Back to Journals » Clinical Interventions in Aging » Volume 16
MALAT1 Regulated mTOR-Mediated Tau Hyperphosphorylation by Acting as a ceRNA of miR144 in Hippocampus Cells Exposed to High Glucose
Authors Lu C, Zhao Y, Cao Y, Liu L, Wu S, Li D, Liu S, Xiao S, Wei Y, Li X
Received 22 February 2021
Accepted for publication 3 June 2021
Published 22 June 2021 Volume 2021:16 Pages 1185—1191
DOI https://doi.org/10.2147/CIA.S304827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Chong Lu,1,* Yikui Zhao,2,* Yan Cao,3,* Li Liu,1 Shanshan Wu,1 Dongbin Li,1 Shuang Liu,1 Shuyuan Xiao,1 Yafen Wei,1 Xinyu Li3
1Department of Neurology, Heilongjiang Provincial Hospital, Harbin, People’s Republic of China; 2HIT Center for Life Sciences, School of Life Science and Technology, Harbin Institute of Technology, Harbin, People’s Republic of China; 3Department of Endocrinology, First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinyu Li; Yafen Wei Tel +86-13796658016
; Tel +86-13796658016
Email [email protected]; [email protected]
Aim: High glucose (HG)-induced activation of mTOR promotes tau phosphorylation and leads to diabetes-associated dementia. This study aimed to explore the role of metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) in HG-induced neuronal cell injury.
Methods: Hippocampus cells were isolated from C57BL/6J mice. After 6 days of culture, the cells were incubated with 5.5 mM glucose in normal medium or 75 mM glucose for 4 days. Cells were transfected with miR-144 mimic, miR-144 inhibitor, siRNA for MALAT1 or corresponding controls. Gene expression was detected by PCR and Western blot analysis.
Results: HG increased the levels of MALAT1 and p-tau in hippocampal cells. Knockdown of MALAT1 partially reversed the effects of HG on mTOR activity and p-tau protein levels. MALAT1 functioned as competing endogenous RNA (ceRNA) for miR-144, and pre-treatment with MALAT1 siRNA decreased mTOR activity and p-tau protein level in HG-treated hippocampal cells, which was significantly attenuated by miR-144 mimics. Moreover, miR-144 negatively regulated the expression of mTOR and knockdown of MALAT1 suppressed mTOR, while overexpression of mTOR abrogated protective effects of MALAT1 knockdown in HG-treated hippocampal cells.
Conclusion: MALAT1 knockdown prevented HG-induced mTOR activation and inhibited tau phosphorylation. MALAT1 may be a therapy target for diabetes associated dementia.
Keywords: diabetes mellitus, tau, MALAT1, mTOR, miR-144
Introduction
Diabetes mellitus (DM) indicates metabolic diseases characterized by high blood glucose level.1 DM includes type 1 and type 2 diabetes. Type 1 diabetes is an autoimmune disease, and insulin-producing cells in the pancreas are destroyed. Patients with type 1 diabetes need take insulin every day, and type 1 diabetes is also called insulin-dependent diabetes. Type 2 diabetes is the most common type of diabetes. The body could not make enough insulin or the cells do not respond normally to the insulin. Hyperglycemia is known to induce diabetes-associated cognitive defects.2,3 To prevent or treat cognitive defects induced by DM, it is important to elucidate the mechanisms how high glucose (HG) promotes neurodegeneration.
Tau plays a crucial role in the regulation of microtubule (MT) in neurons and pathological phosphorylation of tau contributes to neurodegeneration.4 In particular, tau phosphorylation promotes cognitive defects in Alzheimer’s diseases (AD), one of the most common neurodegenerative diseases.5 Interestingly, tau pathology is also observed in DM, and tau hyperphosphorylation was reported in the brains of diabetes animal models and diabetes patients.6 Moreover, long-term exposure to HG could induce tau hyperphosphorylation in primary neurons. Therefore, inhibiting tau hyperphosphorylation is a promising approach to prevent the development of neurodegeneration and cognitive dysfunction.7
Hyperglycemia is known to induce the production of inflammatory cytokines to activate multiple signaling pathway, including mTOR signaling. In AD, the activation of mTOR signaling leads to tau phosphorylation and consequent cognitive defects.8 Several studies demonstrated that mTOR activation promoted tau phosphorylation both in vitro and in vivo.9,10
The metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) is a lncRNA implicated in many diseases, including diabetes and its complications.11 Recently, the role of MALAT1 in AD has been suggested, but whether MALAT1 contributes to HG-induced tau hyperphosphorylation is unclear. The lncRNA can antagonize the function of miRNA by trapping or miRNA sponge to regulate mRNA expression, acting as endogenous competitive RNA (ceRNA).12 As ceRNA, lncRNA regulates many processes involved in metabolic diseases and neurodegenerative diseases.13,14 In a previous study, we have successfully constructed the ceRNA network related to DM based on bioinformatics analysis, and extracted the competitive subnet of MALAT1-miR-144-mTOR.15 Therefore, this study aimed to investigate whether MALAT1 is a ceRNA of miR-144 and whether the silencing of lncRNA MALAT1 could attenuate HG-induced tau hyperphosphorylation via regulating mTOR signaling in hippocampus cells.
