematoxylin, dehydrated, and mounted.
The expression levels of CD24 in the resected specimens from 72 patients with EOC were assessed by a pathologist who was blinded to the clinical information of the patients. The immunoreactivity of the specimens was graded into 2 groups based on the frequency of positively stained cells: low expression, <30% positive staining and high expression, ≥30% positive staining. In addition, 5 random fields from each section were examined at 200× magnification.
Reverse transcription and real-time quantitative PCR analysis
The SLAM and Siglec-10 mRNA levels in Jurkat T cells were analyzed by Q-PCR as previously described.11 Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Paisley, UK) and reverse-transcribed using a HiScript Ⅱ 1st Strand cDNA Synthesis kit (Vazyme Biotech Co., Ltd, Nanjing, China) according to standard protocols. Quantitative RT-PCR analysis was performed using a AceQ Universal SYBR Green qPCR Master Mix (Vazyme Biotech Co., Ltd, Nanjing, China) and 300 mM primers (Table 1). After an initial 95 °C (30 seconds) hot start, 40 cycles of 95 °C (5 seconds) and 55 °C (34 seconds) were performed using an ABI 7500 Real-Time PCR System (Life Technologies, NY, USA). The primer sequences are as follows:
 |
Table 1 Profiler protein array results for EVs-treated PBLs (Malignant EVs group vs Benign EVs group) |
GAPDH, 5-TGAAGGTCGGAGTCAACG-3 and 5-CAAAGTTGTCATGGATGA-3; Siglec-10, 5-CTGCTGGGCCCCTCCTGC-3 and 5-GACGTTCCAGGCCTCACAG-3; SLAM, 5-GTGTATGCTGGGCTGTTAGG-3, 5-AGAGGTAAAACGAACCATTACCA-3.
Assessment of protein kinase C activity
Human Jurkat T cells (purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China) were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum. After pre-treatment with PBS or the isolated EVs (7 malignant or 7 benign, 1×109 EV particles/mL) in Gibco® CTS™ AIM-V™ serum-free medium for 24 h, the Jurkat cells (2×105/mL) were seeded in 6-well plates in the presence or absence of phorbol 12-myristate 13-acetate and ionomycin (PMA+IoM; 10 ng/mL PMA and 300 ng/mL IoM) for 24 h. Protein kinase C (PKC) activity was determined with a PKC Kinase Activity Assay Kit (Catalog number: ab139437, Abcam, Cambridge, UK) according to the manufacturer’s instructions.20 Briefly, the cells were harvested, washed with ice-cold PBS, then lysed in Lysis Buffer (1 mL). After the lysates were subjected to centrifugation at 13,000 rpm for 15 min, the supernatants were collected and the protein concentrations were determined using the bicinchoninic acid method. Then, PKC kinase activity was evaluated in a solid-phase enzyme-linked immunosorbent assay based on the phosphorylation of a specific synthetic peptide by the PKC present in the sample after the addition of adenosine triphosphate. A phospho-specific antibody was used to recognize only the phospho-substrate. The primary antibody was subsequently detected with a horseradish peroxidase (HRP)-conjugated secondary antibody. The assay was finally developed with 3,3′,5,5′-tetramethylbenzidine substrate. The color development was stopped with an acid stop solution. The intensity of the color was measured at 450 nm. PKC activity is expressed relative to the total protein content.
