Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 10
Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia
Authors Ataro Z , Ashenafi W , Fayera J, Abdosh T
Received 11 June 2018
Accepted for publication 31 July 2018
Published 11 October 2018 Volume 2018:10 Pages 181—192
DOI https://doi.org/10.2147/HIV.S176877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Zerihun Ataro,1 Wondimye Ashenafi,2 Jiregna Fayera,3 Tekabe Abdosh3
1Department of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3School of Medicine, College of Health Sciences, Haramaya University, Harar, Ethiopia
Background: People with HIV infection are at increased risk of noncommunicable diseases (NCDs). Diabetes mellitus (DM) and hypertension are recognized as the major NCDs. Except few findings in general population, there is no well-documented evidence on the magnitude of NCDs and associated factors among HIV-positive patients.
Purpose: The aim of this study was to determine the magnitude and associated factors of DM and hypertension among adult HIV-positive subjects receiving highly active antiretroviral therapy (HAART)
Methods: A hospital-based cross-sectional study was conducted from February to April at Jugal Hospital, Harar, Eastern Ethiopia. Sociodemographic and anthropometric data and blood pressure (BP) were collected by senior clinical nurses. A total of 5 mL of venous blood was collected. Serum glucose and lipid profile were measured using the Autolab 18 clinical chemistry analyzer. Data were analyzed using STATA version 13.
Results: A total of 425 HIV-infected individuals taking HAART of age ranging from 18 to 68 years were included. The prevalence of DM and hypertension were 7.1% (95% CI: 4.9–9.9) and 12.7% (95% CI: 9.8–16.2), respectively. Increased blood triglyceride (adjusted odds ratio [AOR] =4.7, 95% CI: 1.7–13.1), high BP (AOR =3.3, 95% CI: 1.1–9.5), and high baseline body mass index (BMI) (AOR =8.7, 95% CI: 2.4–31.8) were significantly associated with DM. In contrast, raised waist–hip ratio (AOR =4.6, 95% CI: 1.6–13.3), raised blood glucose (AOR =3.5, 95% CI: 1.1–11.4), increased total cholesterol (AOR =3.9, 95% CI: 1.3–11.9), high current BMI (AOR =3.8, 95% CI: 1.5–9.6), drinking alcohol (AOR =3.4, 95% CI: 1.5–8.1), CD4 count <500 cell/mL (AOR =2.7, 95% CI: 1.3–5.6), and longer duration of HAART (AOR =2.3, 95% CI: 1.1–5.1) were significantly associated with hypertension.
Conclusion: DM and hypertension were frequent among HIV patients on HAART, and they were linked to the well-known risk factors. Therefore, regular screening and monitoring of DM and hypertension before and after the initiation of HAART is of paramount importance.
Keywords: diabetes mellitus, hypertension, HIV, HAART, Ethiopia
Introduction
Globally, an estimated 36.7 million people were living with HIV, and in eastern and southern Africa, a total of 19 million people were living with HIV in 2016.1 Globally, 20.9 million people living with HIV (PLWHIV) were receiving antiretroviral therapy (ART) in June 2017, which accounts for ~53% of all PLWHIV.2 In eastern and southern Africa, 11.7 million people were accessing ART, which accounts for 60% of all PLWHIV in the region in 2016.2 According to the Ethiopia Demographic and Health Survey (EDHS) report, the estimated prevalence of HIV was 1.2% among men and women aged 15–49 years in Ethiopia.3
HIV and noncommunicable diseases (NCDs) are the major public health concerns worldwide. Studies have reported an increased risk of NCDs such as diabetes mellitus (DM), hypertension, and cardiovascular diseases among PLWHIV.4–9 It was found that there was a linkage between HIV/AIDS and NCDs. The linkage is due to either direct effects of HIV or indirectly by ART regimens.10–12
The introduction of highly active ART (HAART) improved the prognosis of HIV/AIDS. These improvements include suppression of viral load, increase in CD4 count, prolonged life span of HIV-infected individuals, decrease in opportunistic infections, and decline in HIV-associated morbidity and mortality.13,14 However, despite all these benefits, the adverse effects of HAART pose challenges in recent decades with new health issues. The use of such treatment regimen has coincided with the emergency of chronic medical conditions. Studies on HIV-infected individuals have reported a wide spectrum of metabolic alterations associated with HAART, including alteration in lipid and glucose metabolism, peripheral and coronary arterial diseases,15,16 and changes in glucose homeostasis and fat redistribution.17,18
Hypertension,19–21 cardiovascular diseases,22,23 and metabolic syndrome and DM21 were the most common abnormalities related to antiretroviral treatments. A study conducted in Nigeria revealed an increase in the prevalence of hypertension after 2 years of being initiated on HAART.24 The risk of type 2 DM is increased among PLWHIV and is associated with cumulative exposure to HAART.6,7 Furthermore, the therapy seems to affect the risks of developing heart diseases by increasing cholesterol levels and changing fat distribution, particularly increasing abdominal fat.25,26
These noncommunicable chronic diseases have significant undesirable impact on the treatment of PLWHIV and their quality of life, and the resulting metabolic imbalances due to the NCDs could affect the long-term prognosis of the HIV patients.27 Furthermore, coexistence of DM and hypertension in HIV-infected individuals may complicate the management of HIV infection, increasing the risk of morbidity and mortality of these individuals.
