Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Magnitude of Hypertension and Associated Factors Among Human Immunodeficiency Virus Positive Adults Receiving Anti-Retroviral Therapy at Debre Markos Referral Hospital, Northwest, Ethiopia
Authors Sewale Y , Afenigus AD , Haile D , Shiferaw WS
Received 14 September 2020
Accepted for publication 14 October 2020
Published 22 October 2020 Volume 2020:12 Pages 629—637
DOI https://doi.org/10.2147/HIV.S280630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Yihenew Sewale,1 Abebe Dilie Afenigus,2 Dessalegn Haile,2 Wondimeneh Shibabaw Shiferaw1
1Department of Nursing, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Nursing, College of Health Science, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Yihenew Sewale Tel +251921578419
Email [email protected]
Background: People living with human immunodeficiency virus are at increased risk for cardiovascular diseases such as hypertension. Current evidence on the proportion of hypertension is essential to inform policymaker to strengthen interventions and regular monitoring of hypertension, yet information is scarce concerning hypertension in this study area.
Objective: This study aimed to assess the magnitude of hypertension and associated factors among human immunodeficiency virus-positive adults receiving antiretroviral therapy at Debre Markos Referral Hospital, Northwest Ethiopia.
Methods: A facility-based cross-sectional study was employed. Data were collected from systematically selected 412 participants using pre-tested, interviewer administered structured questionnaire. Data were entered and coded using Epi-data version 3.1 and analyzed using STATA version 14. The assumption of the logistic regression model was checked using a correlation matrix and Hosmer and Lemeshow’s tests. Bivariate and multivariate logistic regression analyses were conducted.
Results: In the present study, the prevalence of hypertension among human immunodeficiency virus-positive adults who received antiretroviral therapy was found to be 41.3% (95% CI; 36.7– 46.0). Age groups 35– 45 years (AOR: 2.48, 95% CI: 1.17, 5.27), greater than 45 years (AOR: 5.00, 95% CI: 2.190, 11.44), no physical exercise (AOR: 2.72, 95% CI: 1.33, 5.57), body mass index greater than or equal to 25 kg/m2 (AOR: 2.87 95% CI: 1.52, 5.39), and antiretroviral therapy regimens of 2 h/2f/2e/ABC+3TC+ATV/r (AOR: 3.05, 95% CI: 1.41, 6.60) were significantly associated with hypertension.
Conclusion: In the current study, the magnitude of hypertension was high among HIV-positive adults. Therefore, educating about the use of lifestyle change, counseling the use of regular physical activities, promoting weight reduction, and intervention in this situation are highly recommended.
Keywords: hypertension, HIV, ART
Introduction
Hypertension is defined as systolic blood pressure (SBP) values ≥130 mmHg and/or diastolic blood pressure (DBP) values ≥80 mmHg. It can be divided into stage I which is SBP 130–139 mmHg and/or 80–89 mmHg and Stage II SBP≥140 mmHg and/or DBP ≥90 hypertension.1
Globally, there are an estimated 1.13 billion people with hypertension, of these most are living in low-and middle-income countries.2 It leads to variety of cardiovascular diseases and accounts for about 45% of deaths due to heart disease and 51% of deaths due to stroke.3,4
World Health Organization (WHO) reported that approximately 34% of Ethiopian population died from non-communicable diseases (NCDs). This report also revealed that the magnitude of cardiovascular disease including hypertension was responsible approximately 15%.5
Human Immunodeficiency Virus/Acquired Immune Deficiency syndrome (HIV/AIDS) is a major public health problem in the world. Nearly 1 in every 20 adults living with HIV and accounting for nearly 70% of the people living with HIV worldwide.6 Worldwide, approximately 36.8 million people are living with HIV/AIDS in 2017.7 Sub-Saharan Africa is the most affected region, with 25.6 million people living with HIV and accounts for two-thirds (2/3rd) of the global total of new HIV infections. A study conducted in Vietnam showed that the numbers of new HIV cases, new AIDS case and deaths from AIDS have tended to decline steadily, with annual decreases of 13.98% in people newly infected with HIV, 2.31% in new AIDS patients, and 9.65% in deaths from AIDS.8 The increased ART use has reduced the HIV-related mortality rate, from an estimated 2 million deaths in 2005 to 1 million in 2018.9,10 However, in the same period, cardiovascular disease mortality rates more than doubled in people living with HIV.11
