Back to Journals » Cancer Management and Research » Volume 14
Lymphoepithelioma‑Like Cholangiocarcinoma with Hepatitis C Virus Infection Treated by Microwave Ablation: A Literature Review and Case Report
Authors Li X, Ji H, Zhang D, Jin M, Guo X, Gao P
Received 14 March 2022
Accepted for publication 18 June 2022
Published 4 July 2022 Volume 2022:14 Pages 2155—2160
DOI https://doi.org/10.2147/CMAR.S366419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Xu Li,1 Huifan Ji,1 Dezhi Zhang,2 Meishan Jin,3 Xiaolin Guo,1,* Pujun Gao1,*
1Department of Hepatology, The First Hospital of Jilin University, Jilin University, Changchun, 130021, People’s Republic of China; 2Department of Abdominal Ultrasound, The First Hospital of Jilin University, Jilin University, Changchun, 130021, People’s Republic of China; 3Department of Pathology, The First Hospital of Jilin University, Jilin University, Changchun, 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaolin Guo; Pujun Gao, Email [email protected]
Background: Lymphoepithelioma-like cholangiocarcinoma (LELCC) is a rare type of intrahepatic tumor that is poorly understood. It is not associated with specific physical findings and is usually diagnosed incidentally, resulting in tumors that are often large-sized at diagnosis. At present, the main treatment approach is surgical resection.
Case Presentation: Here, we report the case of a patient with LELCC treated with microwave ablation (MWA). Our patient was a Chinese man with chronic hepatitis C and a 51 mm-diameter intrahepatic tumor. Despite blood testing, gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging, and abdominal ultrasound, the tumor was not well diagnosed. However, the histopathological findings of ultrasound-guided percutaneous tumor biopsy led to a diagnosis of LELCC. The patient was treated with MWA, and no new lesions had occurred at 9 months after treatment.
Conclusion: To our knowledge, this is the first patient with LELCC treated using MWA. Our experience suggests that MWA is an effective new therapeutic method for this disease.
Keywords: Epstein-Barr virus, lymphoepithelioma-like cholangiocarcinoma, microwave ablation, hepatitis C, liver tumor
Introduction
First described in the nasopharynx, lymphoepithelioma-like carcinoma (LELC) is a type of tumor characterized by proliferating undifferentiated epithelial or carcinoma cells with prominent infiltrating lymphocytes.1–3, Primary hepatic LELC, also known as lymphoepithelioma-like hepatocellular carcinoma or lymphoepithelioma-like cholangiocarcinoma (LELCC),1 is exceedingly rare, with fewer than 100 cases reported. Similar to LELC arising from other organs, LELCC is often associated with Epstein-Barr virus (EBV) infection. Due to its rarity, however, there is a lack of information concerning its imaging findings and laboratory test results, making it difficult to establish a diagnosis. There is also no standard treatment plan. Most cases of LELCC are treated surgically, although a few patients have been treated with radiofrequency ablation or chemotherapy. Here, we report the case of a patient with LELCC treated with microwave ablation (MWA).
Case Report
A 55-year-old Chinese man visited our hospital complaining of abdominal distension. He was positive for hepatitis C antibodies and showed elevated hepatitis C virus (HCV) RNA (3.26E+004 IU/mL). The results of liver function tests were as follows: aspartate aminotransferase (AST) 38.2 U/L (reference range: 15–40), alanine aminotransferase (ALT) 47.1 U/L (reference range: 9–50), alkaline phosphatase (ALP) 92.2 U/L (reference range: 45–125), and total bilirubin 12.2 mmol/L (reference range: 0–26). Notably, both gamma-glutamyl transpeptidase (GGT) (108.5 U/L, reference range: 10–60) and alpha-fetoprotein were elevated (22.22 ng/mL, reference range: 0–20), and CA19-9 was normal (13.14 ng/mL, reference range: 0–37). EBV nucleic acid quantitative detection was 4.50E+003 copies/mL. Abdominal ultrasound showed the presence of a hypoechoic lesion in the right posterior lobe of the liver that was 41×51 mm in size. The results of a complete blood count, blood chemistry tests, and urinalysis were normal.
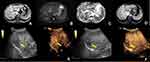
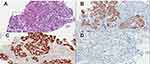
Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI) showed the lesion was hypointense on T1-weighted images and hyperintense on T2-weighted images (Figure 1A and B). The apparent diffusion coefficient (ADC) map showed that the tumor features restricted diffusion (Figure 1C). After administration of contrast material, the lesion showed hypoenhancing against the background (Figure 1D) and a heterogenic perfusion defect in the hepatobiliary phase. Contrast-enhanced ultrasonography (CEUS) showed hyperenhancement in the arterial phase, and after the contrast agent was rapidly cleared (<40s), the portal and delay phases showed hypoenhancement (Figure 1E and F). These findings led us to suspect a malignancy such as hepatocellular carcinoma, due to the patient’s hepatitis C background. An abscess was also considered, although the patient did not have other symptoms, such as fever. After performing ultrasound-guided percutaneous tumor biopsy, examination of biopsy specimens revealed the presence of nodular cirrhosis. Histologically, the tumor consisted of poorly differentiated tumor cells along with intense lymphocytic infiltrates and interstitial fibrous proliferation (Figure 2A). Immunohistochemically, the neoplastic cells exhibited expression of cytokeratin 19 (Figure 2B) and 7 (Figure 2C) and no expression of HepPar-1 (Figure 2D). Epstein-Barr encoding region in situ hybridization produced negative results.