Studies have reported different risk factors associated with NCDs. Most of these risk factors comprise a mixture of irreversible elements such as age, sex, ethnicity, and family history and reversible lifestyle habits (physical activity, smoking, and alcohol use).28,29 Besides these risk factors, some of the HIV-associated factors include the toxic effect of the drug,30 CD4 count, and duration and type of HAART.31 Identification and modification of these factors have important benefits in these patients.
In Ethiopia, although there were few findings on the prevalence of DM and hypertension in general populations,32–35 there is no adequate evidence on the magnitude of DM and hypertension in HIV-infected individuals receiving HAART. Information related to comorbidities of these NCDs in HIV-positive individuals is needed to inform various HIV programs. Moreover, research need to be undertaken to identify the factors associated with these problems and outline those, which are modifiable factors that can reduce the magnitude of the DM and hypertension in HAART receiving individuals. Therefore, this study was conducted to determine the prevalence and associated factors of DM and hypertension among adult HIV-positive subjects receiving HAART.
Methods
Study site
The study was conducted at Jugal Hospital. The hospital is situated in Harar town, and the town is located east of Ethiopia, 526 km away from the capital city, Addis Ababa. According to the 2007 Central Statistical Agency census report, the total projected population of the Harar region is 183,415. The town has four governmental and two nongovernmental hospitals. Government supported free HIV/AIDS care, including that HAART was delivered in all of these institutions. This study was conducted in one of the governmental hospitals, Jugal Hospital, situated in the Harar town.
Study design and period
An institution-based cross-sectional study was conducted between February and April 2017.
Study population
The study population was adult HIV-positive individuals receiving HAART from the ART center of Jugal Hospital.
Inclusion and exclusion criteria
HIV-positive patients who were ≥18 years having documented HIV infection and were on HAART for at least 6 months were included in the study. HIV-positive patients were excluded if they had opportunistic infections, were on hormonal contraceptives or anabolic or corticosteroid agents, were pregnant, critically ill, and unable to undergo the assessment, and had hypertension and/or DM before HAART.
Sample size and sampling techniques
The number of HIV-positive individuals needed for this study was calculated based on the study from Jimma (Southwest Ethiopia), which reported a prevalence of 6.4% for DM among HIV-infected individuals.36 The sample size was calculated by using single-population proportion formula (n=Z2P(1-P)/e2) with the following parameters: the level of statistical significance set up at the level of 95% CI (Z) (1.96) and likelihood error (e) (2.5%). Based on this, the minimum estimated sample size was 369. After adding 20% for anticipated nonresponse, we obtained a total of 443 sample size. The study participants were recruited using the convenient sampling technique.
Data collection
After obtaining consent from the study subjects, socio-demographic characteristics such as age, sex, marital status, educational status, and residence area and behavioral characteristics such as habit of alcohol consumption, smoking, khat chewing, and doing physical activity were collected by interviews using structured questionnaires prepared in local language (Amharic and Afan Oromo). The physical activity section included habit of doing vigorous activity, physical fitness, and walking. For information regarding alcohol consumption, cigarette smoking, and khat chewing, data were collected on current drinking, smoking, and chewing status of the study subjects. These responses were measured dichotomously. Furthermore, anthropometric (weight, height, waist circumference, and hip circumference) and blood pressure (BP) data were collected from the study subjects. From the patient’s health record, clinical characteristics such as CD4+ cell count, WHO stage, baseline body mass index (BMI), and type and duration of HAART were collected using a checklist. All these data were collected by two experienced clinical nurses.
Blood specimen collection
A total of 5 mL of fasting venous blood specimen was collected from each study participant. Serum was separated from the whole blood and analyzed for glucose, total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) at Clinical Chemistry Laboratory of Jugal Hospital, following standard procedures. Blood collection, serum separation, and laboratory analysis were done by two experienced medical laboratory technologists.
Physical measurements
BP measurement
Resting BP was measured by nurses using an automated sphygmomanometry. The BP reading was consistently taken from the left arm, three times at 5 min interval. The average of the two last readings was estimated and used in the analysis. High BP (hypertensive) was classified as a BP of ≥140/90 mmHg.