Evidence showed that approximately 35% of all HIV-positive adults on ART have hypertension, compared with an estimated 30% of persons with not HIV.12 HIV-positive adults receiving antiretroviral therapy with hypertension also have a higher risk of cardiovascular events and mortality than persons without HIV with hypertension.13–16 A study which was conducted in American veterans on HIV-positive adults on ART and uninfected adults showed that HIV-positive adults on ART with hypertension had a 2-fold higher risk for cardiovascular events as compared to HIV-negative adults with hypertension.16
Hypertension is responsible for cardiovascular mortality in HIV-positive adults receiving ant-retroviral therapy.17 The mechanisms of hypertension (HTN) in HIV-positive adults before the introduction of highly active antiretroviral therapy (HAART) were often associated with complications related to HIV.18 Though the origin of progressive vascular damage in HIV-positive adults is not clear, some studies suggested that the possibility of HAART may cause hypertension after HAART initiation.19 A study conducted in Italy confirmed higher rates of hypertension among HIV-positive adults on ART compared to uninfected adults.20 Likewise, the studies which are conducted in Sub-Sahara Africa showed that prevalence of hypertension among HIV-positive adults was high.21,22 However, the study conducted in South Africa also showed that hypertension was less common among HIV-positive adults.23
Though factors responsible for hypertension are multifactorial, evidence showed that pro-inflammatory impact induced by HIV infection on vascular endothelium would increase the risk of hypertension.24 Several studies showed that smoking,25 stress,26 and kidney dysfunction27 were significantly associated factors for hypertension in people living with HIV.
Studies conducted in Ethiopia found that the magnitude of hypertension was ranged from 12.7%28 to 29.7%.29 These studies documented factors associated with hypertension such as diabetes mellitus, body mass index (BMI), drinking alcohol, CD4 count <500 cell/mL, and duration of HAART.28,29 In Ethiopia, the government design different strategies to achieve sustainable development goal (SDG) #3. For example, national consolidated ART care and reduced by one-third premature mortality from non-communicable diseases, yielding marked success in reduction of HIV and hypertension patient morbidity and mortality, respectively.30,31
Despite the seriousness of the problem, a few studies have been conducted to evaluate factors that responsible to hypertension among HIV-positive adults. Therefore, this study aimed to identify factors associated with hypertension and its magnitude. The results obtained from the current study will improve the quality care of clients in the study area and similar settings of Ethiopia.
Methods
Study Design and Setting
A facility-based cross-sectional study was employed at Debre Markos Referral Hospital from February to March 2020. The Hospital is located in Debre Markos Town, northwest of Ethiopia and which is 300 km away from the capital city of Addis Ababa. It was constructed in 1957 to work for approximately 25,000 people, but now it is serving more than 3.5 million people. In the hospital, about 3220 patients were HIV/AIDS-infected adults who received antiretroviral therapy and had to undergo follow-up care, of these 1220 males and 2000 females.32
Population
In this study, the source and study population were all HIV-positive adults who received anti-retroviral therapy and available during the data collection period at the Debre Markos Referral Hospital ART clinic. We excluded patients who were pregnant and developed hypertension before antiretroviral therapy.
Sample Size Determination and Sampling Procedures
We estimated the adequate sample size based on the study objectives. For the first objective, the sample size was calculated using a single population proportion formula by considering the following statistical assumptions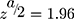 = at confidence level of 95%, d= margin error = 5%, p= population proportion (29.7%) of hypertension among HIV-positive adults receiving antiretroviral therapy which was taken from a study conducted in northeast Ethiopia,29
= at confidence level of 95%, d= margin error = 5%, p= population proportion (29.7%) of hypertension among HIV-positive adults receiving antiretroviral therapy which was taken from a study conducted in northeast Ethiopia,29 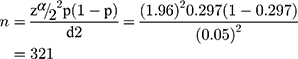 Finally, after adding a 10% non-response rate, the final sample size for the first objective was 353.
