Back to Journals » Clinical Ophthalmology » Volume 13
Main Complications of Photorefractive Keratectomy and their Management
Authors Spadea L , Giovannetti F
Received 4 October 2019
Accepted for publication 6 November 2019
Published 27 November 2019 Volume 2019:13 Pages 2305—2315
DOI https://doi.org/10.2147/OPTH.S233125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Leopoldo Spadea, Francesca Giovannetti
Eye Clinic, Policlinico Umberto 1, Department of Sensory Organs, “La Sapienza” University of Rome, Rome, Italy
Correspondence: Leopoldo Spadea
Head Eye Clinic, Policlinico Umberto 1, “Sapienza” University of Rome, Via Benozzo Gozzoli 34, Rome 00142, Italy
Tel +39 06 519 3220
Fax +39 06 8865 7818
Email [email protected]
Abstract: Photorefractive keratectomy (PRK) was the first surface ablation procedure introduced for the treatment of refractive errors and has been proven to be effective and safe. In some cases, however, the patient may not be totally satisfied with the final result and retreatment may be necessary. We performed a literature review to describe the main conditions that may arise following PRK that may require retreatment and new promising techniques to allow customized and effective treatments for patients. There is currently no gold standard for retreatment of residual refractive error after PRK. The surgeon must take into account the patient’s history and type of problem when choosing the most appropriate technique. LASIK and PRK are the main options. Haze can be treated with good results with phototherapeutic keratectomy and mytomicin C. High order aberrations and decentration may be addressed with topographically-guided excimer photoablation or with wavefront-guided PRK.
Keywords: photorefractive keratectomy, enhancement, corneal wound healing, regression, haze, decentration, HOA, topographically-guided excimer laser photoablation
Introduction
Spectacles and contact lenses are the most common modalities used for correction of refractive errors. Surgical treatment is another option and aims to overcome some of the limitations of spectacles and contact lenses, such as glasses being uncomfortable and not being practical for playing sports, and contact lenses being associated with corneal infections. Surgical procedures that involve operating on the cornea using an excimer laser are known as laser vision corrections and include two main procedure groups: laser-assisted in situ keratomileusis (LASIK) and surface treatments.
Photorefractive keratectomy (PRK) was the first type of surface treatment introduced. In this procedure, the ultraviolet beam generated by a 193nm argon fluoride excimer laser is irradiated to the corneal stroma, after epithelium removal, to reshape the anterior corneal stroma by photoablation and correct the ametropia. PRK has been studied since the 1980s1,2 and has been proven to be predictable, effective and safe in the treatment of low to moderate myopia, hyperopia and astigmatism.3–6
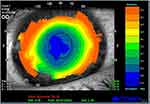
Since its introduction, the procedure and laser technology used have been improved substantially. By avoiding the creation of a lamellar flap and its associated risks, the procedure is particularly indicated in patients with thinner corneas, greater pupillary diameter and low to moderate myopia. Shortt et al in 2006 performed a metanalysis of PRK results which were published in a Cochrane report. The procedure was found to be highly predictable, accurate, and reproducible and the authors concluded that there was stability of refraction at three months follow-up, with minimal regression after this time.7 (Figure 1) However, the wound-healing response caused by ablation of the central Bowman layer and anterior stroma, that plays a role in the final result, can in some cases lead to subepithelial haze formation or to regression of the initial correction. In particular, the higher the ametropia that has to be corrected, the higher the possibility of haze development.8
In this article we will discuss the main complications that may arise following PRK and their current treatment options.
A literature review was performed to identify relevant publications addressing complications of PRK and their treatment. Pubmed, Scopus and Cochrane databases were searched for articles published in English and Italian, from 1999 to 2019, using the following search terms, either singly or in combination: “photorefractive keratectomy”, “complications”, “management”, “treatment”, “enhancement”, “haze”, “regression”, “decentration”, “dry eye”, “retreatment”, “topographically-guided ablation”, “wave-front guided ablation”. An initial screening of articles’ titles and abstracts was carried out by both reviewers. This yielded a total 80 articles, of which full text was further examined. The selection was restricted to articles that described clinical studies with follow-up periods longer than six months and that evaluated at least 20 eyes, systematic reviews of the literature regarding retreatment after PRK, and case reports from our own experience. Additional studies were identified using reference lists to find other relevant articles that might have been overlooked in our database search. The screening led to a selection of fifty-eight articles in total.