Materials and Methods
Cell Culture
All animal experiments were approved by Animal Care and Use Committee of First Affiliated Hospital of Harbin Medical University and were performed following Chinese National Guidelines for Experimental Animal Welfare. Hippocampus cells were isolated from C57BL/6J mice, and after 6 days of culture hippocampus cells were validated by staining with neuron marker NeuN, following standard protocols.16 Cell were incubated with 5.5 mM glucose in normal medium (control group) or 75 mM glucose (HG group).
Cell Transfection
miR-144 mimic, NC mimic, miR-144 inhibitor and NC inhibitor were provided by Thermo Fisher. The small interfering RNA (siRNA) for MALAT1 (si-MALAT1) and negative control (si-NC) were provided by Ribobio (Guangzhou, China). The empty vector (pcDNA3.1) and mTOR overexpression vector (pcDNA3.1-mTOR) were provided by BlueGene (Shanghai, China). Hippocampus cells were seeded in 12-well plates at a density of 120,000 cells per well, and transfected with oligonucleotides or plasmids using Lipofectamine RNAi Max (Life Tech, Carlsbad, CA, USA) following the manufacturer’s protocols. The cells were collected 24 h after transfection for further analysis.
Real-Time PCR
RNA was extracted from hippocampus cell using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and was reversely transcribed using PrimeScript RT Kit or Mir-XTM miRNA First-Strand Synthesis Kit (Takara, Dalian, China). Real-time PCR was performed using TB Green Fast qPCR Mix (Takara). Primer sequences were as follows: MALAT1-F, CACTTGTGGGGAGACCTTGT; MALAT1-R, TGTGGCAAGAATCAAGCAAG; mTOR-F, ATGACGAGACCCAGGCTAAG; mTOR-R, GCCAGTCCATGCCATCAG; tau-F, ATGGCTGAGCCCCGCCAGGAGTTCG; tau-R, CACAAACCCTGCTTGGCCAGGG; GAPDH-F, TTCACCACCATGGAGAAGGC; GAPDH-R, GGCATGGACTGTGGTCATGA; miR-144-F, CGGTACAGTAGATGTACT; miR-144-R, Uni-miR qPCR primer; U6 F, CTCGCTTCGGCAGCACA; U6 R, AACGCTTCACGAATTTGCGT. MALAT1 and mTOR expression levels were normalized based on glyceraldehyde 3-phosphate dehydrogenase and miR-144 expression level was normalized based on U6.
Western Blot Analysis
Proteins were extracted from hippocampus cells by lysis in radio-immunoprecipitation assay buffer (Sigma-Aldrich), and then were separated by gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were incubated with 5% non-fat milk at room temperature for 1 h, and then incubated with primary antibodies for mTOR, tau, p-tau and β-actin (dilution 1:1,000, Cell Signaling, Cambridge, MA, USA) overnight at 4°C. The membranes were washed and incubated with secondary antibodies conjugated to horseradish peroxidase (dilution 1:2,000, Cell Signaling) for 1 h at room temperature. The membranes were developed with enhanced chemiluminescence kit (Thermo Fisher).
Statistical Analysis
Data were shown as mean ± standard derivation (mean ± SD) and analyzed with SPSS 13 version software (SPSS, Chicago, IL, USA). t tTest or one-way ANOVA was used for group comparison. A two-tailed p< 0.05 was considered to be significant difference.
Results
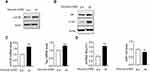
MALAT1, mTOR and p-Tau Were Significantly Upregulated in HG-Treated Hippocampus Cells
The primary culture hippocampal cells were treated with high glucose (75 mM). Based on Western blot analysis, we found that protein levels of mTOR, tau and p-tau were significantly higher in HG-treated hippocampus cells compared to control (Figure 1A and B). In addition, mTOR and tau mRNA levels and lncRNA MALAT1 levels were higher, while miR-144 level was lower in HG-treated hippocampus cells compared to control (Figure 1C and D).