Finally, after adding a 10% non-response rate, the final sample size for the first objective was 353.
For the second objective, the sample size was calculated by considering age, sex and duration of HAART as the major statistical significant variables29 using Epi info version 7 statistical software. This calculation provided a sample size of 412 patients (Table 1). Finally, we took the largest sample size, and a total of 412 patients attended the ART clinic of Debre Markos Referral Hospital.
 |
Table 1 Sample Size Calculations to Assess the Magnitude of Hypertension and Associated Factors Among HIV-Positive Adults Who Received Antiretroviral Therapy at Debre Markos Referral Hospital, 2020 |
Sampling Procedures
An average patient who was attending an ART clinic for the last six months (July–November 2019) was 3220 on ART care in the study facility, of which 412 participants were selected. A systematic random sampling was employed to select the participants. The sampling interval (kth) was estimated by dividing the total HIV-positive adults receiving anti-retroviral therapy hospital allocated sample size. 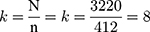 The first sample was selected randomly from attending patients, and then every (kth) sample was selected for collecting information until the required sample was achieved from the study participant of daily follow-up.
The first sample was selected randomly from attending patients, and then every (kth) sample was selected for collecting information until the required sample was achieved from the study participant of daily follow-up.
Covariates of the Study
The main outcome variable for this study was hypertension. The explanatory variables were sociodemographic characteristics, including age of the participants, sex, residence, occupation educational status and family size. Clinical-related factor information included CD4 count, viral load, ART adherence, WHO stage, OI, ART duration, ART regimen, prophylactic and comorbidity diseases, and lifestyle-related factors such as smoking, alcohol consumption, physical inactivity, chat chewing, and stress.
Operational Definitions
- Hypertension: a patient having a systolic blood pressure (SBP) of ≥130 and/or a diastolic blood pressure (DBP) of ≥80 mm Hg with two measurements apart of 2 minutes.1
- Adherence to ART: The recent adherence status of the adult to ART is recorded as poor when an adult takes less than 85% of the dose, fair when he/she takes 85–94% of the dose and good when he/she takes 95% and above of the dose.30
- Comorbid disease: a chronic disease with a confirmed diagnosis of the disease other than HIV infection.30
- Opportunistic infections/diseases: is the list of opportunistic diseases documented on national comprehensive HIV prevention, care, and treatment.30
Data Collection Procedures and Quality Control
An interviewer administered and structured questionnaire was employed to collect the data. The questionnaires were adapted and modified into the local context from previous works in the literature.16,33–41 To measure blood pressure the patient’s arm was bare and supported, use a cuff size appropriate for the patient arm (length of the bladder should be 80%, and the width of the bladder should be at least 40% of the circumference of the upper arm), the lower edge of cuff 3 cm above elbow crease, did not talk or moved before or during the measurement, legs uncrossed and feet flat on the floor. The average of at least two BP measurements spaced 2 minutes apart should be taken, and the average was used in the analysis.42 To assure data quality, the tool was first prepared in English and then translated to Amharic and back to English. In addition, daily supervision was performed by principal investigators. Two-day training was given for data collectors concerning the data collection tool and data collection process. The tool was pretested on 10% of the total sample size at Debre Markos Health Center. Data were collected at the time of ART care follow-up by interviewing all participants who were attended to the ART clinic of Debre Markos Referral Hospital. Additionally, clinical data were obtained by reviewing the chart. Four bachelors degree nurses working in the ART clinic of Debre Markos Referral Hospital were participated as data collectors. Furthermore, collected data were checked for completeness and consistency during the data management, storage, and analysis by the research team members.