Conditions Following PRK That May Require Retreatment
Excimer laser treatments have become consistent and reliable procedures, and patients report high satisfaction rates. Visual acuity outcomes have become fairly standardized due to the many improvements that have been made in laser applications and accuracy, as well as in the mode of corneal flap removal and in the introduction of customized algorithms. However, several complications can occur. In the short-term, patients can experience pain, delayed visual recovery, and haze.3 Under- or over-correction, regression, decentration, haze, corneal ectasia and dry eye are among the most common long-term complications.
Under/Over-Correction and Regression
Under- or over-correction and regression are mainly due to an abnormal healing process or to biomechanical changes in the cornea. When surgeons started to use excimer laser surface ablation, initial hyperopic overcorrection was more common; later on, the enlargement to 6.00mm of the optical zone reduced the variance in refractive outcomes.9 Refractive regression is defined as the gradual, partial or complete loss of the attempted correction, that affects the predictability of refractive surgery procedures, and is mainly due to epithelial hyperplasia and stromal remodeling.10 Primary undercorrection also depends on the epithelial and stromal healing response.11 An increase in axial myopia, lenticular myopia, and corneal reshaping are possible causes of these residual refractive errors. Pregnancy or endocrinal disorders can also play a role in these late changes.12 Naderi et al examined 150 eyes with refractive error regression following PRK to analyze the factors linked to regression. They found a 19% rate of regression and concluded that an increase in the variables “simulated keratometry astigmatism” and “5mm irregularity” was associated with refractive error regression. Their results also indicate that age and sex of patients, type of refractive error and surgeon specialty, could also play a role.13
The percentage of patients that need retreatment after PRK myopic correction with an excimer laser is 6.8% (range from 3.8% to 20.8%).12,14,15
Various authors have followed-up patients after PRK treatment, to evaluate its efficacy. Alio et al found that the higher the degree of myopia, the lower the percentage of patients who achieve a good uncorrected visual acuity. In the low–moderate myopia group, 0.49 diopter (D) of myopic regression was seen over ten years, whereas 1.33D regression was observed in the high myopia group. Most cases of myopic regression developed over the first three months after surgery, with only slight change after the first three months and up to ten years. The authors hypothesized that the observed regression was due to epithelial hyperplasia and to corneal steeping due to corneal thinning, or to an increase in axial myopia and lenticular myopia. Another known possible complication, haze, appeared in less than 2% of patients in the low myopia group compared with 8.6% in the high myopia group.16,17 Shojaei et al reported myopic regression over the first year and a half after surgery analyzing 371 myopic eyes who had undergone PRK, following which no further regression was observed at 7.5 years. Only 4.3% of eyes required retreatment. Greater myopic corrections were associated with greater regression rates and higher rates of haze development.14
Besides refractive correction larger than 5.00D, other factors associated with the need of retreatment after PRK include the use of Mitomycin,18 a small optical zone (<6.00mm) and unstable fixation during laser ablation.19 De Benito-llopis et al examined 75 eyes of 49 patients ten years after having undergone PRK with de-epithelialization performed by laser to correct myopia in thin corneas (<500μm). By leaving thicker residual stroma than LASIK, PRK is less involved in the development of postoperative ectasia and therefore can be considered the technique of choice for patients with thin corneas and also for patients with recurrent erosion syndrome, corneal dystrophies, high intraocular pressure, dry eye disease or patients who are more at risk for trauma, like athletes and pilots. In this study thirty eyes (40%) needed enhancement, due to early undercorrection or to late regression, or both.20 It is important to note that other studies with normal thickness corneas show similar safety and efficacy indices on a long-term follow-up.21
Corneal Haze
The possible development of corneal opacity (haze) is one of the limits of PRK treatment and an important long-term complication. Limiting haze formation is important in improving visual outcomes in surface ablation techniques: when it develops patients may experience refractive regression and glare. Two types of haze have been described: type 1 tends to appear 1–3 months after surgery and then disappear after one year; type 2, defined as “late-onset corneal haze”, appears after three months and persists for more than three years.22,23 Several factors are involved in haze formation: the greater ablation depth required in the treatment of higher grades of myopia, the integrity of the epithelial basement membrane, and the deposition of abnormal extracellular matrix as part of the corneal wound-healing process.24 Stromal surface irregularities leave persistent defects of basal membrane that promote the passage of TGF-β, involved in myofibroblast proliferation.25 When the epithelial basal membrane is restored, the levels of TGF-β decrease, IL-1 causes the apoptosis of myofibroblast and the cornea re-acquires its transparency. This process can last several weeks to several months.26