Anthropometric parameters’ measurement
The height and body weight were measured to a precision of 0.1 cm and 0.1 kg, respectively, using a digital balance with height measurement attached to it. Weight was measured by placing the weighing balance on a flat hard surface and height was measured while a patient is facing directly ahead. BMI was calculated as a ratio between the weight in kilograms and the square of the height in meters. The BMI results were categorized as follows: obesity if the BMI is ≥30 kg/m2, overweight if the BMI is 25–29.99 kg/m2, normal if the BMI is 18.5–24.99 kg/m2, and underweight if BMI is <18.5 kg/m2.37
Waist and hip circumferences were measured to the nearest 0.1 cm using a nonstretchable tape. Waist circumference was made at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest while subjects were standing and breathing normally. Hip circumference measurements were taken at the point yielding the maximum circumference over the buttocks with the tape in a horizontal plane, touching but not compressing the skin. The waist-to-hip ratio (WHR) was determined by dividing the mean waist circumference (cm) by the mean hip circumference (cm). We used the European cutoff to interpret the waist circumference measurements as per WHO. According to the above guidelines, the abnormal waist circumferences of male and female are ≥94 and ≥80 cm, respectively.38
Laboratory tests
The glucose and lipid profile tests were analyzed using an automated clinical chemistry analyzer (Autolab 18; Boehringer-Mannheim Diagnostics, Indianapolis, IN, USA). They were measured by the direct end point enzymatic method using test reagents from the HUMAN Company (Human Biological Diagnostic, Magdeburg, Germany). Serum glucose level was estimated by glucose oxidase-phenol amino phenazone (GOD-PAP) method. Total cholesterol was determined by the cholesterol oxidase-peroxidase (CHOD-POD) method, and serum triglyceride was measured using the glycerol phosphate oxidase-p-aminophenazone peroxidase (GPO-PAP) method. Precipitation/enzymatic method was used for the measurement of HDL-C. The low-density lipoprotein cholesterol (LDL-C) was calculated by using Friedewald equation, LDL = TC HDL - (TG/5), where TC represents total cholesterol and TG represents triglyceride.39 The study participants were classified as diabetic using WHO diagnostic criteria when fasting plasma glucose was ≥126 mg/dL.40 The lipid profile measurements were classified as high total cholesterol if the value was ≥200 mg/dL, high triglyceride if the value was ≥200 mg/dL, high LDL-C if the calculated LDL value was ≥130 mg/dL, and low HDL-C if the HDL-C value was <40 mg/dL.
Data processing and analysis
All the data were cleaned, double-entered into Microsoft Excel spreadsheets, and analyzed using the STATA software Version 13 (StataCorp LP, College Station, TX, USA). Descriptive summaries were presented in terms of mean, SD, median, interquartile range (IQR), and proportions depending on the scale of the variable. Frequencies of diabetes and hypertension were compared according to the main characteristics of the study sample using chi-squared test. For variables that do not satisfy the assumptions of the chi-squared test, Fisher’s exact test was used instead of Pearson’s chi-squared test.
Logistic regression was used to identify factors associated with diabetes and hypertension. Bivariate and multivariable logistic regression analyses were used. Age, sex, educational status, occupational status, marital status, waist circumference (WC), WHR, current BMI, baseline BMI, WHO clinical stage, current CD4 cell count, baseline CD4 count, total cholesterol level, triglyceride level, HDL cholesterol level, LDL cholesterol level, duration of HAART treatment, the habit of smoking, physical activity, alcohol consumption, chewing khat, and eating fruit/vegetables were included in a bivariate analysis. All variables with P-value <0.25 in bivariate logistic regression analysis were further entered into a multivariable logistic regression model to control confounding effects. Crude odds ratio and adjusted odds ratio (AOR) with 95% CI were reported. All statistical tests were two-sided and considered statistically significant at a P-value of <0.05.
Ethics approval
The study proposal was approved by Institutional Research and Ethics Review Committee of College of Medical and Health Sciences, Haramaya University. All participants gave informed written consent to take part in this study. Each study subject was informed of the aim and purpose of the study, risks and benefits of participating in the study, and that his/her participation was purely voluntary. Confidentiality was maintained throughout the study period. Each study participant was informed of his/her physical assessment and biochemical test results. Abnormal findings were communicated to their clinician for better evaluation and management.
Results
Sociodemographic characteristics
The sociodemographic characteristics of the study participants are shown in Table 1. A total of 425 study participants (95.9% response rate) were included in this study. The majority of the respondents were female 297 (69.9%). The overall age range was between 19 and 68 years with mean ± SD 39.7±8.9 years. The mean age of male was 41.8±9.7 years, and that of female was 38.8±8.5 years. The majority was middle-aged adults (31–50 years) accounting for 70% of the total samples. The majority of the study participants were married (46.6%), orthodox in religion (261 [61.4%]), resided in urban (395 [92.9%]), and Amhara by ethnicity (233 [54.8%]). One hundred thirty (30.6%) of the study participants were labor worker in the occupation. About one-third of the respondents (37.2%) had attained primary level of education.
  | Table 1 Sociodemographic characteristics of the study subjects at Jugal Hospital, Harar, Eastern Ethiopia |
Behavioral and anthropometric characteristics
Behavioral and anthropometric characteristics of the study subjects are shown in Table 2. Of the 455 participants, 241 (56.7%) and 297 (69.9%) participants had an abnormal waist circumference and WHR, respectively. Thirty (7.1%), 66 (15.5%), and 122 (28.7%) participants had a habit of smoking, alcohol drinking, and chewing khat, respectively. The majority of the participants had poor participation on doing physical activity. A total of 301 (70.8%) participants were not involved in vigorous activities such as carrying or lifting heavy loads and construction works, and only 48 (11.3%) participants were involved in the physical fitness exercise regularly. Regarding dietary habits of the respondents, 35 (8.2%) and 49 (11.5%) respondents reported that they never ate vegetables and fruits at all, respectively.