Western blot analysis
After treatment with PBS or the isolated EVs (7 malignant or 7 benign, 1×109 EV particles/mL) in Gibco® CTS™ AIM-V™ serum-free medium for 24 h, Jurkat cells were activated with anti-human CD3 antibody (50 μg/mL; clone: UCHT-1).21 We incubated the cells for 5 min at 37 °C and stopped the activation by quickly freezing the cells in liquid nitrogen. Then, the Jurkat cells were lysed in buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.02% NaN3, 0.1% sodium dodecyl sulfate (SDS), 100 mg/L phenylmethylsulfonyl fluoride, 1 mg/L aprotinin, and 1% Triton™ X-100. For total ZAP-70 protein immunoprecipitation, we incubated equal volumes of cell lysates on a rotator platform at 4 °C for 2 h in the presence of the rabbit monoclonal anti-ZAP-70 antibody (5 μL; catalog number: ab32429, Abcam, Cambridge, UK), then incubated with protein G-coupled sepharose beads (20 μL) for 1 h. After washing 5 times with ice-cold washing buffer, we re-suspended the beads in 2× sample loading buffer (100 μL) and boiled for 10 min. After centrifugation, the supernatants were subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Burlington, MA, USA) via wet transfer. After blocking for 1 h in PBS with TWEEN® 20 containing 2% non-fat dry milk, we incubated the membranes with antibodies specific for ZAP-70 and phospho-ZAP-70 (Y292) (Catalog number: ab199245 Abcam, Cambridge, UK), then HRP-conjugated secondary antibodies (anti-rabbit). The proteins were detected using the enhanced chemiluminescence reaction (Beyotime, Haimen, China).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 5 (San Diego, CA, USA). Two-tailed Student’s t-tests were used to compare experimental groups. Statistical significance was defined at greater than or equal to a 95% confidence interval or a P-value <0.05. The data shown are the mean ± standard deviation of 3 repeated experiments.
Results
Production and characterization of EVs
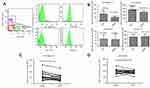
We first removed cells and debris from the crude samples of the ascites or peritoneal washes by differential centrifugation, then isolated the EVs by ultracentrifugation. We identified the EVs by TEM, which showed that EVs were membrane bound, round, and heterogeneous in size (Figure 1A and C). Although the 2 kinds of EVs had similar particle size ranges (30–250 nm), the vesicles from the malignant ascites were larger than those from the peritoneal washes (mean sizes: 138.9 and 86.4 nm, respectively; Figure 1B and D). The morphology of the EVs resembled that of exosomes.
Multiple PBL-derived cytokines are altered by exposure to EVs
We used Quantibody® Human Cytokine Antibody Array Q440 to simultaneously measure the concentrations of 440 cancer- and immunity-related cytokines in the cell lysates of normal PBLs exposed to benign or malignant EVs. As shown in the heat maps (Figure 2A), in the group exposed to malignant EVs, the levels of 48 cytokines differed (21 upregulated and 27 downregulated) from those in the benign EV control group (Table 1). Among those cytokines, there were marked increases in the concentrations of CEACAM-5, GCP-2, WISP-1, ErbB3, midkine, Siglec-10, and PD-1 and significant decreases in the concentrations of SALM, PARC, TIM-3, SDF-1α, and MCP-4 in the malignant EV group compared to the benign EV group. Interestingly, the expression of 4 immunosuppressive receptors (PD-1, Siglec-10, SLAM, and TIM-3) on normal lymphocytes significantly differed in the benign and malignant EV groups (Figure 2B).
Heterogeneous expression of Siglec-10 and SLAM on T lymphocyte subsets in the ovarian cancer and control groups
To explore the molecular mechanism of tumor immune escape facilitated by malignant exosomes, we assessed the expression of the immunosuppressive receptors PD-1, Siglec-10, SLAM, and TIM-3 in CD3+ T lymphocytes isolated from the blood of 39 EOC patients and 30 healthy controls by flow cytometry (Figure 3A). In the peripheral blood samples, we found that the expression of Siglec-10 and SLAM on CD3+ T lymphocytes was significantly higher in EOC patients than in healthy controls; we did not detect significant differences in PD-1 and TIM-3 expression on CD3+ T cells (Figure 3B) and CD19+ B cells (Figure S1). Next, we found that the percentage of Siglec-10+ CD3+ T cells was higher in the malignant ascites of 19 EOC patients than in their blood (Figure 3C). Given that CD24 is the ligand of Siglec-10,22 we further evaluated the expression and prognostic utility of CD24 by clinical pathology. As shown in Table 2, we found that 47% of 72 EOC patients had high CD24 expression (≥30% positive staining). Moreover, the expression of CD24 was markedly associated with advanced disease stage (P=0.004) and cancer cell metastasis (P=0.015). No correlation was found between CD24 expression and any of the other clinical pathologic parameters examined.