Statistical Analysis
Data were entered into EPI DATA version 3.1, and further analysis was performed using STATA version 14.1 statistical packages. Descriptive statistics, like frequency and percentage, were used depending on the nature of the variable. The assumption of the logistic regression model was checked using a correlation matrix and Hosmer and Lemeshow's tests. Binary logistic regression analysis was computed to assess the associations of the independent variables with hypertension. In the bivariable analysis variables with a p-value of less than 0.2 were entered into the final model. Variables with a p-value of ≤0.05 at multivariable analysis were considered as significantly associated with the outcome variable. The strength of association between independent and outcome variables was assessed using adjusted odds ratio (AOR) with 95% confidence interval (CI).
Results
Sociodemographic Characteristics of the Study Participants
A total of 412 HIV-positive adults attending the ART clinic at Debre Markos Referral Hospital were included. The response rate of this study was 100%. About 56.3% of respondents were females and the majority (82.2%) from urban areas. The mean (±SD) age of the study respondents was 43.12 (±11.29) years. Regarding marital status, approximately 43.9% were married, more than one-fifth (21.8%) were governmentally employed, less than one-third (30.6%) were unable to write and read, nearly two-thirds (61.9) had a family size of 1–3, more than two-thirds (69.9%) were nutritionally unsupported and counseled, and the majority (84.2%) had no family history of hypertension (Table 2).
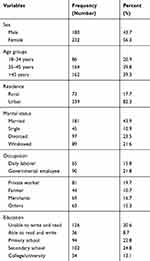 |
Table 2 Socio-Demographic Characteristics of the Study Participants at Debre Markos Referral Hospital Northwest, Ethiopia 2020 |
Lifestyle-Related Characteristics
Most (95.1%) of the participants were non-smokers. One-fourth (26.9%) of the participants used alcohol, and the majority (87.1%) and 96.4% of the participants did not undergo physical exercise, and did not chew chat, respectively. Among all participants, one –fourth (26.5%) had symptoms of stress, of which more than half (67.9%) experienced symptoms within less than six months duration, more than one –fourth (26.5%) of participants experienced symptoms of frequency once a month, and approximately half (48%) participants were at a moderate (4–7) level of stress (Table 3).
 |
Table 3 Lifestyle-Related Characteristics of Study Participants at Debre Markos Referal Hospital Northwest, Ethiopia, 2020 |
Clinical-Related Characteristics
The majority (83%) of participants were in the world health organization stage of I&II, more than one-fourth (27.9%) had less than 350 cell/µL CD4, more than one-third (38.4) of participants had received ART for greater than or equal to 10 years, and approximately one-fifth (22.6%) had taken prophylaxis, of which the majority (80.6%) were taking CPT. Approximately fifth (5.1%) of the participants had diabetes millets, of which the majority (85.3%) were type II diabetes millets. The majority (88.1%) and one-tenth (11.9%) of participants had good and fair/poor ART adherence, respectively. More than one-third (44.9%) were low literacy due to fair/poor adherence. Most (97.8%) participants did not develop comorbidities. Among participants in the study, 337 (81.80%) had a body mass index less than twenty-five, and one-third (30.6%) developed OI, of which more than one-third (34.9%) of participants developed herpes zoster and URTI. Among the participants in the study, one-third (32.5%) were taking the HAART regimen of 1e (TDF+3TC+EFV), two-fifths (38.1%) were taking 1J (TDF+3TC+DTG), and one-fifth (18%) were taking 2h (TDF+3TC+ATV/r)/2f (AZT+3TC+ATV/r)/2e (AZT+3TC+LPV/r)/(ABC+3TC+LPV/r). The majority (92.7%) of respondents did not develop comorbidities.
Magnitude of Hypertension Among HIV-Infected Patients
In the present study, the overall magnitude of hypertension was 41.3% (95% CI: 36.7–46.0), of these 86 (20.9%) stage I and 84 (20.4%) stage II hypertension among HIV positive adults who received antiretroviral therapy.