A better understanding of the phenomena involved in corneal healing can surely improve the effectiveness and safety of refractive surgery procedures.27 The debridement conducted during PRK leaves the epithelium thinner; however, a gradual thickening occurs up to 12 months after surgery.28 The wound healing processes of keratocyte activation and myofibroblast proliferation are particularly evident after the treatment of high-grade myopia.29 High myopia or astigmatism, autoimmune conditions, age and high exposure to ultraviolet radiation are common risk factors for haze development. An environment with high UV radiation levels is associated with an increased risk of late-onset corneal haze, as reported by Stojanovic and Nitter in a study published in 2001, suggesting that the use of UV protective eyewear during the first year after surgery may be recommended.30
Topical steroids after surgery are able to prevent corneal haze for the first three months, but are not effective in preventing late-onset haze, except in eyes with high myopia.31
The use of mitomycin C (MMC) in humans after refractive surgery was documented for the first time in 2000 by Majmudar et al.32 MMC modulates the corneal wound-healing process by blocking keratocyte activation and proliferation and myofibroblast differentiation. However, the intraoperative use of MMC is linked to increased endothelial cell loss and variability in refractive outcomes and corneal haze may still appear despite its use.18
Corneal Ectasia
Another, less frequent, long-term complication that can be induced by PRK is corneal ectasia. In the studies we previously analyzed, none of the eyes developed postoperative ectasia. The risk for ectasia appears to be lower after surface ablation than after LASIK. Randleman et al performed a literature search of reported cases of ectasia from 1997 to 2005, and found that 96% of cases of ectasia occurred after LASIK and only 4% occurred after surface ablation.33 It is very important to rule out post-refractive surgery ectasia when it comes to deciding for enhancement treatment after refractive surgery: only small topographical changes may be seen, especially in the initial stages, and these can be mistaken for regression.34
Other Conditions
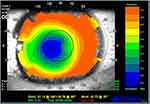
Other conditions that may determine a need for retreatment include decentration of treatment, the presence of a small optical zone (evaluated through computerized corneal topography), and aberrations.35 Slightly decentered ablation is quite common, but it becomes clinically significant when the decentration is greater than 0.5mm from the visual axis. Signs and symptoms may be irregular astigmatism, reduced contrast sensibility, glare and halos, reduced visual acuity, undercorrection.36 (Figure 2) Decentered treatments may be associated with poor postoperative visual acuity and lower patient satisfaction: the cornea can result highly multifocal, with suboptimal results regarding visual acuity, and patients can experience subjective symptoms in scotopic and mesopic conditions due to induced astigmatism.37 Aberrations can occur after PRK but are less common than after LASIK treatment, especially in patients with greater pupillary diameters.
Another common cause of patient dissatisfaction and one of the most common complications after PRK and LASIK is dry eye. Tear production, as measured by Schirmer test, was lower following LASIK than after PRK.38 Both techniques impair corneal innervation, crucial for ocular surface homeostasis, and cause ocular surface inflammation. After surgery, patients may experience foreign body sensation, blurred vision and excessive tearing. These symptoms usually persist up to three months in PRK whereas after LASIK last until six months.39 In vivo confocal microscopy of corneal nerves studies have shown that regeneration of normal nerve density may be faster after PRK than LASIK, but after both procedures, corneal sensitivity eventually returns to normal.40
Therefore, in patients with preoperative dry eye, PRK appears to be the technique of choice.
Treatment Options
Management of Over/Under Correction, Regression, Aberrations and Decentration
Although the accuracy of refractive surgical procedures is increasing, it is not uncommon to consider retreatment after PRK when there is a residual refractive error such as over/under correction, regression, aberrations and decentration, that interfere with the patient’s quality of life.41 As some patients may adapt to small amounts of residual error, the main indication to consider retreatment is patient dissatisfaction with visual acuity. A metanalysis establishing a gold standard treatment for retreatment (also known as enhancement) has not been performed yet. In 2014 Parikh et al published a review of the various steps and methods in managing residual refractive error after LASIK and PRK.42 The authors highlighted the importance of approaching systematically the management of residual refractive error after LASIK and PRK in order to decide which technique can result the most efficacious for the patient. The first step when considering enhancement surgery is a thorough patient evaluation. A complete ocular examination, corneal topography and pachymetry and uncorrected and best-corrected visual acuity examination must be performed, and the surgical records from the previous excimer laser treatment must be reviewed.