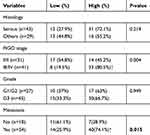 |
Table 2 Clinicopathological analysis of the CD24 expression in surgically resected epithelial ovarian cancer |
Malignant ascites-derived EVs upregulated Siglec-10 expression on Jurkat T cells and inhibited Jurkat T cell activation
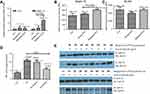
Our results suggest that malignant EVs may inhibit T cell activity to promote tumor immune escape by upregulating the expression of the suppressive receptor Siglec-10. To test this hypothesis, we collected EVs from the malignant ascites of 7 EOC patients and the peritoneal washes of 7 benign ovarian cyst patients and analyzed their effects on Siglec-10 expression on Jurkat T cells. As shown in Figure 4, treatment with malignant EVs significantly increased the mRNA and protein expression of Siglec-10 on Jurkat T cells compared to treatment with the benign peritoneal wash EVs or the PBS negative control (P<0.05; Figure 4A and B), whereas there was no statistical difference in the expression of SLAM (Figure 4A and C). Although we did not observe differences in IL-2, IL-4, IFN-γ, and TNF-α mRNA levels in the human Jurkat T cells stimulated with the different EVs (data not shown), we found that pre-treatment with malignant EVs, but not benign EVs, inhibited PMA+IoM-enhanced PKC activity (P<0.05; Figure 4D). Furthermore, compared to benign EVs, malignant EVs impaired the phosphorylation of the tyrosine kinase ZAP-70, which mediates T cell receptor-elicited T cell activation, in anti-CD3 activated Jurkat cells (Figure 4E).
Discussion
Many studies have demonstrated that malignant EVs help tumor cells evade host immunosurveillance and develop unchecked in the microenvironment.23,24 However, many questions remain about the trigger mechanisms for cancer immune escape.25 We aimed to clarify the effects of malignant ascites-derived EVs on the expression of key immunomodulatory molecules on lymphocytes, especially T cells, and to determine the clinical relevance of EVs.
Recently, a novel mechanism of biological regulation has attracted the attention of researchers: EVs derived from various cells in the blood, urine, ascites, and other body fluids can transfer bioactive molecules, including proteins and nucleic acids, between different cell types and across species.26,27 Depending on their biophysical properties and biogenesis, EVs are mainly divided into exosomes (a homogeneous population 40–100 nm in diameter, characterized by CD9/CD63 expression) and macrovesicles (a heterogeneous population 50–1,000 nm in diameter, no unique markers).28,29 In this study, the EVs ranged from 30 to 250 nm in diameter and the mean size of the malignant ascites-derived EVs (138.9 nm) was greater than that of the benign EVs (86.4 nm), which may indicate that they carry different proteins and nucleic acids. Although the morphology of the EVs resembled that of exosomes, due to the limitations of the available techniques, we cannot exclude the possibility of the presence of a very small proportion of macrovesicles in the exosome samples. Therefore, we collectively referred to our samples as EVs.
Microarray analysis facilitates the assessment of many molecules simultaneously, which dramatically accelerates the pace of discovery of key molecules, signaling pathways, and regulatory mechanisms.30,31 In this study, we developed a cytokine profile of EV-mediated immune evasion by subjecting normal PBLs that had been cultured with benign or malignant EVs to microarray to simultaneously detect the concentrations of 440 cancer- and immunity-related cytokines. We selected 4 (PD-1, Siglec-10, SLAM, and TIM-3) of the 48 differentially regulated genes and assessed their roles in ovarian cancer immune escape and their potential values as prognostic indicators for EOC by evaluating their expression in the blood and malignant ascites samples of EOC patients and the blood of healthy controls. We found that Siglec-10 and SLAM expression levels on CD3+ T lymphocytes in the blood were significantly higher in the EOC samples and that Siglec-10 levels on CD3+ T cells in the tumor microenvironment were higher than the levels on the T cells in the blood. Moreover, the expression of the Siglec-10 ligand CD24 was associated with advanced disease stage and cancer cell metastasis. However, due to the complicated nature and individual variations of the immune system, further studies should be performed with larger cohorts to determine the clinical relevance of Siglec-10 and CD24 in the tumor microenvironment with the aim to develop biomarkers for the evaluation of tumor immune escape.