Associated Factors of Hypertension Among HIV-Positive Adults
In bivariable logistic regression analysis, variables with (P-valve <0.2) were age, sex, marital status, occupation, educational status, household family size, nutritional support and counseling, body mass index, family history of hypertension, alcohol, physical inactivity, stress, duration of HAART, prophylaxis therapy, HAART regimen, OI, DM, drugs for comorbidities, CD4, viral load and stage of WHO. In the multivariable analysis, only four variables were identified as significantly associated factors of hypertension. Age group (35–45) and (>45) years participants were 2.48 (AOR=2.48, 95% CI: 1.17, 5.27) and 5.00 (AOR, 5.00, 95% CI: 2.19, 11.43) times more likely to develop hypertension, respectively, compared to age group (18–34) years. Participants who did not have regular physical exercise were 2.72 times higher risk of hypertension compared with those who did physical exercise (AOR=2.72, 95% CI: 1.33, 5.57). Moreover, participants who had a body mass index greater than or equal to twenty-five (≥25 kg/m2) were 2.87 (AOR=2.87, 95% CI: 1.52, 5.39) higher odds of hypertension compared to less than twenty-five. Finally, this study found that participants who were taking the regimen of (2 h (TDF+3TC+ATV/r)/2f (AZT+3TC+ATV/r)/2e (AZT+3TC+LPV/r)/ABC+3TC+ATV/r) were 3.058 (AOR=3.058, 95% CI: 1.416, 6.605) times more likely to develop hypertension compared to participants who were taking regimens of 1e (TDF+3TC+EFV) (Table 4).
 |
Table 4 The Bivariable and Multivariable Logistic Regression Analysis of Associated Factors of Hypertension at Debre Markos Referral Hospital Northwest, Ethiopia, 2020 |
Discussion
This study aimed to evaluate the proportion and associated factors of hypertension among HIV-positive adults who received antiretroviral therapy at Debre Markos Referral Hospital, Northwest Ethiopia. In line with the study objective, it has demonstrated that a magnitude of hypertension was found to be 41.3% (95% CI: 36.7, 46.0). This finding is in line with previous studies conducted in Cameroon 36.44% (95% CI: 30.15–43.10)43 and USA at the University of Florida 34.7% (95% CI: 27.4–42.8).44 However, our finding was lower than studies conducted in Malaysians (45.60%)45 and in South Africa (73.8%).46 Conversely, this study finding is much higher than studies conducted in Ethiopia 12.7% (95% CI: 9.8–16.2),28 northeast Ethiopia 29.7% (95% CI, 25.3–35.0%),29 Cameroon 24.8% (95% CI: 20.1–30.0),47 Malawi 23.7% (95% CI: 21.1–26.6),39 and United States 22% (95% CI: 21–24).48 The above disparity between studies could be elucidated by the study settings, cut-off point of blood pressure, ART duration and regimens, and life style related factors of the study participants.
The current study shows that older age, no physical exercise, BMI≥25 kg/m2, and regimen of HAART were significant factors associated with hypertension. The current study showed that older age was significantly associated with hypertension. The finding is consistent with studies reported from Ethiopia,29 Zimbabwe,36 Malaysia,45 and the US.48 This would be explained that aging causes a loss in vessel function by stiffening of the arterial vasculature, and vascular changes include the advanced reduction in viscoelastic properties of vessels, progressive atherosclerotic arterial disease, and hypertrophy/sclerosis of muscular arteries and arterioles, which narrow the vessel wall and make resistance to blood pressure.49
The present study found that physical inactivity was statistically associated with hypertension. This finding is in line with previous studies conducted in southern Ethiopia,50 Uganda,51 and the USA.52 Evidence also support that physical exercise help to prevent the development of hypertension by decreasing vascular resistance, arterial stiffness, oxidative stress, decreasing body weight/mass, rennin-angiotensin system activity, decreasing stress and increasing endothelial function, and lumen diameter.42 Additionally, a high body mass index was statistically associated with hypertension. This finding is in consistent with studies conducted in Ethiopia,28,29 Uganda,51 Zimbabwe,36 Cameroon,43 and Malaysia.45 A possible explanation for this finding is that an increase in body mass index leads to activation of the RAAS, renal compression, increased plasma leptin and induced metabolic disorders that result in increased blood pressure.53
