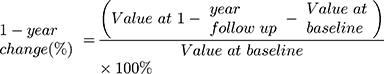Back to Journals » International Journal of General Medicine » Volume 13
MAGGIC Risk Model Predicts Adverse Events and Left Ventricular Remodeling in Non-Ischemic Dilated Cardiomyopathy
Authors Dong Y, Wang D, Lv J, Pan Z, Xu R, Ding J, Cui X, Xie X, Guo X
Received 26 October 2020
Accepted for publication 18 November 2020
Published 10 December 2020 Volume 2020:13 Pages 1477—1486
DOI https://doi.org/10.2147/IJGM.S288732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yang Dong,* Dongfei Wang,* Jialan Lv, Zhicheng Pan, Rui Xu, Jie Ding, Xiao Cui, Xudong Xie, Xiaogang Guo
Department of Cardiology, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaogang Guo
Department of Cardiology, The First Affiliated Hospital of Zhejiang University School of Medicine, No. 79 Qing Chun Road, Hangzhou 310006, People’s Republic of China
Tel + 86-13867441856
Email [email protected]
Purpose: We aimed to study the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) risk model’s prognostic value and relationship with left ventricular remodeling in dilated cardiomyopathy.
Patients and Methods: Dilated cardiomyopathy patients were prospectively recruited and underwent clinical assessments. MAGGIC risk score was calculated. Patients were followed up for adverse events and echocardiography. Primary endpoints were all-cause mortality and first rehospitalization due to heart failure. Secondary endpoint was left ventricular remodeling defined as a decline in left ventricular ejection fraction > 10% or an increase in left ventricular end-diastolic diameter > 10%. Survival status was examined using Cox regression analysis. The model’s ability to discriminate adverse events and left ventricular remodeling was calculated using a receiver operating characteristics curve.
Results: In total, 114 patients were included (median follow-up time = 31 months). The risk score was independently related to adverse events (2-year all-cause mortality: hazard ratio [HR] = 1.122; 95% confidence interval [CI], 1.043– 1.208; 1-year first rehospitalization due to heart failure: HR = 1.094; 95% CI, 1.032– 1.158; 2-year first rehospitalization due to heart failure: HR = 1.088; 95% CI, 1.033– 1.147, all P < 0.05). One-year change in left ventricular end-diastolic diameter was correlated with the risk score (r = 0.305, P = 0.002). The model demonstrated modest ability in discriminating adverse events and left ventricular remodeling (all areas under the curve were 0.6– 0.7).
Conclusion: The MAGGIC risk score was related to adverse events and left ventricular remodeling in dilated cardiomyopathy.
Keywords: dilated cardiomyopathy, left ventricular remodeling, prognosis, risk model
Introduction
Non-ischemic dilated cardiomyopathy (DCM) is a progressive disease presenting with left ventricle dilation as well as decreased systolic function excluding abnormal loading conditions and coronary artery disease.1 As an important cause of heart failure (HF), DCM is responsible for a substantial proportion of adverse events such as cardiac morbidity and mortality.2,3 Left ventricular remodeling plays an important role in the pathophysiological progression of DCM, which can appear as a change in left ventricular diameter and left ventricular systolic function on echocardiography.1,4–6 Drugs currently treating DCM, including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and beta blockers are used to halt or reverse left ventricular remodeling.2,7 Some researchers also found that short-term left ventricular remodeling or left ventricular reverse remodeling was related to long-term prognosis.8–11 There is a demand for an easily available tool to predict left ventricular remodeling in DCM patients at baseline to guide treatment.
The Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score emerged in 2013 and has since been validated by several studies as a good risk stratification tool for predicting the prognosis of HF patients.12–15 Moneghetti et al also reported that the MAGGIC risk score was independently related with the outcomes in DCM patients.16 However, most studies focused on patients with HF of heterogenous etiology and lacked validation of the MAGGIC risk model’s prognostic value in DCM patients only. Studies were also lacking on the relationship between MAGGIC risk score and left ventricular remodeling.
Here we aimed to calculate the baseline MAGGIC risk score and study its prognostic value and relationship with left ventricular remodeling in a cohort of DCM patients.