Zhang et al reported that Siglec-10 was predominantly expressed on NK cells,15 moderately on Kupffer cells and B cells, and minimally on T cells and monocyte-derived dendritic cells. In addition, the percentage of Siglec-10+ NK cells is higher in human hepatocellular carcinoma tissues than in the surrounding non-tumor tissues. Here, we found that the mean fluorescence intensity representing Siglec-10 expression on CD3+ T cells was higher in the malignant ascites samples from EOC patients than in their blood. Furthermore, Bandala-Sanchez et al reported that Siglec-10 expressed on the T cell surface inhibits the phosphorylation of the T cell receptor-associated kinase ZAP-70 and T cell activation.14,32 In this study, compared to benign EVs, malignant EVs significantly increased the mRNA and protein expression of Siglec-10 on Jurkat T cells, inhibited PMA+IoM-enhanced PKC activity, and impaired anti-CD3 activated phosphorylation of ZAP-70 in Jurkat T cells. These results indicate that malignant EVs could increase Siglec-10 expression in T cells to inhibit T cell activation in the tumor microenvironment.
Frankly, the current study has several limitations. First, no perfect method has been identified to isolate all exosomes from 30 to 150 nm in various body fluids including ascites without any impurities. Our study is also limited by the methods used in this study that may also precipitate non-exosome debris in benign washing and malignant ascites samples. Second, as carriers for a variety of biological signaling molecules including proteins, lipids, and miRNA/mRNA/DNAs, the administration of EVs undoubtedly can trigger many regulatory mechanisms on immune cells. Due to the lack of well-established methods to analyze the effective ingredients in EVs, here we only discuss the synergistic regulatory effects on T cells leaded by benign EVs and malignant EVs in vitro.
Taken together, our findings reveal that malignant cell-secreted EVs in the tumor microenvironment stimulate lymphocytes to suppress anti-tumor immunity and promote tumor progression. Importantly, malignant EVs upregulate Siglec-10 expression on T cells to impair T cell function in the ovarian tumor microenvironment. Moreover, CD24 expression in ovarian tumors is associated with advanced cancer stage and metastasis. Thus, the levels of Siglec-10 and CD24 in the ovarian tumor microenvironment may represent novel biomarkers for the evaluation of tumor immune escape and serve as targets for the development of better immunotherapeutic strategies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers: 81572557 and 81872114) State Key Laboratory of Reproductive Medicine (SKLRM-K201507) and the Medical Science and Technology Development Foundation of Nanjing (Department of Health, grant numbers: ZKX15029, YKK16122 and YKK17126).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dinkelspiel HE, Champer M, Hou J, et al. Long-term mortality among women with epithelial ovarian cancer. Gynecol Oncol. 2015;138(2):421–428. doi:10.1016/j.ygyno.2015.06.005
2. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–773. doi:10.7150/jca.17648
3. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi:10.1111/imm.2007.121.issue-1
4. Stanske M, Wienert S, Castillo-Tong DC, et al. Dynamics of the intratumoral immune response during progression of high-grade serous ovarian cancer. Neoplasia. 2018;20(3):280–288. doi:10.1016/j.neo.2018.01.007
5. Pogge von Strandmann E, Reinartz S, Wager U, et al. Tumor–host cell interactions in ovarian cancer: pathways to therapy failure. Trends Cancer. 2017;3(2):137–148. doi:10.1016/j.trecan.2016.12.005
6. Sullivan R, Maresh G, Zhang X, et al. The emerging roles of extracellular vesicles as communication vehicles within the tumor microenvironment and beyond. Front Endocrinol (Lausanne). 2017;8:194. doi:10.3389/fendo.2017.00194
7. Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi:10.1016/j.tcb.2016.11.003
8. Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi:10.1016/j.ccell.2016.10.009
9. Cohen IJ, Blasberg R. Impact of the tumor microenvironment on tumor-infiltrating lymphocytes: focus on breast cancer. Breast Cancer (Auckl). 2017;11:1178223417731565.