Participants who used a combination of nucleoside reverse transcriptase inhibitors (NRTI) with protease inhibitors (PI) (2h/2f/2e/ABC+3TC+ATV/r) were at higher risk of developing hypertension compared with those who used a combination of nucleoside reverse transcriptase inhibitors with nonnucleoside reverse transcriptase inhibitors or integral transfer strand inhibitors. This finding is in line with studies conducted in southern Malawi,39 Cameroon,43 and Zimbabwe.36 The possible explanation may be that PI combinations ritonavir/lopinavir and ritonavir/atazanavir were revealed to stimulate adipokine-mediated inflammatory pathways that lead to the activation of adipose RAAS, endothelial dysfunction, arterial stiffness, lipodystrophy, and dyslipidemia.54 However, the contradicts findings reported from a study conducted in Maryland.41 The possible variation might be related to HIV infection has been linked to arterial stiffness and pro-inflammatory responses; hence, the virus may lead to premature vascular dysfunction causing elevated blood pressure, endothelial dysfunction, and subclinical HIV-associated kidney disease may be responsible for high rates of hypertension among HIV-positive adults.24,45,55
The current study demonstrated that a BMI ≥25 kg/m2 was strongly associated with hypertension among HIV-positive adults. This finding is in line with a study conducted in Brazil.56 Therefore, obesity of individuals with HIV/AIDS may be associated with excessive weight gain during treatment with HAART and should be avoided.
Limitation of the Study
The study has a number of limitations. These are there may be measurement bias, and information on lipid profile, renal function, and other lab results were not collected.
Conclusion
In the present study, more than two-third of the study participants was developed hypertension among HIV-positive adults. No physical exercise, age group 35–45, body mass index >25kg/m2, HAART regimen 1J(TDF+3TC+DTG and 2h/2f/2e/ABC+3TC+ATV/r) increased the risk of hypertension among peoples living with HIV/AIDS. Therefore, policymakers and clinicians should emphasize on educating about the use of lifestyle change, counseling the use of regular physical activities, promoting weight reduction, and intervention in this situation are highly recommended.
Abbreviations
AIDS, acquired immune deficiency syndrome; ART, anti-retroviral therapy; HAART, hyperactive anti-retroviral therapy; HTN, hypertension; WHO, World Health Organization.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the ethical review committee of Health Science Debre Markos University with institutional research ethics review committee number of HSC/R/C/SE/PG/Co/422/11/12. Permission was obtained from hospital administrators. All participants were informed about the purpose of the study prior to consenting to participate. Verbal consent was obtained from each participant prior to data collection. The Ethical Review Committee of Health Science Debre Markos University approved the informed verbal consent process. A participant name was not included in the data collection format, and the data were not disclosed to any person other than principal investigators. This study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable.
Acknowledgments
We would like to acknowledge Debre Markos Referral Hospital officials to allow us to conduct this project on their customers. We extend our special thanks to all data collectors and respondents.
Author Contributions
YS: Conception of the research protocol, literature review, study design, data analysis, interpretation and drafting of the manuscript. YS, ADA, WSS and DH: Data analysis, interpretation, and manuscript review. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have declared that they have no competing interests.
References
1. American Cardiology of Physicians. What’s in the new hypertension guidelines? Am Cadiol. 2018.
2. World Health Organization (WHO). Hypertension in the world; 2019. Available from: https://wwwwhoint/news-room/fact-sheets/detail/hypertension.
3. WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva, Switzerland: World Health Organization; 2013.
4. Lloyd-Sherlock P, Ebrahim S, Grosskurth H. Is hypertension the new HIV epidemic? Int J Epidemiol. 2014;43(1):8–10. doi:10.1093/ije/dyu019
5. WHO. Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: World Health Organization; 2014.
6. UNAIDS. Global AIDS Update. UNAIDS; 2018.
7. Frank TD, Carter A, Jahagirdar D, et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831–e859. doi:10.1016/S2352-3018(19)30196-1
8. Chu DT, Vo Truong Nhu N, Tao Y, Le Hoang S. Achievements and challenges in HIV/AIDS control in Vietnam. HIV Med. 2018;19(9):e75–e76. doi:10.1111/hiv.12631
9. World Health Organization (WHO). HIV/AIDS Fact Sheet; 2018. Available from: http://wwwwhoint/en/newsroom/fact-sheets/detail/hiv-aids.