Patients and Methods
Study Population
This observational study was approved by Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University. Written informed consents were obtained from all included patients according to the Helsinki Declaration. DCM patients were prospectively recruited from June 2015 to June 2018. DCM was diagnosed as increasing left ventricular end-diastolic diameter (LVEDD) (>55 mm in men or >50 mm in women) and decreasing left ventricular ejection fraction (LVEF) <45% on echocardiography. Patients with coronary artery disease and abnormal loading conditions, such as hypertension, congenital heart disease, or valvular disease, were excluded.17 All patients underwent coronary angiography or coronary computed tomography angiography. Patients with a significant stenosis of coronary artery >50% were excluded.18 The demographic characteristics and clinical data of every patient were collected. All DCM patients received standard drug therapy according to the current guideline.19
Echocardiography
All patients underwent two-dimensional echocardiography using a dedicated ultrasound system (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). Cardiac structure and function were assessed according to the former scan protocol of the American Society of Echocardiography.20 Left atrial diameter, LVEDD and LVEF were collected to evaluate the status of left heart of DCM patients. Left atrial diameter and LVEDD were obtained using two-dimensional ultrasonography in the parasternal long-axis 2-chamber view. Left atrial diameter was obtained at the end of left ventricle systole, while LVEDD was obtained at the end of left ventricle diastole. LVEF was estimated using the biplane Simpson method. All echocardiographic scans and measurements, including scans on baseline and scans during follow-up, were performed by an experienced research sonographer blinded to the patients’ other clinical data. Twenty percent of total subjects were randomly selected from the DCM cohort by random numbers to evaluate intra-observer variabilities. The sonographer repeated the measurements by using the identical method at least 6 months later.
MAGGIC Risk Model
The MAGGIC risk score was calculated at baseline when each DCM patient was included. The risk model comprises 13 variables including LVEF (from 0 to 7 points according to 6 different groups of LVEF), LVEF extra for age (from 0 to 15 points according to 7 different groups of age combining with 3 different groups of LVEF), LVEF extra for systolic blood pressure (from 0 to 5 points according to 6 different groups of systolic blood pressure combining with 3 different groups of LVEF), body mass index (from 0 to 6 points according to 5 different groups of body mass index), creatinine (from 0 to 8 points according to 8 different groups of creatinine), New York Heart Association class (from 0 to 8 points according to 4 different groups of New York Heart Association class), male sex (one point), current smoker (one point), diabetes (three points), chronic obstructive pulmonary disease (two points), first diagnosis of HF within the past 18 months (two points), not on beta-blocker use (three points), and not on angiotensin converting enzyme inhibitor/angiotensin receptor blocker use (one point).15 All patients were divided into two groups, including M1 group and M2 group, by the median of all MAGGIC risk scores.
Follow-Up
Patients were followed up every 6 months to record adverse events. All living patients underwent echocardiography within 2 weeks of the 1-year follow-up. Primary endpoints were all-cause mortality and first rehospitalization due to HF. First rehospitalization due to HF was defined as worsening symptoms and signs of HF, such as dyspnea or signs of congestion, which require urgent treatment in hospital for the first time after patients’ baseline admissions.21 The secondary endpoint was left ventricular remodeling during follow-up, which was defined as a decline of LVEF >10% or increase of LVEDD >10%.7 The 1-year change of left heart remodeling parameters (left atrial diameter, LVEDD, and LVEF) were calculated using the formula:
Statistical Analysis