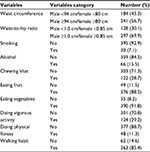  | Table 2 Behavioral and anthropometric characteristics of the study subjects at Jugal Hospital, Harar, Eastern Ethiopia |
Clinical characteristics
At baseline, the median BMI was 19.9 (IQR; 17.6–22.7). About half of the study participants (51.5%) had normal weight. The rest half of the study subjects were underweight (34.6), overweight (12.5%), and obese (1.4%). After starting the HAART, there was a decrease in the proportion of underweight subjects (17.2%). However, there was an increase in the proportion of overweight (23.1%) and obese (7.1%) subjects.
At baseline, majority of the HIV patients had WHO stage III (54.4%) and CD4 <200 cells/μL (61.7%). However, at the time of the study, majority of the subjects had WHO stage I (96.9%) and CD4 >500 cells/μL (47.4%). The median treatment duration was 72 months (6 years). At baseline, the most commonly prescribed regimen was 1e (TDF–3TC–EFV) (40.9%) (Table 3).
Prevalence of DM and hypertension
Among the 425 patients, 30 patients had DM, corresponding to a prevalence of 7.1% (95% CI: 4.9–9.9). Forty-three (10.1%) and 10 (2.4%) study participants had impaired fasting glucose value (111–125 mg/dL) and hypoglycemic (<70 mg/dL), respectively. The prevalence of hypertension (BP ≥140/90 mmHg) among the study participants was 12.7% (54/425) (95% CI: 9.8–16.2).
Factors associated with DM
Bivariate and multivariable logistic regression analyses on the associated factors of DM are shown in Table 4. Variables associated with diabetes in bivariate analyses were age, baseline BMI, BP, the level of total cholesterol, triglycerides, and LDL cholesterol. In the multivariable regression analysis, high BP (AOR =3.3, 95% CI 1.1–9.5), increased blood triglyceride (AOR =4.7, 95% CI 1.7–13.1), and high baseline BMI (overweight/obesity) (AOR =8.7, 95% CI 2.4–31.8) were the factors significantly associated with DM.
Factors associated with hypertension
Bivariate and multivariable logistic regression analyses on the associated factors of hypertension are shown in Table 5. Variables associated with hypertension in bivariate analyses were age, WC, WHR, baseline WHO clinical stage, current BMI, current CD4 count, HAART duration, habit of alcohol drink, and level of total cholesterol, triglycerides, LDL cholesterol, and glucose. In the multivariable regression analysis, raised WHR (AOR =4.6, 95% CI: 1.6–13.3), raised blood glucose (AOR =3.5, 95% CI: 1.1–11.4), increased total cholesterol (AOR =3.9, 95% CI: 1.3–11.9), high current BMI (overweight/obesity) (AOR =3.8, 95% CI: 1.5–9.6), drinking alcohol (AOR =3.4, 95% CI: 1.5–8.1), current CD4 count <500 cells/μL (AOR =2.7, 95% CI: 1.3–5.6), and longer duration of HAART (AOR =2.3, 95% CI: 1.1–5.1) were the factors significantly associated with hypertension.
Discussion
This study was conducted with the aim of determining the prevalence and associated factors of DM and hypertension in HIV-infected patients taking combined ART. In the present study, after starting the HAART, there was a decrease in the proportion of underweight subjects from 34.6% to 17.2% and an increase in the proportion of overweight/obese subjects from 13.9% to 30.2%. There was also an increase in median CD4 count (from 180 cells/μL at baseline to 480 cells/μL during follow-up) and an improvement in WHO clinical stage. Similar improvement and changes have been observed in other studies.41–43 This is more likely to reflect the efficacy of ART, suggesting good control of HIV disease by the HAART.
The estimated prevalence of DM in the present study was 7.1%. In Ethiopia, according to WHO report of diabetes country profile in 2016, the national prevalence of DM was estimated to be 3.8% in the general population,44 and the total prevalence of DM in Ethiopian adult population (20–79 years) was found to be 5.2%.45 A cross-sectional study conducted in Jimma, southwestern Ethiopia, reported a DM prevalence of 5.3% in general population.33 Therefore, the present study has a high prevalence of DM among HIV-positive patients compared to the general population of Ethiopia. This could be due to people with HIV having an increased risk of developing a number of serious health problems due to a compromised immune system. Furthermore, HAART might have an impact on the cause of diabetes. These justifications have been supported by studies from developed countries, with prevalence rates of DM among PLWHIV reported to be higher than the general population.6,9,46,47 In the multicenter AIDS cohort study, the relative risk of diabetes among HIV-infected men taking HAART was more than four times that of HIV seronegative.6 Therefore, being HIV positive and exposure to HAART were found to be the factors associated with diabetes.
Few similar studies conducted in Ethiopia were available for possible comparison of the DM prevalence on the same study population (HIV patients). Finding of the prevalence of DM in the present study was comparable to two studies from Ethiopia conducted on HIV-infected patients taking combined ART, ie, from Jimma (6.4%)36 and Wolaita Sodo (8%).48
When we compare our result with studies conducted in developing and developed countries, our finding is comparable to the 7.6% and 7% prevalence of diabetes observed in HIV-infected patients taking combined ART in Benin41 and Slovenia,49 respectively. The prevalence of DM in this study was lower than the study conducted from Iran (11.6%),50 Senegal (14.5%),21 Tanzania (24.7%),51 Israel (22.9%),52 California, USA (12%),43 and Romania (13%).53 In contrast, the prevalence observed in this study was higher than the study conducted in Taiwan (3.5%),54 South Africa (1.3%),55 India (2.1%),42 Kenya (1.5%),56 and Thailand (4.6%).57 The difference could be attributed to the difference in the participant characteristics that influence these disease conditions. These include variation in life style, HAART regimens (drug-specific effects), and age and sex distribution of the HIV-infected individuals.