- Enhancements can be performed with a second PRK procedure. In 1999 George and Johnson performed a retrospective analysis of 224 eyes that had undergone PRK surgery. These were divided into two groups: group 1 had a one-step PTK removal of the epithelium, group 2 had a two-step laser removal of the epithelium (first PRK and then PTK). Laser epithelial removal resulted in an even and smooth stroma, reducing the risk of corneal irregularities. The two-step procedure was found to provide a more even and confluent removal of the epithelium, resulting in less risk of haze formation and of loss in visual acuity and therefore in a reasonably safe and effective procedure for PRK enhancements.11
- Laser in situ keratomileusis (LASIK) enhancement after PRK is also considered safe, predictable, and effective. The surgeon however must use precautions in order to prevent corneal haze after LASIK, which can develop probably from increased keratocyte reactivation as a result of loss of Bowman layer and prolonged wound healing with previous PRK surgery: the use of a 180μm flap and intensive topical corticosteroid therapy may help in preventing the development of the opacities.43
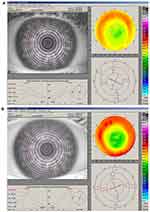
- Topographically-guided transepithelial excimer laser photoablation, in which the individual patient corneal topography is measured and converted to a custom ablation profile, can be effective and safe for the treatment of the residual myopia or hyperopia after primary myopic or hyperopic PRK.44 Reliable detection of corneal irregularities is required to allow the laser to perform customized corneal ablation. The laser ablation is defined as the volume of tissue which must be subtracted to bring the detected corneal shape at the ideal shape which is needed to optimize the quality of vision. Indications for customized corneal ablation treatments include congenital defects, eye injuries, burns, scarring after infections, corneal ulcers and prior eye surgery.45 Topographically- guided customized ablation is an effective option for patients who underwent prior eye surgery that resulted in decentered ablation, central islands, post-keratoplasty astigmatism. This procedure was evaluated in a study that enrolled 37 eyes of 32 myopic or hyperopic patients who requested retreatment after PRK. The minimum follow-up was three years. The main indications for retreatment in this study were significant regression or overcorrection relative to the original refractive defect. After undergoing this procedure, all patients were within +0.75D of emmetropia in manifest refractive spherical error (MRSE). The information provided by the videokeratographic system included evaluation of simulated keratometry (SimK), of refractive astigmatism and qualitative–quantitative morphological information of the topographic indices surface asymmetry index (SAI) and surface regularity index (SRI). The topographic patterns improved in all eyes. (Figures 3 and 4) Anterior stromal haze was evaluated using Heitzmann criteria (from 0 to 5)46 and no patient presented a haze score greater than 1 in the last examination at 3 years.40 The transepithelial technique is useful because epithelium can mask stromal irregularities. Therefore excimer laser customized ablation seems to be a powerful technique to treat corneal irregularities and may increase the success rate in retreatments.37 Ghoreishi et al agree that topography-guided PRK can significantly reduce irregular astigmatism and increase the uncorrected visual acuity (UCVA) and distance corrected visual acuity (DCVA). In a study published in 2014, in which 28 consecutive eyes of 26 patients with irregular astigmatism after radial keratotomy, corneal transplant, small hyperopic and myopic excimer laser optical zones, and corneal scars were operated, UCVA improved significantly and refractive cylinder decreased greatly.47 Reinstein et al studied the transepithelial-PTK technique and state that it can be a safe and effective method of reducing stromal surface irregularities by taking advantage of the natural masking effect of the epithelium. In their retrospective analysis of 41 procedures, after 12 months corrected distance visual acuity had improved by one or more lines in 58% of eyes, whereas one eye had lost one line and no eyes had lost two lines, and significant stromal surface regularization was achieved.48
Enhancements for decentration have been described only anecdotally and include arcuate cuts and laser photoablative techniques, such as diametral ablation, masked ablations through collagen based lenticules, and the Nizzola and Vinciguerra technique. Topography-linked excimer laser is a technique for treating decentrations. Alessio et al demonstrated its effectiveness in a study that analyzed 32 patients with irregular or asymmetric corneal astigmatism caused by decentration after excimer laser treatment who underwent Corneal Interactive Programmed Topical Ablation (CIPTA, LIGI, Taranto, Italy). Topographic analysis showed that the average decentration was reduced and the UCVA improved in all the patients.37 This technique may increase the success rate in retreatments, which is generally lower with respect to the primary procedure. Anyway, the rate of enhancements performed for decentration is decreasing thanks to wider optical zones (6–8mm), eye tracker technology and greater experience.45