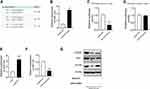
Silencing of MALAT1 Attenuated the Effects of HG on mTOR and p-Tau Levels in Hippocampus Cells
MALAT1 expression level was significantly lower in hippocampus cells treated with si-MALAT1 compared to control cells treated with si-NC (Figure 2A). However, in cells treated with si-NC, HG increased protein levels of mTOR, tau and p-tau, while si-MALAT1 significantly abrogated the effects of HG on mTOR, tau and p-tau levels in hippocampus cells (Figure 2B and C).
MALAT1 Acted as a Sponge for miR144 in Hippocampus Cells
In a previous study, we constructed MALAT1-miR144-mTOR competitive sub network.15 We identified complementary binding sites between miR-144 and MALAT1 fragments (Figure 3A). miR-144 mimic led to significantly higher miR-144 expression level in hippocampus cells (Figure 3B). Furthermore, miR-144 overexpression inhibited luciferase activity of wild-type MALAT1 but not mutant MALAT1 reporter (Figure 3C and D). In addition, silencing of MALAT1 led to higher miR-144 expression level in hippocampus cells (Figure 3E and F). Moreover, si-MALAT1 pretreatment decreased mTOR, tau and p-tau levels in HG-treated hippocampus cells, but this could be reversed by miR-144 inhibitor (Figure 3G).
MALAT1 Was ceRNA of miR-144 and Regulated the Effects of HG on Tau Phosphorylation in Hippocampus Cells
We further identified complementary binding sites between mTOR 3ʹUTR and miR-144 (Figure 4A). miR-144 overexpression inhibited luciferase activity of wild type mTOR 3ʹUTR but not mutant mTOR 3ʹUTR reporter vector in hippocampus cells (Figure 4B and C). Moreover, mTOR mRNA expression level was significantly lower in cells treated with miR-144 mimic or si-MALAT1 (Figure 4D and E). After transfection with mTOR overexpression vector, we found higher mTOR mRNA and protein levels in hippocampus cells (Figure 4F). In addition, si-MALAT1 pretreatment decreased protein levels of tau and p-tau in HG-treated hippocampus cells, but mTOR overexpression partially reversed the effects in hippocampus cells (Figure 4G).
Discussion
Hyperglycemia is known to be associated with tau phosphorylation and cognitive defects.17 Moreover, HG was shown to induce tau phosphorylation in primary hippocampal neurons.18 Therefore, this study focused on the mechanism by which HG induced tau phosphorylation in hippocampal cells. We found that HG induced mTOR activation to promote tau phosphorylation and this was regulated by MALAT1-miR144-mTOR network.
MALAT1 is an lncRNA involved in many diseases.19,20 In this study, for the first time we showed that knockdown of MALAT1 suppressed tau phosphorylation in HG-treated hippocampus cells. In addition, Western blot analysis showed that HG increased mTOR protein level in hippocampus cells, consistent with the role of HG in inflammation.21 MALAT1 acts as a ceRNA for miRNAs, which could inhibit the expression of target genes.22
Based on our previous findings on MALAT1-miR144-mTOR network, we performed luciferase assay to show that MALAT1 was a ceRNA for miR-144, and MALAT1 knockdown led to higher expression of miR-144. MiR-144 is a negative regulator of a disintegrin and metalloprotease 10 (ADAM10). ADAM10 is implicated in AD pathogenesis because proteolytic processing of amyloid precursor protein (APP) by ADAM10 produces a secreted ectodomain fragment of APP that has neuroprotective effects.22 Moreover, miR-144 regulates oxidative stress tolerance via controlling the generation of glutathione in AD.23 We found that miR-144 inhibitor abrogated the effects of MALAT1 knockdown on the expression of mTOR in hippocampus cells, suggesting that MALAT1 mediated neuronal damage in HG-treated hippocampus cells via suppressing miR-144.
mTOR plays a key role in cell growth, development and aging.24 Compelling evidence indicates the key role of mTOR in the regulation of tau-mediated pathological process in AD.25 In this study, we showed that mTOR was a target of miR-144. More importantly, overexpression of mTOR abrogated protective effects of MALAT1 knockdown on hippocampus cells injured by HG. Collectively, these data indicate that HG could induce the activation of mTOR, which then promotes the phosphorylation of tau and neuron injury. However, MALAT1 knockdown could reduce HG-induced injury in neurons by targeting miR-144.
In conclusion, we reported the upregulation of MALAT1 in in vitro cell model of diabetes-associated neurodegeneration. MALAT1 knockdown could attenuate HG-induced neuron injury, and this is achieved by the regulation of mTOR expression as a ceRNA for miR-144.
Acknowledgments
This study was supported by the National Natural Science Foundation of Heilongjiang Province (No. LH2020H115), the scientific research project of Heilongjiang Health Committee (No. 2019-019), the scientific research project of Heilongjiang Health Committee (No. 2020-244) and the special funds for postdoctoral research of Heilongjiang Province (No. LBH-Q20114). We thank Lin Zijing for the contribution to this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kumar P, Raman T, Swain MM, Mishra R, Pal A. Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented Tuberous Sclerosis Complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol. 2017;54(1):238–254.