The prevalence and severity of hypertension among HIV-positive patients in low- and middle-income countries is only recently gaining attention. Hypertension is among the leading causes of death globally. It increases the risks of stroke, heart diseases, and other diseases.58 The estimated prevalence of hypertension in the present study was 12.7%, which was lower than the prevalence from the general Ethiopian population. The national prevalence of raised BP (hypertension) was estimated to be 31.2% (95% CI: 23.7–38.9) in the general population aged ≥18 years.59 The prevalence observed in this study was higher than reports from Jimma, Southwest Ethiopia, in general adult population (9.3%),60 whereas it was lower than the prevalence from a community-based cross-sectional study from Gondar, northwest Ethiopia (28.3%).34
There was a discrepancy in the prevalence of hypertension among HIV-positive patients and general population (HIV-negative patients). Different studies have reported that HIV patients are at a higher risk of becoming hypertensive than the general population15,31 or HIV-negative individuals,61 whereas others report lower BP in HIV-positive patients or no difference in BP between HIV-positive and HIV-negative patients.62,63
Studies on hypertension among PLWHIV reported a wide range of prevalence. When the finding of this study is compared with studies conducted among HIV-positive patients in Ethiopia, the finding is comparable to the study done in Wolaita Sodo, Ethiopia (15.9%)48 but much lower than a study conducted in another part of the country (Jimma, southwestern Ethiopia) (34%).36 The finding of this study is also higher compared to studies conducted in Uganda (27.9%),20 South Africa (19.1%),55 Senegal (28.1%),21 Tanzania (48%),51 Malaysia (19.7%),64 and Brazil (25.6%).65 Comparable to the findings of this study, a study from Kenya described hypertension prevalence rates of 11.2% and 7.4% among HIV-positive men and women, respectively.66
In developed countries (Norway, Spain, Italy, and the USA), the prevalence ranges between 20% and 40% among populations exposed to HAART,19,67–69 which are higher than the finding of this study. The study of the South American cohort reported a prevalence of 31.5%, but used a different definition (systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg) than that of our study.70 The observed difference could be due to variation in types of HAART, duration of HIV infection itself, stage of HIV infection, sex, lifestyle, and age difference of the study participants. The other possible explanations for these discrepant results include differences in study design, methodological aspects, and the cutoff values for hypertension.
The bivariate analysis of the present study revealed that being aged >40 years, high BP, increased baseline BMI, increased total cholesterol, increased triglycerides, and increased LDL cholesterol remained to be predictors of DM. Nevertheless, only increased BP, triglycerides, and baseline BMI were found to be predictors of DM in the final model (multivariable analysis). Other sociodemographic and clinical characteristics did not have a significant association with DM.
Different studies reported that the prevalence of DM increased when age increased. It was reported that alterations in glucose tolerance were more prevalent among patients who were older age.48,71–74 In the present study, though age was not found to be a risk factor for diabetes, it was strongly predicted in bivariate analysis. In contrast, higher BMI was found to be a predictor of DM. This finding was in agreement with the study from Denmark,73 the USA,75 and France,76 indicating that HIV-infected individuals showed an increasing risk of DM with increasing BMI. Therefore, older age and adiposity were linked with higher risk of diabetes among PLWHIV.
Different studies reported that the prevalence of DM was higher among patients with longer ART duration compared to those with shorter ART duration; however, in the present study, the difference was not statistically significant. A study from Ethiopia (Jimma) reported 11.6% DM prevalence among participants who had >5-year HAART duration and 2% DM prevalence in those who had shorter duration of HAART (<5 years).36 A similar study done in Wolaita Sodo, Ethiopia, reported a DM prevalence of 6.8% and 1.1% among individuals with a long HAART duration (>4 years) and shorter HAART duration (<4 years), respectively.48 In the study from D:A:D cohort, the incidence of diabetes was found to increase with cumulative exposure to combination antiretroviral treatment after adjustment for the potential risk factors of diabetes.77
Most of the DM participants in this study had higher levels of total cholesterol, triglyceride, and LDL-C, and low level of HDL-C, though only triglyceride showed significant differences in multivariate analysis. This was consistent with the studies done in Malaysia,64 Nigeria,63 and Australia.78 This could be due to the similar risk factors of DM. The abnormal level of lipids could partly be due to the effects of the ART drugs itself, as most of the ART drugs have been linked with low blood levels of HDL cholesterol, raised blood levels of triglyceride, LDL cholesterol, and raised total cholesterol.79,80
The comparison between nonhypertensive and hypertensive HIV patients using bivariate analysis showed that the risk factors associated with the presence of hypertension were older age, overweight/obesity, raised WC, increased WHR, decreased CD4 count, longer HAART duration, habit of alcohol use, and increased level of total cholesterol, LDL cholesterol, triglycerides, and glucose. However, raised WHR, raised blood glucose, increased total cholesterol, overweight/obesity, alcohol use, current CD4 count <500 cells/μL, and longer duration of HAART remained to be factors associated with hypertension in the final multiple regression analysis.