- Wavefront-guided retreatment. Refractive surgery can sometimes induce high order aberrations (HOAs), especially spherical aberrations and coma, which can decrease quality of vision.49 In fact the air-cornea interface and the anterior corneal surface contribute greatly to optical errors. By using corneal elevation data from corneal topography and clinical data, it is possible to create customized treatments to minimize 2nd order aberrations and HOAs. Several studies have shown that wavefront-guided retreatments can reduce HOAs and corneal spherical aberrations and therefore improve visual acuity.21,50 By measuring how the optical system alters a wavefront of light entering the eye, wavefront aberrometry can detect subtle aberrations of the eye.51 Aliò et al demonstrated that corneal wavefront-guided PRK enhancement with the custom ablation manager software ORK-CAM system (Schwind Eye Tech Solutions, Kleinostham, Germany), a software for customized ablation design, minimized corneal HOAs in eyes with previous unsuccessful keratorefractive surgery. They examined 25 eyes of 20 patients and reported a statistically significant improvement in UCVA and DCVA at six months.21 Reductions in total, primary coma, spherical-like, and coma-like aberrations at six months were also statistically significant. This technique was shown also to improve night vision symptoms in patients with high positive spherical aberration after myopic laser refractive surgery.52 Night time glare and halo symptoms subjectively improved to none or mild (in all patients at the six-month follow-up examination) and spherical aberration was significantly reduced. In highly aberrated corneas that were subjected to previous keratorefractive procedures, aberrations generated with the surgery are the most important source of visual disturbances. Retreatment using ablations based on corneal customization or topography guidance is an option to consider to restore quality of vision.
Management of Corneal Haze
As previously stated, the occurrence of corneal haze is the major complication of surface ablation. There are several medical and surgical methods for managing haze, according to the haze grade. In 1993 Heitzmann proposed a corneal haze grading system: grade 0 clear cornea, grade 0.5 haze slightly detectable on slit lamp examination, grade 1 reticular haze, easily detectable on slit lamp examination, grade 2 clinically significant haze with areas of focal confluency, grade 3 clinically significant haze with areas of diffuse confluency, grade 4 confluent and diffuse haze leading to difficulties in examining the iris, grade 5 corneal opacity that does not allow to explore the iris.46 Initially (grades 0–2) the haze can be treated with topical steroids and topical non-steroidal anti-inflammatory drugs, especially in myopic eyes, but their efficacy over a long-time period is still controversial as they may also increase intraocular pressure. Haze grade 2–4 can be treated with mechanical epithelial debridement or with laser-scrape. Success of these procedures may depend on the morphology of the haze and on the patient’s individual wound healing response. Manual debridement and mitomycin-C (MMC) application can also be performed.53 (Figure 5). Excimer laser treatment with phototherapeutic keratectomy (PTK) can be considered and it was shown to be effective.35 MMC can be employed during this treatment to prevent recurrent haze development. Corneal opacities ultimately need corneal transplant.
Management of Dry Eye and Corneal Ectasia
Dry eye after PRK and LASIK must be promptly recognized and addressed, as the tear film contributes to refraction. Ophthalmologists should maximize tear film stability preoperatively and minimizes dry eye postoperative, since it is one of the main caused of patient dissatisfaction after refractive surgery. Careful assessment of the eyelid, Tear film break up time, Rose Bengal staining, corneal esthesiometry and Schirmer test should be performed. Initial management with preservative-free artificial tear supplements, treatment of pre-existing lid disease and cyclosporine 0.05% eye drops can be effective.7 Khalil et al treated patients with lower refractive errors with punctal plug placement, obtaining an improvement in visual acuity.54
Corneal ectasia that may develop after refractive surgery has traditionally been treated with rigid gas-permeable contact lenses and with intracorneal ring segments, although corneal transplantation is often needed.55 Recently, crosslinking treatment and intracorneal ring surgery, also combined, have shown good results.56,57
Conclusions
Many variables need to be considered when approaching a patient with residual refractive error. The main indication for enhancement treatment is patient dissatisfaction with the refractive surgery results. A thorough preoperative exam must be conducted. Refraction should be stable since at least six months and the risk of ectasia must be evaluated with corneal topography and pachymetry. If dry eye has developed after surgery, it must be addressed.