2. Guo C, Zhang S, Li JY, et al. Chronic hyperglycemia induced via the heterozygous knockout of Pdx1 worsens neuropathological lesion in an Alzheimer mouse model. Sci Rep. 2016;6:29396.
3. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604.
4. Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12(6):609–622.
5. Wang F, Zhao M, Han Z, et al. Long-term subclinical hyperglycemia and hypoglycemia as independent risk factors for mild cognitive impairment in elderly people. Tohoku J Exp Med. 2017;242(2):121–128.
6. Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem. 2009;111(1):242–249.
7. Ma YQ, Wu DK, Liu JK. mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Mol Med Rep. 2013;7(2):623–627.
8. Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology. 2010;151(8):3752–3763.
9. Wang S, Zhou SL, Min FY, et al. mTOR-mediated hyperphosphorylation of tau in the hippocampus is involved in cognitive deficits in streptozotocin-induced diabetic mice. Metab Brain Dis. 2014;29(3):729–736.
10. Wu J, Zhou SL, Pi LH, et al. High glucose induces formation of tau hyperphosphorylation via Cav-1- mTOR pathway: a potential molecular mechanism for diabetes-induced cognitive dysfunction. Oncotarget. 2017;8(25):40843–40856.
11. Abdulle LE, Hao JL, Pant OP, et al. MALAT1 as a diagnostic and therapeutic target in diabetes-related complications: a promising long-noncoding RNA. Int J Med Sci. 2019;16(4):548–555.
12. Yao K, Wang Q, Jia J, Zhao H. A competing endogenous RNA network identifies novel mRNA, miRNA and lncRNA markers for the prognosis of diabetic pancreatic cancer. Tumour Biol. 2017;39(6):1010428317707882.
13. Feng SD, Yang JH, Yao CH, et al. Potential regulatory mechanisms of lncRNA in diabetes and its complications. Biochem Cell Biol. 2017;95(3):361–367.
14. Wu P, Zuo X, Deng H, Liu X, Liu L, Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res Bull. 2013;97:69–80.
15. Lin Z, Li X, Zhan X, et al. Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. J Cell Mol Med. 2017;21(12):3204–3213.
16. Li J, Zhang Y, Zhang T, et al. Analysis of isolation of cerebral cortical neurons in rats by different methods. Biocell. 2020;44(2):209–215.
17. Huang R, Tian S, Zhang H, Zhu W, Wang S. Chronic hyperglycemia induces tau hyperphosphorylation by downregulating OGT-involved O-GlcNAcylation in vivo and in vitro. Brain Res Bull. 2020;156:76–85.
18. Sun Y, Xiao Q, Luo C, et al. High-glucose induces tau hyperphosphorylation through activation of TLR9-P38MAPK pathway. Exp Cell Res. 2017;359(2):312–318.
19. Tang D, Yang Z, Long F, et al. Inhibition of MALAT1 reduces tumor growth and metastasis and promotes drug sensitivity in colorectal cancer. Cell Signal. 2019;57:21–28.
20. Riva P, Ratti A, Venturin M. The long non-coding RNAs in neurodegenerative diseases: novel mechanisms of pathogenesis. Curr Alzheimer Res. 2016;13(11):1219–1231.
21. Dai J, Jiang C, Chen H, Chai Y. Rapamycin attenuates high glucose-induced inflammation through modulation of mTOR/NF-κB pathways in macrophages. Front Pharmacol. 2019;10:1292.
22. Zheng Y, Zhang P, Zhu J, Zhang L, Zeng W. MicroRNA-382 inhibits the proliferation of mouse spermatogonia by targeting Kmt5a. Biocell. 2020;44(2):201–207.
23. Cheng C, Li W, Zhang Z, et al. MicroRNA-144 is regulated by activator protein-1 (AP-1) and decreases expression of Alzheimer disease-related a disintegrin and metalloprotease 10 (ADAM10). J Biol Chem. 2013;288(19):13748–13761.
24. Zhou C, Zhao L, Zheng J, et al. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci Lett. 2017;655:21–27.
25. Shafei MA, Harris M, Conway ME. Divergent metabolic regulation of autophagy and mTORC1-early events in alzheimer’s Disease? Front Aging Neurosci. 2017;9:173.
26. Tramutola A, Lanzillotta C, Di Domenico F. Targeting mTOR to reduce Alzheimer-related cognitive decline: from current hits to future therapies. Expert Rev Neurother. 2017;17(1):33–45.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.