This study showed that subjects who were on HAART for longer duration (≥5 years) remained to be at a significantly increased risk of developing hypertension. The role of HAART for the development of hypertension remains controversial. A report from Norway showed that the duration of HAART was associated with the hypertension.19 A study from Spain reported a rise in BP after 48 weeks of ART. The authors suggested that the treatment had a partial role in raising BP by improving the patient’s health condition.67
As many studies showed, the likelihood of hypertension increased with advancing age.65,81,82 However, data from the present study did not show significant difference in multivariable regression analysis. In this study, being obese was significantly associated with hypertension compared to having normal BMI. This finding was in agreement with findings reported elsewhere.19,65–67 Obesity of individuals with HIV/AIDS may be associated with excessive weight gain during treatment with HAART and should be avoided.
Different studies have shown that abnormal level of lipid profile was associated with hypertension.19,65,67 In this study, though raised total cholesterol, triglyceride, and LDL cholesterol and decreased HDL cholesterol lipid profile were observed among individuals with high BP, the difference was significant only for total cholesterol. Risky behavior such as alcohol use was significantly associated with hypertension in this study. The prevalence of hypertension was somewhat higher in smoker patients compared to nonsmoker patients, but the difference was not statistically significant. This may be due to the low prevalence of these factors in the population studied.
The strengths of this study include that it provides the opportunity of evaluating the prevalence of hypertension and DM in a rarely studied population (HIV-infected patients receiving HAART). This contributes to improved assessment and better analysis of this problem in the context of resource-constrained countries where study on this topic is rare. However, there are some limitations to the present study. Our study design was cross-sectional and, as a result, it could only identify associated factors and not risk factors. Our sampling technique was convenient; therefore, our sample cannot be considered representative of all HIV-infected patients receiving treatment in the country. Furthermore, this study cannot confirm that the prevalence of DM and hypertension observed in this study is because of HAART. There might be different risk factors responsible for this.
Conclusion and recommendation
DM and hypertension are common in HIV-infected patients receiving HAART, accounting 7.1% and 12.7%, respectively. Hypertension in patients with HIV/AIDS was associated with factors such as raised WHR, BMI >25 (overweight/obesity), alcohol use, CD4 count <500 cells/μL, longer duration of HAART treatment, increased blood glucose level, and increased total cholesterol level, whereas increased blood triglyceride, high BP, and high baseline BMI were the factors associated with DM.
Based on our findings, we recommend regular monitoring and screening of HIV-infected individuals receiving HAART for DM and hypertension for the early detection of diseases and the prevention of comorbidities of HIV and the NCDs. We also recommend the control of the reversible factors associated with DM and hypertension, particularly the reinforcement of dietary advice, guidance, and prevention of excessive weight gain for the prevention of NCDs’ morbidity among PLWHIV/AIDS. The health professionals who are working in the hospital should counsel the subjects with respect to risky behaviors such as alcohol use, smoking, and physical activity and should create awareness about the prevention of hypertension and DM to prevent further complication. Future researches that investigate the impact of DM and hypertension on the adherence to the antiretroviral medication and quality of life need to be conducted in this population.
Acknowledgments
The authors greatly acknowledge Haramaya University for funding this research. Our special thanks go to our study participants for willing to participate in this study. We would like to express our sincere thanks to data collectors for their management, collection, and analysis of blood specimen. We are thankful to the staff of Jugal Hospital for their support during data collection.
Author contributions
ZA designed the study, participated in data collection and analysis, and drafted the manuscript. WA, TA, and JF participated in study design, analysis, and write-up, and critically revised the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
UNAIDS. Global AIDS Update 2016. Geneva: UNAIDS; 2016. | ||
Joint United Nations Programme on HIV/AIDS. Fact Sheet-Latest Statistics on the Status of the AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2017. | ||
EDHS. Ethiopia Demographic and Health Survey, 2016 HIV prevalence report: Central Statistical Authority; Addis Ababa, Ethiopia, and Rockville, Maryland, USA; CSA and ICF; 2016 | ||
Medina-Torne S, Ganesan A, Barahona I, Crum-Cianflone NF. Hypertension is common among HIV-infected persons, but not associated with HAART. J Int Assoc Physicians AIDS Care. 2012;11(1):20–25. | ||
Nüesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS). J Acquir Immune Defic Syndr. 2013;62(4):396–404. | ||
Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. | ||
Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45(1):111–119. | ||
Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. | ||
Wand H, Calmy A, Carey DL, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21(18):2445–2453. | ||
Rabkin M, Nishtar S. Scaling up chronic care systems: leveraging HIV programs to support noncommunicable disease services. J Acquir Immune Defic Syndr. 2011;57(Suppl 2):S87–S90. | ||
Nigatu T. Integration of HIV and noncommunicable diseases in health care delivery in low- and middle-income countries. Prev Chronic Dis. 2012;9:E93. | ||
Smart T. HIV and non communicable diseases (NCDs). London, UK; NAM; 2011 | ||
Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. | ||
Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87–95. | ||
Aoun S, Ramos E. Hypertension in the HIV-infected patient. Curr Hypertens Rep. 2000;2(5):478–481. | ||
Tershakovec AM, Frank I, Rader D. HIV-related lipodystrophy and related factors. Atherosclerosis. 2004;174(1):1–10. | ||
Calza L, Manfredi R, Chiodo F. Insulin resistance and diabetes mellitus in HIV-infected patients receiving antiretroviral therapy. Metab Syndr Relat Disord. 2004;2(4):241–250. | ||
Grinspoon S. Mechanisms and strategies for insulin resistance in acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(Suppl 2):S85–S90. | ||
Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26(11):2126–2133. | ||
Mateen FJ, Kanters S, Kalyesubula R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31(7):1372–1378. | ||
Diouf A, Cournil A, Ba-Fall K, et al. Diabetes and hypertension among patients receiving antiretroviral treatment since 1998 in Senegal: prevalence and associated factors. Isrn Aids. 2012;2012:1–8. | ||
Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D, Clinical Epidemiology Group from the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17(17):2479–2486. | ||
DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. | ||
Denue A, Muazu J, Gashau W, Nkami D, Ajayi NA. Effects of highly active antiretroviral therapy (HAART) on blood pressure changes and its associated factors in HAART naive HIV-infected patients in north eastern Nigeria. Arch Appl Sci Res. 2012;4(3):1447–1452. | ||
Currier J, Scherzer R, Bacchetti P, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48(1):35–43. | ||
Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(Suppl 2):S79–S85. | ||
Shenoy A, Ramapuram JT, Unnikrishan B, et al. Effect of lipodystrophy on the quality of life among people living with HIV (PLHIV) on highly active antiretroviral therapy. J Int Assoc Provid AIDS Care. 2014;13(5):471–475. | ||
Daly R, Freemantle J, Koh G, Stradling C, van der Linde M, Taylor S. Are traditional risk factors associated with cardiovascular events in HIV positive subjects. HIV Med. 2012;13:52–53. | ||
Factor SH, Lo Y, Schoenbaum E, Klein RS. Incident hypertension in older women and men with or at risk for HIV infection. HIV Med. 2013;14(6):337–346. | ||
Hadigan C. Dietary habits and their association with metabolic abnormalities in human immunodeficiency virus-related lipodystrophy. Clin Infect Dis. 2003;37(Suppl 2):S101–S104. | ||
Savès M, Raffi F, Capeau J, François R, Jacqueline C, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34(10):1396–1405. | ||
Kibret KT, Mesfin YM. Prevalence of hypertension in Ethiopia: a systematic meta-analysis. Public Health Rev. 2015;36(1):14. | ||
Yemane T, Belachew T, Asaminew B, Befekadu O. Type II diabetes mellitus in Jimma Town, southwest Ethiopia. Ethiopia J Health Sci. 2007;17(2). | ||
Awoke A, Awoke T, Alemu S, Megabiaw B. Prevalence and associated factors of hypertension among adults in Gondar, Northwest Ethiopia: a community based cross-sectional study. BMC Cardiovasc Disord. 2012;12(1):113. | ||
Misganaw A, Mariam DH, Ali A, Araya T. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J Health Popul Nutr. 2014;32(1):1. | ||
Mohammed AE, Shenkute TY, Gebisa WC. Diabetes mellitus and risk factors in human immunodeficiency virus-infected individuals at Jimma University Specialized Hospital, Southwest Ethiopia. Diabetes Metab Syndr Obes. 2015;8:197. | ||
WHO. Global Database on Body Mass Index. Geneva: WHO; 2011. | ||
WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. Geneva: WHO; 2011. | ||
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. | ||
WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: World Hearth Organization; 2006. | ||
Zannou DM, Denoeud L, Lacombe K, et al. Incidence of lipodystrophy and metabolic disorders in patients starting non-nucleoside reverse transcriptase inhibitors in Benin. Antivir Ther. 2009;14(3):371–380. | ||
Gupta V, Biswas A, Sharma SK. Metabolic and body composition changes after six months of highly active antiretroviral therapy in northern Indian patients. Int J STD AIDS. 2011;22(1):46–49. | ||
Salehian B, Bilas J, Bazargan M, Abbasian M. Prevalence and incidence of diabetes in HIV-infected minority patients on protease inhibitors. J Natl Med Assoc. 2005;97(8):1088. | ||
WHO. Diabetes Country Profiles. Geneva: WHO; 2016. | ||
IDF. IDF Diabetes Atlas. Brussels: International Diabetes Federation; 2017. | ||
Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV Study. AIDS. 2007;21(13):1739–1745. | ||