No procedure is considered the safest or most efficacious for enhancement, so the best method must be determined considering the individual patient needs. If there is enough stromal bed, a surface ablation procedure can be considered. LASIK and secondary PRK are the main options. When haze is present, MMC, LASIK or PTK/PRK are options to consider. If there is ectasia, CXL and ICRS can be considered.41 Based on our literature review, the topographically guided techniques have also been shown to be useful in improving visual acuity and reducing refractive cylinder in a large percentage of patients, while minimizing the scarring often associated with repeated surface ablations.58 Treatment must be customized for each patient after a careful eye examination, in order to address the various types of complications that may develop after refractive surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goodman GL, Trokel SL, Stark WJ, Munnerlyn CR, Green WR. Corneal healing following laser refractive keratectomy. Arch Ophthalmol. 1989;107(12):1799–1803. doi:10.1001/archopht.1989.01070020881031
2. Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. 1988;14(1):46–52. doi:10.1016/S0886-3350(88)80063-4
3. Gartry DS, Kerr Muir MG, Marshall J. Excimer laser photorefractive keratectomy. 18-month follow-up. Ophthalmology. 1992;99(8):1209–1219. doi:10.1016/S0161-6420(92)31821-4
4. Seiler T, Wollensak J. Myopic photorefractive keratectomy with the excimer laser. One-year follow-up. Ophthalmology. 1991;98(8):1156–1163. doi:10.1016/S0161-6420(91)32157-2
5. Liu YL, Tseng CC, Lin CP. Visual performance after excimer laser photorefractive keratectomy for high myopia. Taiwan J Ophthalmol. 2017;7(2):82–88. doi:10.4103/tjo.tjo_6_17
6. Sher NA, Barak M, Daya S, et al. Excimer laser photorefractive keratectomy in high myopia. Arch Ophthalmol. 1992;110(7):935–943. doi:10.1001/archopht.1992.01080190041027
7. Shortt AJ, Allan BDS. Photorefractive keratectomy (PRK) versus laser-assisted in-situ keratomileusis (LASIK) for myopia. Cochrane Database Syst Rev. 2006;2:CD005135.
8. Taneri S, Weisberg M, Azar DT. Surface ablation techniques. J Cataract Refract Surg. 2011;37(2):392–408. doi:10.1016/j.jcrs.2010.11.013
9. O’Brart DP. Excimer laser surface ablation: a review of recent literature. Clin Exp Optom. 2013;97(1):12–17. doi:10.1111/cxo.12061
10. Spadea L, Fasciani R, Necozione S, et al. Role of the corneal epithelium in refractive changes following laser in situ keratomileusis for high myopia. J Refract Surg. 2000;16:133–139.
11. George Stan P, Johnson Donald G. Photorefractive keratectomy retreatments, comparison of two methods of excimer laser epithelium removal. Ophthalmology. 1999;106:1469–1480. doi:10.1016/S0161-6420(99)90439-6
12. Randleman JB, White AJ
13. Naderi M, Sabour S, Khodakarim S, Daneshgar F. Studying the factors related to refractive error regression after PRK surgery. BMC Ophthalmol. 2018;18(1):198. doi:10.1186/s12886-018-0879-y
14. Shojaei A, Mohammad-Rabei H, Eslani M, Elahi B, Noorizadeh F. Long-term evaluation of complications and results of photorefractive keratectomy in myopia: an 8-year follow-up. Cornea. 2009;28(3):304–310. doi:10.1097/ICO.0b013e3181896767
15. Wagoner MD, Wickard JC, Wandling GR
16. Alio JL, Muftuoglu O, Ortiz D, et al. Ten-year follow-up of photorefractive keratectomy for myopia of less than 6 diopters. Am J Ophthalmol. 2008;145:29–36. doi:10.1016/j.ajo.2007.09.007
17. Alio JL, Muftuoglu O, Ortiz D, et al. Ten-year follow-up of photorefractive keratectomy for myopia of more than 6 diopters. Am J Ophthalmol. 2008;145:37–45. doi:10.1016/j.ajo.2007.09.009
18. Sy ME, Zhang L, Yeroushalmi A, et al. Effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2014;40:1980–1984. doi:10.1016/j.jcrs.2014.02.048
19. Mohammadi SF, Nabovati P, Mirzajani A, Ashrafi E, Vakilian B. Risk factors of regression and undercorrection in photorefractive keratectomy. Int J Ophthamol. 2015;8(5):933–937.