10. World Health Organization (WHO). Global health sector response to HIV, 2000–2015: focus on innovations in Africa. http://wwwwhoint/hiv/pub/progressreports/2015-progress-report/en/.
11. Feinstein MJ, Bahiru E, Achenbach C. 2016 ea: patterns of cardiovascular mortality for HIV-infected adults in the United States. Am J Cardiol. 2016.
12. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV. J Am Soc Hyperten. 2017;11:530–540. doi:10.1016/j.jash.2017.06.004
13. Nguyen KA, Peer N, Mills EJ. Burden, determinants, and pharmacological management of hypertension in HIV-positive patients and populations: a systematic narrative review. AIDS Rev. 2015.
14. Nüesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS). J Acquir Immune Defic Syndr. 2013;62(4):396–404. doi:10.1097/QAI.0b013e3182847cd0
15. Bloomfield GS, Hogan JW, Keter A, Holland TL, Sang E, Kimaiyo S. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14(1). doi:10.1186/1471-2334-14-284
16. Armah KA, Chang CC, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58:121–129. doi:10.1093/cid/cit652
17. Global Health Risks. Mortality and burden of disease attributable to selected major risks. The World Health Organization; 2009. Available from: http://wwwwhoint/healthinfo/global_burden_disease/GlobalHealthRisks_report.
18. Winston J. HIV-associated nephropathy. Mt Sinai J Med. 2015.
19. Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti ACS. Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2010.
20. Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2013.
21. Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6(7):e22288. doi:10.1371/journal.pone.0022288
22. Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31(7):1372–1378. doi:10.1097/HJH.0b013e328360de1c
23. Malaza A, Mossong J, Bärnighausen T, Newell M-L. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One. 2012;7(10):e47761. doi:10.1371/journal.pone.0047761
24. Dube MP, Lipshultz SE, Fichtenbaum CJ, et al. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation. 2018;2:1–21.
25. Calvo-Sanchez M, Perello R, Perez I, et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 2013;14(1):40–48. doi:10.1111/j.1468-1293.2012.01057.x
26. Burchell AN, Calzavara LM, Myers T, et al. Stress and increased HIV infection risk among gay and bisexual men. AIDS. 2010;24(11):1757–1764. doi:10.1097/QAD.0b013e32833af7c9
27. Tobin SC. Cumulative antiretroviral therapy exposure increases annual incidence of chronic kidney disease in a large international cohort study. AIDS. 2016;30(11):N16.
28. Ataro Z, Ashenafi W, Jiregna Fayera TA. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV‑positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. HIV/AIDS. Res Palliat Care. 2018;10.
29. Fiseha T, Belete AG, Dereje H, Dires A. Hypertension in HIV-infected patients receiving antiretroviral therapy in Northeast Ethiopia. Int J Hypertens. 2019;2019:1–8. doi:10.1155/2019/4103604
30. Federal Ministry of Health. National comprehensive HIV prevention, care and treatment training for health care providers. Participant Manual. 2018;1–123.
31. WHO. Health in the Sustainable Development Goals, Good Health and Well-Being. World Health Organization, Regional Office for South-East Asia; 2016.
32. Debre Markos Referral Hospital. Annual report. 2019.
33. Tesfaw A, Jara D, Temesgen H. Dietary diversity and associated factors among HIV positive adult patients attending public health facilities in Motta Town, East Gojjam Zone, Northwest Ethiopia. Hindawi Adv Public Health. 2018;8.
34. Woldemariam AT, Yusuf ME, Beyen TK, Yenit MK. Factors associated with dietary diversity among HIV positive adults (≥18 years) attending ART clinic at Mettema Hospital, Northwest Ethiopia. AIDS Clin Res. 2015;06(08). doi:10.4172/2155-6113.1000490
35. Arruda Junior ER, Lacerda HR, Moura LC, et al. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14(3). doi:10.1590/S1413-86702010000300014.