MedCalc version 13.0 (Med-Calc Software; Ostend, Belgium) and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA) were used to perform statistical analysis. The Kolmogorov–Smirnov test was used to check the distribution of continuous variables. Normally distributed continuous variables were described as mean ± SD, while non-normally distributed data were presented as median and interquartile range. Intergroup differences of continuous variables were analyzed by Student’s t-test or the Mann–Whitney U-test. Differences between two groups of categorical variables were tested by the Chi-square test. Survival curves of 2-year all-cause mortality as well as 1-year and 2-year first rehospitalization due to HF were created using the Kaplan–Meier method. Survival curves between different groups of MAGGIC risk scores were compared using the Log rank test. Univariate and multivariate Cox regression analyses were used to examine relationships between the clinical characteristics and adverse events, and hazard ratio (HR) was calculated. Spearman correlation analysis was performed to evaluate the relationship between left heart remodeling parameter changes and MAGGIC risk scores. The diagnostic ability of the MAGGIC risk model for discriminating adverse events and left ventricular remodeling were calculated by receiver operating characteristics curve, and the areas under the curve were calculated. The intra-observer variability was calculated by mean bias from Bland–Altman analyses, coefficient of variation (COV, %), and intra-class correlation coefficient (ICC). ICC calculation used two-way mixed, absolute agreement single measure model. Values of P < 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics
A total of 217 patients were recruited according to echocardiography findings. One hundred and three patients were excluded due to coronary artery disease according to coronary angiography or coronary computed tomography angiography findings. Ultimately, 114 DCM patients were included in the study. The median follow-up time was 31 months (interquartile range, 26–34 months). After following up the adverse events of all included DCM patients, 10 patients (<10% of all included patients) died within the first year after inclusion, while 21 patients died within the first 2 years after inclusion. Thirty-one patients experienced the first rehospitalization due to HF in the first year after inclusion, while 44 patients experienced the first rehospitalization due to HF within the first 2 years after inclusion. The patients’ demographic and clinical characteristics stratified by endpoint are shown in Table 1. Age, sex, body mass index, systolic blood pressure, N-terminal–pro-brain natriuretic peptide level, ratio of current smokers, diagnosis of chronic obstructive pulmonary disease, angiotensin receptor blocker use, LVEDD, and MAGGIC risk score differed significantly among the endpoint groups.
 |
Table 1 Comparison of Baseline Demographic Characteristics and Clinical Data Between Dilated Cardiomyopathy Patients with and without Adverse Events in 1-Year and 2-Year Follow-Up |
Relationship Between MAGGIC Risk Score and Adverse Events
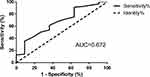
Patients in M1 group (5–20 points, n = 58) had significantly better survival status than that in M2 (21–37 points, n = 56) group (Figure 1). Cox regression analysis showed that MAGGIC risk score was independently related to adverse events in DCM patients (Table 2). However, the MAGGIC risk model demonstrated modest ability to discriminate adverse events (Figure 2 and Supplementary Table 1).
 |
Table 2 Cox Regression of Adverse Events in Dilated Cardiomyopathy Patients in 1-Year and 2-Year Follow-Up |
Relationship Between MAGGIC Risk Score and Left Ventricular Remodeling Status
Since 10 patients died within the first year of follow-up, 104 patients underwent 1-year follow-up echocardiography. Patients showed significant left heart reverse remodeling after 1 year as demonstrated by the decreased left atrial diameter (baseline vs 1-year follow-up: 45 ± 7 mm vs 42 ± 9 mm, P = 0.017), LVEDD (baseline vs 1-year follow-up: 64 ± 8 mm vs 59 ± 9 mm, P < 0.001) and increased LVEF (baseline vs 1-year follow-up: 32 ± 11% vs 41 ± 14%, P < 0.001) (Supplementary Figure 1). Comparison of the 1-year changes in left heart remodeling parameters between M1 and M2 groups revealed that change in LVEDD was significantly less over 1 year in the M1 group than in the M2 group (M1 (n = 54) vs M2 (n = 50): −9 [−16 to −2] % vs −3 [−10 to 4] %, P = 0.006) (Supplementary Figure 2). One-year change of left atrial diameter and LVEF did not differ significantly between the two groups. Spearman correlation revealed that the 1-year change in LVEDD was significantly correlated with MAGGIC risk score (Supplementary Table 2). The MAGGIC risk model demonstrated modest ability to discriminate left ventricular remodeling (Figure 3 and Supplementary Table 3). Echocardiography parameters measurement demonstrated good consistency in 23 randomly selected patients (Supplementary Table 4).