20. De Benito-llopis L, Alió JL, Ortiz D, Teus MA, Artola A. Ten-year follow-up of excimer laser surface ablation for myopia in thin corneas. Am J Ophthalmol. 2009;147(5):768–773. doi:10.1016/j.ajo.2008.12.022
21. Alió JL, Piñero DP, Plaza Puche AB. Corneal wavefront-guided photorefractive keratectomy in patients with irregular corneas after corneal refractive surgery. J Cataract Refract Surg. 2008;34(10):1727–1735. doi:10.1016/j.jcrs.2008.06.025
22. Meyer JC, Stulting RD, Thompson KP, et al. Late onset of corneal scar after excimer laser photorefractive keratectomy. Am J Ophthalmol. 1996;121:529–539. doi:10.1016/S0002-9394(14)75427-3
23. Lipshitz I, Loewenstein A, Varssano D, et al. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology. 1997;104:369–373. doi:10.1016/S0161-6420(97)30306-6
24. Moller-Pedersen T, Cavanagh HD, Petroll WM, et al. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi:10.1097/00003226-199811000-00011
25. Netto MV, Mohan RR, Sinha S, et al. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi:10.1016/j.exer.2005.09.021
26. Barbosa FL, Chaurasia SS, Kaur H, et al. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp Eye Res. 2010;91:456–461. doi:10.1016/j.exer.2010.06.023
27. Spadea L, Giammaria D, Trabucco P. Corneal wound healing after laser vision correction. Br J Ophthalmol. 2016;100:28–33. doi:10.1136/bjophthalmol-2015-306770
28. Ivarsen A, Fledelius W, Hjortdal JO. Three-year changes in epithelial and stromal thickness after PRK or LASIK for high myopia. Invest Ophthalmol Vis Sci. 2009;50:2061–2066. doi:10.1167/iovs.08-2853
29. Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi:10.1016/S0014-4835(02)00251-8
30. Stojanovic A, Nitter TA. Correlation between ultraviolet radiation level and the incidence of late-onset corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 2001;27:404–410. doi:10.1016/S0886-3350(00)00742-2
31. Kaiserman I, Sadi N, Mimouni M, Sela T, Munzer G, Levartovsky S. Corneal breakthrough haze after photorefractive keratectomy with mitomycin C. Cornea. 2017;36(8):961–966. doi:10.1097/ICO.0000000000001231
32. Majmudar PA, Forstot SL, Dennis RF, et al. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi:10.1016/S0161-6420(99)00019-6
33. Randleman JB, Stulting RD. Ectasia after photorefractive keratectomy [letter]. Ophthalmology. 2007;114:396. doi:10.1016/j.ophtha.2006.09.008
34. Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi:10.1016/j.ophtha.2007.03.073
35. Spadea L, Bianco G, Balestrazzi E. Four techniques for retreatment after excimer laser photorefractive keratectomy. J Refract Surg. 1996;12(6):693–696.