da Silva EF, Bassichetto KC, Lewi DS. Lipid profile, cardiovascular risk factors and metabolic syndrome in a group of AIDS patients. Arq Bras Cardiol. 2009;93(2):113–118. | ||
Sachithananthan V, Loha E, Gose M. Prevalence of Diabetes Mellitus, Hypertension and Lipodystrophy in HAART Receiving HIV Patients in Southern Ethiopia. Int STD Res Rev. 2013;1(1):1–11. | ||
Tomažic˘ J, Silic˘ A, Karner P, et al. Lipodystrophy and metabolic abnormalities in Slovenian HIV-infected patients. Wien Klin Wochenschr. 2004;116(21–22):755–759. | ||
Ramezani A, Mohraz M, Yadegarinia D, et al. Prevalence of dyslipidemia and metabolic abnormalities in HIV-infected patients. Acta Medica Iranica. 2009;47(2):83–88. | ||
Kabati C, Maurice H, Mselle T, Urio M. Evaluation of the prevalence of insulin dependent diabetes mellitus in HIV/AIDS patients in Muhimbili National Hospital, Dar es Salaam, Tanzania. TaJONAS: Tanzania Journal of Natural and Applied Sciences. 2012;1(2):164–173. | ||
Tzur F, Chowers M, Mekori Y, Hershko A. Prevalence of diabetes mellitus among Ethiopian-born HIV patients in Israel. J Int AIDS Soc. 2012;15(Suppl 4):1–1. | ||
Danciulescu R, Musat M, Cristescu V, Poiana C. The prevalence of diabetes mellitus in patients infected with human immunodeficiency virus on treatment with antiretoviral drugs. Endocrine Abstracts. 2011;26:P736. | ||
Wu P-Y, Hung C-C, Liu W-C, et al. Metabolic syndrome among HIV-infected Taiwanese patients in the era of highly active antiretroviral therapy: prevalence and associated factors. J Antimicrob Chemother. 2012;67(4):1001–1009. | ||
Julius H, Basu D, Ricci E, et al. The burden of metabolic diseases amongst HIV positive patients on HAART attending The Johannesburg Hospital. Curr HIV Res. 2011;9(4):247–252. | ||
Manuthu EM, Joshi MD, Lule GN, Karari E. Prevalence of dyslipidemia and dysglycaemia in HIV infected patients. East Afr Med J. 2008;85(1):10–17. | ||
Puttawong S, Prasithsirikul W, Vadcharavivad S. Prevalence of lipodystrophy in Thai-HIV infected patients. J Med Assoc Thai. 2004;87(6):605–611. | ||
Howell SJ, Sear JW, Foëx P. Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaesth. 2004;92(4):570–583. | ||
Mendis S. Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization; 2014. | ||
Muluneh AT, Haileamlak A, Tessema F, et al. Population based survey of chronic non-communicable diseases at gilgel gibe field research center, southwest Ethiopia. Ethiopia J Health Sci. 2012;22(4):7–18. | ||
Nyabera R, Yonga G, Mwangemi F, Bukachi F. Evaluation of a project integrating cardiovascular care into HIV programmes. Cardiovasc J Afr. 2011;14(3):S17. | ||
Sani M, Muhammad S, Okeahialam B. Effects of HAART on cardiovascular risk profile of HIV/AIDS patients in Aminu Kano Teaching Hospital, Kano, Nigeria. Cardiovasc J Afr. 2011;22:S22. | ||
Adewole O, Eze S, Betiku Y, et al. Lipid profile in HIV/AIDS patients in Nigeria. Afr Health Sci. 2010;10(2):144–149. | ||
Hejazi N, Rajikan R, Kwok Choong CL, Sahar S. Metabolic abnormalities in adult HIV infected population on antiretroviral medication in Malaysia: a cross-sectional survey. BMC Public Health. 2013;13(1):758. | ||
Arruda Junior ER, Lacerda HR, Moura LC, et al. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14(3):281–287. | ||
Bloomfield GS, Hogan JW, Keter A, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6(7):e22288. | ||
Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7(1):10–15. | ||
Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naïve and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22(12):731–736. | ||
Coloma AC, Alvarez MA, Roca-Cusachs AC, Domingo PP, Puig MC. Prevalence of arterial hypertension and lipid profile in HIV patients. Medicina clinica. 2008;131(18):681–684. | ||
Cahn P, Leite O, Rosales A, et al. Metabolic profile and cardiovascular risk factors among Latin American HIV-infected patients receiving HAART. Braz J Infect Dis. 2010;14(2):158–166. | ||
Nsakashalo-Senkwe M, Siziya S, Goma FM, Songolo P, Mukonka V, Babaniyi O. Combined prevalence of impaired glucose level or diabetes and its correlates in Lusaka urban district, Zambia: a population based survey. Int Arch Med. 2011;4(1):2. | ||
Butt AA, Mcginnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. | ||
Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLoS One. 2012;7(9):e44575. | ||
Howard AA, Floris-Moore M, Arnsten JH, et al. Disorders of glucose metabolism among HIV-infected women. Clin Infect Dis. 2005;40(10):1492–1499. | ||
Justman JE, Benning L, Danoff A, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(3):298–302. | ||
Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26(3):303–314. | ||
de Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–1229. | ||
Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30(1):113–119. | ||
Omech B, Sempa J, Castelnuovo B, et al. Prevalence of HIV-associated metabolic abnormalities among patients taking first-line antiretroviral therapy in Uganda. Isrn Aids. 2012;2012(4):1–6. | ||
Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011;25(2):185–195. | ||
Shishani K, Dajani R, Khader Y. Hypertension risk assessment in the largest ethnic groups in Jordan. J Immigr Minor Health. 2013;15(1):43–48. | ||
Ahaneku G, Osuji CU, Anisiuba BC, Ikeh VO, Oguejiofor OC, Ahaneku JE. Evaluation of blood pressure and indices of obesity in a typical rural community in eastern Nigeria. Ann Afr Med. 2011;10(2):120. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.