Discussion
In this study, we found that MAGGIC risk score was independently related to adverse events in a cohort of DCM patients and appeared to have modest ability to discriminate adverse events in DCM patients. Meanwhile, MAGGIC risk score was also independently related to left ventricular remodeling parameters of DCM patients and had modest ability to discriminate left ventricular remodeling in DCM patients.
MAGGIC Risk Score and Adverse Events
Since its inception in 2013, the MAGGIC risk score has been validated several times for its ability to discriminate adverse events in HF patients with the ejection fraction reducing, ejection fraction preserving, and mixed types.14,15,22–25 Here we validated the prognostic value of MAGGIC risk score for adverse events of DCM patients. Our results were consistent with those of DCM patients reported by Moneghetti et al demonstrating that MAGGIC risk score was independently related to adverse events.16 However, the MAGGIC risk model demonstrated modest ability to discriminate adverse events in our study. Sawano et al reported that the MAGGIC risk score showed modest ability to discriminate 1-year mortality (area under the curve = 0.71), while Sartipy et al reported that the MAGGIC risk score was able to discriminate 1-year mortality (area under the curve = 0.743) in HF patients.13,25 The MAGGIC risk model could not predict adverse events in DCM patients in this cohort as strongly as in HF patients. Due to the small sample size of our study, future studies might examine this difference in predictive ability of adverse events in DCM versus HF patients.
MAGGIC Risk Score and Left Ventricular Remodeling
There has been a lack of studies examining the relationship between MAGGIC risk score and left ventricular remodeling in DCM patients. Some researchers reported that some biomarkers such as tenascin-c and galectin-3 were predictors of left ventricular remodeling or reverse remodeling in DCM patients.26,27 Other researchers reported that genotypes were predictors of left ventricular reverse remodeling in DCM patients.7,28,29 However, these biomarkers require extra examination and increase patient financial burdens, while all MAGGIC model risk factors are obtained from routine evaluations of DCM patients. We found that the MAGGIC risk score was related to left ventricular enlargement and demonstrated modest discrimination ability on left ventricular remodeling in DCM patients. This implies that the MAGGIC risk score could become a reference for the short-term evaluation of left ventricular remodeling status which is an indicator of the long-term prognosis of DCM patients.8–11
MAGGIC Risk Model and Risk Factors Included
Risk factors included in the MAGGIC risk model were proven associated with adverse events in HF patients as well as DCM patients.16,30–33 And we could also infer that these risk factors were associated with left ventricular remodeling in DCM patients as well, for some of these risk factors were previously proven associated with left ventricular remodeling.6,34,35 Thus, the MAGGIC risk model generates a good combination of these risk factors for predicting adverse events and left ventricular remodeling. The easy availability of the model makes it a choice for evaluating DCM patients. Although there is limited translational benefit from the model’s use in clinical practice, this study has inspirational meaning for future research on the model’s usage. Notably, some researchers adjusted the MAGGIC risk model by including N-terminal–pro-brain natriuretic peptide or brain natriuretic peptide in the MAGGIC model, and the revised models demonstrated better prognostic value than original MAGGIC model in HF patients.12,13,36 Exploration of a unified model combining natriuretic peptide with the original MAGGIC risk model and validation of the adjusted model’s prediction ability in DCM patients are also works that need to be done, especially in a DCM cohort with large sample size, in the future.
Limitations
This study has some limitations. First, it was a single-center study with a small sample size. And since the discriminative capacity of the model was simply weak, there is limited translational benefit from the model’s use in clinical practice for now. More studies with large sample size and multi-center studies are needed to uncover the accurate discrimination ability of the model in adverse events and left ventricular remodeling. Second, DCM patients included in the study did not use a unified treatment plan, which could have created bias in our study. However, since personalized medicine becoming increasingly more popular, it would be difficult to ensure that all DCM patients use the same treatment approach. Our study could reveal a meaningful regular pattern in the real world. Third, the follow-up time in this study was short. The life expectancy of DCM patients would be prolonged with treatment advances. The MAGGIC risk model’s ability to predict long-term survival could be tested in the future.
Conclusions
In conclusion, the MAGGIC risk score was related to adverse events and left ventricular remodeling but demonstrated modest ability to discriminate adverse events and left ventricular remodeling in DCM patients. It has the potential to be a choice for evaluating the prognosis of DCM patients.