36. Chireshe R. Hypertension among human immunodeficiency virus infected patients on treatment at Parirenyatwa Hospital. Afr J Prm Health Care Fam Med Care Res Rev. 2019;11(1):a1974. doi:10.4102/phcfm.v11i1.1974
37. Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2012.
38. Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS One. 2018;13(10):e0204020. doi:10.1371/journal.pone.0204020
39. Divala HO, Amberbir A, Ismail Z, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care. BMC Public Health. 2016;16:1243. doi:10.1186/s12889-016-3916-x
40. Njelekela M, Muhihi A, Aveika A, et al. Prevalence of hypertension and its associated risk factors among 34,111 HAART Na\ve HIV-infected adults in Dar es Salaam, Tanzania. Int J Hypertens. 2016;9:5958382.
41. Medina-Torne S, Ganesan A, Barahona I, et al. Hypertension is common among HIV-infected persons, but not associated with HAART. J Int Assoc Physicians AIDS Care. 2012;11(1):20–25. doi:10.1177/1545109711418361
42. FMOH. Guidelines on Clinical and Programmatic Management of Major Non Communicable Diseases, Ethiopia. 2016.
43. Pangmekeh PJ, Awolu MM, Gustave S, Gladys T, Cumber SN. Association between highly active antiretroviral therapy (HAART) and hypertension in persons living with HIV/AIDS at the Bamenda regional hospital, Cameroon. Pan Afri Med J. 2019;1–8.
44. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV. J Am Soc Hyperten. 2017;1–12.
45. Hejazi N, Huang MSL, Lin KG, Choong LCK. Hypertension among HIV-infected Adults Receiving Highly Active Antiretroviral Therapy (HAART) in Malaysia. Glob J Health Sci. 2014;6(2):1–14.
46. Ataklte F, Erqou S, Kaptoge S, et al. Burden of undiagnosed hypertension in sub-Saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65(2):291–298. doi:10.1161/HYPERTENSIONAHA.114.04394
47. Ngu RC, Choukem S-P, Dimala CA, Ngu JN, Monekosso GL. Prevalence and determinants of selected cardio‑metabolic risk factors among people living with HIV/AIDS and receiving care in the South West Regional Hospitals of Cameroon. BMC Res. 2018;11:305. doi:10.1186/s13104-018-3444-0
48. Krauskopf K, Natta MLV. Correlates of hypertension in patients with AIDS in the era of highly-active antiretroviral therapy United States. J Int Assoc Provid AIDS Care. 2013;1–18.
49. Messerli F, Ventura H, Glade L, Sundgaard-Riise D, Frohlich E, Frohlich E. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet. 1983;322(8357):983–986. doi:10.1016/S0140-6736(83)90977-7
50. Helelo TP, Gelaw YA, Adane AA. Prevalence and associated factors of hypertension among adults in Durame Town, Southern Ethiopia. PLoS One. 2014;9(11):e112790. doi:10.1371/journal.pone.0112790
51. Dalsone K, Laura B, David H, et al. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLoS One. 2016.
52. Buchacz K, Baker RK, Palella FJ, et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther. 2013.
53. Vaneˇcˇkova´ I, Maletı´nska´ L, Veronika Nagelova´ MB, Zicha J, Kunes J. Obesity-related hypertension: possible pathophysiological mechanisms. http://joeendocrinology-journalsorg. Printed in Great Britain Published by Bioscientifica Ltd. 2014:1–16.
54. Boccara F, Auclair M, Cohen A, et al. HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2016;15:363–375. doi:10.3851/IMP1533
55. Fourie CMT, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240(1):154–160. doi:10.1016/j.atherosclerosis.2015.03.015
56. de Arruda Junior ER, Lacerda HR, Moura LCRV, et al. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14(3):281–287. doi:10.1016/S1413-8670(10)70057-X
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