36. Mulhern MG, Foley-Nolan A, O’Keefe M, Condon PI. Topographical analysis of ablation centration after excimer laser photorefractive keratectomy and laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 1997;23(4):488–494. doi:10.1016/S0886-3350(97)80204-0
37. Alessio G, Boscia F, La Tegola MG, Sborgia C. Topography-driven excimer laser for the retreatment of decentralized myopic photorefractive keratectomy. Ophthalmology. 2001;108(9):1695–1703. doi:10.1016/S0161-6420(01)00706-0
38. Lee JB, Ryu CH, Kim J, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:1326–1331. doi:10.1016/S0886-3350(00)00566-6
39. Pérez-Santonja JJ, Sakla HF, Cardona C, et al. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999;127(5):497–504. doi:10.1016/S0002-9394(98)00444-9
40. Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi:10.3109/08820538.2010.518133
41. Parikh NB. Management of residual refractive error after laser in situ keratomileusis and photorefractive keratectomy. Curr Opin Ophthalmol. 2014;25(4):275–280. doi:10.1097/ICU.0000000000000059
42. Lazaro C, Castillo A, Hernandez-Matamoros JL, et al. Laser in situ keratomileusis enhancement after photorefractive keratectomy. Ophthalmology. 2001;108(8):1423–1429. doi:10.1016/S0161-6420(01)00635-2
43. Alió JL, Artola A, Attia WH, et al. Laser in situ keratomileusis for treatment of residual myopia after photorefractive keratectomy. Am J Ophthalmol. 2001;132(2):196–203. doi:10.1016/S0002-9394(01)01004-2
44. Spadea L, Di Gregorio A. Enhancement outcomes after photorefractive keratectomy and laser in situ keratomileusis using topographically guided excimer laser photoablation. J Cataract Refract Surg. 2005;31(12):2306–2312. doi:10.1016/j.jcrs.2005.08.055
45. Spadea L, Bianco G, Balestrazzi E. Topographically guided excimer laser photorefractive keratectomy to treat superficial corneal opacities. Ophthalmology. 2004;111(3):458–462. doi:10.1016/j.ophtha.2003.06.002
46. Heitzmann J, Binder PS, Kassar BS, Nordan LT. The correction of high myopia using the excimer laser. Arch Ophthalmol. 1993;111:1627–1634. doi:10.1001/archopht.1993.01090120049021
47. Ghoreishi M, Naderi Beni A, Naderi Beni Z. Visual outcomes of topography-guided excimer laser surgery for treatment of patients with irregular astigmatism. Lasers Med Sci. 2014;29(1):105–111. doi:10.1007/s10103-013-1282-9
48. Reinstein DZ, Archer TJ, Dickeson ZI, Gobbe M. Transepithelial phototherapeutic keratectomy protocol for treating irregular astigmatism based on population epithelial thickness measurements by artemis very high-frequency digital ultrasound. J Refract Surg. 2014;30(6):380–387. doi:10.3928/1081597X-20140508-01
49. Ivarsen A, Hjortdal J. Seven-year changes in corneal power and aberrations after PRK or LASIK. Invest Ophthalmol Vis Sci. 2012;53:6011–6016. doi:10.1167/iovs.12-10208
50. Kanellopoulos AJ, Pe LH. Wavefront-guided enhancements using the wave- light excimer laser in symptomatic eyes previously treated with LASIK. J Refract Surg. 2006;22:345–349. doi:10.3928/1081-597X-20060401-08
51. Sakimoto T, Rosenblatt MI, Azar DT. Laser eye surgery for refractive errors. Lancet. 2006;367(9520):1432–1447. doi:10.1016/S0140-6736(06)68275-5
52. Alio ́ JL, Pin ̃ero D, Muftuoglu O. Corneal wavefront-guided retreatments for significant night vision symptoms after myopic laser refractive surgery. Am J Ophthalmol. 2008;145:65–74. doi:10.1016/j.ajo.2007.08.025
53. Spadea L, Verrecchia V. Effectiveness of scraping and mitomycin C to treat haze after myopic photorefractive keratectomy. Open Ophthalmol J. 2011;5:63–65. doi:10.2174/1874364101105010063
54. Khalil MB, Latkany RA, Speaker MG, Yu G. Effect of punctal plugs in patients with low refractive errors considering refractive surgery. J Refract Surg. 2007;23:467–471. doi:10.3928/1081-597X-20070501-08
55. Randleman JB. Post-laser in-situ keratomileusis ectasia: current understanding ad future directions. Curr Opin Ophthalmol. 2006;17:406–412. doi:10.1097/01.icu.0000233963.26628.f0
56. Spadea L. Collagen crosslinking for ectasia following PRK performed in excimer laser–assisted keratoplasty for keratoconus. Eur J Ophthalmol. 2012;22(2):274–277. doi:10.5301/ejo.5000019
57. Yildirim A, Uslu H, Kara N, et al. Same-day intrastromal corneal ring segment and collagen crosslinking for post-LASIK ectasia: long-term results. Am J Ophthalmol. 2014;157:1070–1076. doi:10.1016/j.ajo.2014.02.011
58. Knorz MC, Jendritza B. Topographically-guided laser in situ keratomileusis to treat corneal irregularities. Ophthalmology. 2000;107(6):1138–1143. doi:10.1016/S0161-6420(00)00094-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.