Funding
This research was supported by grants from the Program of National Natural Science Foundation of China [No.81470370]; the Key Projects of Zhejiang Medical and Health Science and Technology Plan (Provincial and Ministerial Co-construction) [No. WKJ-ZJ-1819]; and the Fundamental Research Funds for the Zhejiang Province Universities [No. 2019XZZX003-15].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390:400–414. doi:10.1016/S0140-6736(16)31713-5
2. Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: A review and meta-analysis. JACC Cardiovasc Imaging. 2018;11:1274–1284. doi:10.1016/j.jcmg.2018.03.006
3. Koutalas E, Kanoupakis E, Vardas P. Sudden cardiac death in non-ischemic dilated cardiomyopathy: A critical appraisal of existing and potential risk stratification tools. Int J Cardiol. 2013;167:335–341. doi:10.1016/j.ijcard.2012.07.014
4. Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi:10.1016/j.jacc.2019.01.056
5. Fabiani I, Pugliese NR. The assessment of pressure-volume relationship during exercise stress echocardiography predicts left ventricular remodeling and eccentric hypertrophy in patients with chronic heart failure. Cardiovasc Ultrasound. 2019;17:6. doi:10.1186/s12947-019-0157-z
6. Wu J, Wu C, Fan W, Zhou J, Xu L. Incidence and predictors of left ventricular remodeling among elderly asian women: A community-based cohort study. BMC Geriatr. 2017;17:21. doi:10.1186/s12877-017-0411-x
7. Dal Ferro M, Stolfo D, Altinier A, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017;103:1704–1710. doi:10.1136/heartjnl-2016-311017
8. Izumi C, Kitai T, Kume T, et al. Effect of left ventricular reverse remodeling on long-term outcomes after aortic valve replacement. Am J Cardiol. 2019;124:105–112. doi:10.1016/j.amjcard.2019.04.010
9. Adamo M, Godino C, Giannini C, et al. Left ventricular reverse remodelling predicts long-term outcomes in patients with functional mitral regurgitation undergoing mitraclip therapy: results from a multicentre registry. Eur J Heart Fail. 2019;21:196–204.
10. Rickard J, Johnston DR, Price J, et al. Reverse ventricular remodeling and long-term survival in patients undergoing cardiac resynchronization with surgically versus percutaneously placed left ventricular pacing leads. Heart Rhythm. 2015;12:517–523. doi:10.1016/j.hrthm.2014.11.013
11. Bax JJ, Schinkel AF, Boersma E, et al. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation. 2004;110:Ii18–22. doi:10.1161/01.CIR.0000138195.33452.b0
12. Michaels A, Aurora L, Peterson E, et al. Risk prediction in transition: maggic score performance at discharge and incremental utility of natriuretic peptides. J Card Fail. 2020;26:52–60. doi:10.1016/j.cardfail.2019.11.016
13. Sawano M, Shiraishi Y, Kohsaka S, et al. Performance of the maggic heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018;5:610–619. doi:10.1002/ehf2.12278
14. Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta-analysis global group in chronic (maggic) heart failure risk score: validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7:e009594. doi:10.1161/JAHA.118.009594
15. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. doi:10.1093/eurheartj/ehs337
16. Moneghetti KJ, Giraldeau G, Wheeler MT, et al. Incremental value of right heart metrics and exercise performance to well-validated risk scores in dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2018;19:916–925. doi:10.1093/ehjci/jex187
17. Mathew T, Williams L, Navaratnam G, et al. Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the british society of echocardiography. Echo Res Pract. 2017;4:G1–g13. doi:10.1530/ERP-16-0037
18. Andreini D, Pontone G, Pepi M, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:2044–2050. doi:10.1016/j.jacc.2007.01.086
19. Yancy CW, Jessup M, Bozkurt B, et al. acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. 2017;136:e137–e161. doi:10.1161/CIR.0000000000000509
20. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi:10.1016/j.echo.2008.11.023
21. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. doi:10.1016/j.jacc.2012.09.038
22. Tsutsui H, Momomura SI, Saito Y, et al. Angiotensin receptor neprilysin inhibitor in japanese patients with heart failure and reduced ejection fraction - baseline characteristics and treatment of parallel-hf trial. Circ J. 2018;82:2575–2583. doi:10.1253/circj.CJ-17-1424
23. Szczurek W, Szyguła-Jurkiewicz B, Zakliczyński MW, Król B, Gąsior M, Zembala M. Prognostic value of selected risk scales in patients with end-stage heart failure. Kardiol Pol. 2018;76:1320–1326. doi:10.5603/KP.a2018.0090
24. Sawano M, Shiraishi Y, Kohsaka S, et al. Performance of the maggic heart failure risk score and its modification with the addition of discharge natriuretic peptides. J Am Heart Assoc. 2018;5:610–619.
25. Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: validation of the maggic heart failure risk score in 51,043 patients from the swedish heart failure registry. Eur J Heart Fail. 2014;16:173–179. doi:10.1111/ejhf.32
26. Sarli B, Topsakal R, Kaya EG, Akpek M, Lam YY, Kaya MG. Tenascin-c as predictor of left ventricular remodeling and mortality in patients with dilated cardiomyopathy. J Investig Med. 2013;61:728–732. doi:10.2310/JIM.0b013e3182880c11
27. Karatolios K, Chatzis G, Holzendorf V, et al. Galectin-3 as a predictor of left ventricular reverse remodeling in recent-onset dilated cardiomyopathy. Dis Markers. 2018;2018:2958219. doi:10.1155/2018/2958219
28. Verdonschot JAJ, Hazebroek MR, Wang P, et al. Clinical phenotype and genotype associations with improvement in left ventricular function in dilated cardiomyopathy. Circ Heart Fail. 2018;11:e005220. doi:10.1161/CIRCHEARTFAILURE.118.005220
29. Tobita T, Nomura S, Fujita T, et al. Genetic basis of cardiomyopathy and the genotypes involved in prognosis and left ventricular reverse remodeling. Sci Rep. 2018;8:1998. doi:10.1038/s41598-018-20114-9
30. Sinagra G, Merlo M, Cannatà A. Gender medicine in dilated cardiomyopathy: pride and prejudice. Eur J Heart Fail. 2018;20:1401–1403. doi:10.1002/ejhf.1280
31. Park J, Lee HJ, Kim SK, et al. Smoking aggravates ventricular arrhythmic events in non-ischemic dilated cardiomyopathy associated with a late gadolinium enhancement in cardiac mri. Sci Rep. 2018;8:15609. doi:10.1038/s41598-018-34145-9
32. Sawamura A, Okumura T, Takeshita K, et al. Abnormal circadian blood pressure profile as a prognostic marker in patients with nonischemic dilated cardiomyopathy. Cardiology. 2017;136:1–9. doi:10.1159/000446868
33. Wojciechowska C, Jacheć W, Romuk E, Nowalany-Kozielska E, Tomasik A, Siemińska L. The effect of bmi, serum leptin, and adiponectin levels on prognosis in patients with non-ischaemic dilated cardiomyopathy. Endokrynol Pol. 2017;68:26–34. doi:10.5603/EP.2017.0005
34. Marcolan Quitete CM, Marcolan Salvany A, de Andrade Martins W, Mesquita ET. Left ventricular remodeling and diastolic function in chronic hypertensive pregnant women. Pregnancy Hypertens. 2015;5:187–192. doi:10.1016/j.preghy.2015.01.007
35. Dong Y, Yang D, Han Y, et al. Age and gender impact the measurement of myocardial interstitial fibrosis in a healthy adult chinese population: A cardiac magnetic resonance study. Front Physiol. 2018;9:140. doi:10.3389/fphys.2018.00140
36. Khanam SS, Choi E. Validation of the maggic (meta-analysis global group in chronic heart failure) heart failure risk score and the effect of adding natriuretic peptide for predicting mortality after discharge in hospitalized patients with heart failure. PLoS One. 2018;13:e0206380. doi:10.1371/journal.pone.0206380
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.