10. Nawaz M, Fatima F, Nazarenko I, et al. Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics. 2016;13(4):395–409. doi:10.1586/14789450.2016.1165613
11. Li Y, Yang Y, Xiong A, et al. Comparative gene expression analysis of lymphocytes treated with exosomes derived from ovarian cancer and ovarian cysts. Front Immunol. 2017;8:607. doi:10.3389/fimmu.2017.00607
12. Macauley MS, Crocker PR, Paulson JC. Siglec regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–666. doi:10.1038/nri3737
13. Liu YC, Yu MM, Chai YF, Shou ST. Sialic acids in the immune response during sepsis. Front Immunol. 2017;8:1601. doi:10.3389/fimmu.2017.01601
14. Zhao Y, Su H, Shen X, et al. The immunological function of CD52 and its targeting in organ transplantation. Inflamm Res. 2017;66(7):571–578. doi:10.1007/s00011-017-1032-8
15. Zhang P, Lu X, Tao K, et al. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J Surg Res. 2015;194(1):107–113. doi:10.1016/j.jss.2014.09.035
16. Yamamoto CM, Oakes ML, Murakami T, et al. Comparison of benign peritoneal fluid- and ovarian cancer ascites-derived extracellular vesicle RNA biomarkers. J Ovarian Res. 2018;11(1):20. doi:10.1186/s13048-018-0391-2
17. Yokoi A, Yoshioka Y, Yamamoto Y, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. doi:10.1038/ncomms14470
18. Zhou Z, Zhang J, Li X, et al. Protein microarray analysis identifies key cytokines associated with malignant middle cerebral artery infarction. Brain Behav. 2017;7(8):e00746. doi:10.1002/brb3.746
19. Cornfield DB, Gheith SM. Flow cytometric quantitation of natural killer cells and T lymphocytes expressing T-cell receptors alpha/beta and gamma/delta is not helpful in distinguishing benign from malignant body cavity effusions. Cytometry B Clin Cytom. 2009;76(3):213–217. doi:10.1002/cyto.b.20455
20. Aherne SA, O’Brien NM. Modulation of cytokine production by plant sterols in stimulated human Jurkat T cells. Mol Nutr Food Res. 2008;52(6):664–673. doi:10.1002/mnfr.200700385
21. Bartis D, Boldizsár F, Szabó M, et al. Dexamethasone induces rapid tyrosine-phosphorylation of ZAP-70 in Jurkat cells. J Steroid Biochem Mol Biol. 2006;98(2–3):147–154. doi:10.1016/j.jsbmb.2005.01.032
22. Chen GY, Tang J, Zheng P, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi:10.1126/science.1168988
23. O’Loghlen A. Role for extracellular vesicles in the tumour microenvironment. Philos Trans R Soc Lond B Biol Sci. 2018;373(1737):20160488. doi:10.1098/rstb.2017.0072
24. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi:10.1016/j.semcdb.2015.02.009
25. Vanichapol T, Chutipongtanate S, Anurathapan U, Hongeng S. Immune escape mechanisms and future prospects for immunotherapy in neuroblastoma. Biomed Res Int. 2018;2018:1812535. doi:10.1155/2018/1812535
26. Junker K, Heinzelmann J, Beckham C, Ochiya T, Jenster G. Extracellular vesicles and their role in urologic malignancies. Eur Urol. 2016;70(2):323–331. doi:10.1016/j.eururo.2016.02.046
27. D’Asti E, Chennakrishnaiah S, Lee TH, Rak J. Extracellular vesicles in brain tumor progression. Cell Mol Neurobiol. 2016;36(3):383–407. doi:10.1007/s10571-015-0296-1
28. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. BioScience. 2015;65(8):783–797. doi:10.1093/biosci/biv084
29. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi:10.1007/s11060-013-1084-8
30. Aguilar-Mahecha A, Hassan S, Ferrario C, Basik M. Microarrays as validation strategies in clinical samples: tissue and protein microarrays. OMICS. 2006;10(3):311–326. doi:10.1089/omi.2006.10.311
31. Al Kuraya K, Simon R, Sauter G. Tissue microarrays for high-throughput molecular pathology. Ann Saudi Med. 2004;24(3):169–174. doi:10.5144/0256-4947.2004.169
32. Bandala-Sanchez E, Zhang Y, Reinwald S, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol. 2013;14(7):741–748. doi:10.1038/ni.2610
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.