The patient was diagnosed with LELCC and hepatitis C-related liver cirrhosis. He underwent MWA (Figure 3) without adjuvant therapy and was given anti-HCV medication (400 mg sofosbuvir and 100 mg velpatasvir tablets once per day). Under ultrasound guidance, two microwave antenna (ECO-100C18 MWA antenna (Yigao, Nanjing, China)) were used for ablation together. First, feeding artery was ablated 4 min at 55 W. Second, tumor was ablated with 0.5cm safety margin. After ablation, ablation efficacy was evaluated by CEUS immediately.
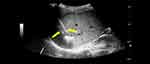 |
Figure 3 Ultrasound-guided microwave ablation was performed and electrodes were placed inside the tumor (arrow). |
AST and ALT were elevated at 47 U/L and 71.9 U/L, respectively, on postoperative day 1. GGT was also slightly elevated at 110.4 U/L. Pleural effusion occurred, and pleural drainage was performed on day 3 after MWA. The patient recovered and was discharged on postoperative day 7. Four months after MWA, laboratory tests showed HCV RNA < 100 IU/mL, alpha-fetoprotein 6.77 ng/mL, normal liver function, and EBV nucleic acid was not detected. MRI showed hyperintense on T1-weighted images and slightly hyperintense on T2-weighted images, foci measuring 6.0 cm in the right posterior segment of the liver (Figure 4A and B). The ADC map did not show that the lesion features restricted diffusion (Figure 4C). The lesion exhibited mild peripheral enhancement in the arterial phase and low enhancement in venous and delay phases (Figure 4D). The patient has been followed-up with regular outpatient visits and no new lesions have occurred within 9 months after MWA (Figure 5).
 |
Figure 4 After microwave ablation, MRI showed: (A) T1-weighted image, (B) T2-weighted image, (C) ADC map, (D) enhanced image. |
 |
Figure 5 Timeline summarizing the main events of this case report. |
Discussion
Our review of the literature uncovered 99 documented cases of LELCC since its first report in 1996 (Supplementary 1). Most cases have occurred in Asian countries, although recently two White patients and two African-American patients were described.4–6
Our case summary indicates that the median age of LELCC patients is around 55 years, and the ratio of female to male patients is 1.7:1. A large proportion (43.4%) of LELCC patients had hepatitis B virus (HBV) infection, whereas only 12.1% had liver cirrhosis. Most LELCC patients (75.8%) had EBV infection. Tumors occurred most frequently in the right lobe of the liver. As most patients show no or unspecified symptoms and information on imaging findings and laboratory test results is lacking, a diagnosis is difficult to establish without postoperative histopathologic and immunohistochemical examination. This may explain why LELCC tumors are large upon diagnosis, with a median size of 42 mm (range: 8–130 mm).
Similar to previous research,1,7 our analysis of existing cases indicates that 75.8% of LELCC patients have EBV infection. EBV is generally regarded to play an important role in LELCC tumorigenesis, possibly via the immune response. In LELCC patients with EBV infection, infiltrating lymphocytes produce several cytokines and chemokines8 that promote tumor progression. However, it should be noted that Adachi et al found no obvious histopathologic differences between EBV-positive and -negative patients with intrahepatic cholangiocarcinoma.9 In our case, Epstein-Barr encoding region in situ hybridization was negative, but blood EBV nucleic acid quantitative detection was positive. Therefore, the association between EBV and LELCC remains unclear.
The 43.4% rate of HBV infection observed in our case summary is higher than that reported by Ding et al in 2019 (26.9%, n = 26 cases),8 Zhang et al in 2020 (39%, n = 34 cases),7 and Nogami et al in 2021 (39.3%, n = 61 cases).10 These findings suggest that the HBV infection rate may be on the rise and indicate that HBV may play a role in the tumorigenesis of LELCC, similar to EBV. In our case summary, only 12.1% of patients had liver cirrhosis, similar to the rate reported by Zhang et al (14.7%)7 and Nogami et al (11.5%).10 The high incidence of HBV and EBV and low incidence of liver cirrhosis indicate that virus infection may play a larger role than liver cirrhosis in the pathogenesis of LELCC, which would be a major difference from hepatocellular carcinoma. Further studies are needed to test this hypothesis.8
Due to its rarity, LELCC does not yet have a standard treatment protocol. Our case summary indicates that most documented cases of LELCC involved early-stage tumors that were treated with liver resection (75.8%), although a few patients received chemotherapy (5.1%) or radiofrequency ablation (4.0%). Tumor ablation is a first-line treatment for many patients with small hepatocellular carcinomas11 or an alternative treatment for patients who are not good candidates for surgical resection or for whom chemotherapy failed.12 The most clinically verified and utilized ablation modalities are radiofrequency and MWA.13 Most LELCC patients undergoing liver resection (64%, 48 of 75) or radiofrequency ablation (100%, 4 of 4) survived without disease (Supplementary 1). MWA is a new procedure that has similar benefits as radiofrequency ablation, with several advantages, such as treatment of a larger area because it produces a larger necrotic region.11,14 Overall survival in patients with hepatocellular carcinoma (focal lesion >3 cm) treated with MWA was also longer than with radiofrequency ablation.11 In a systematic review and meta-analysis of 28 randomized and observational studies, the risk of local tumor progression was reduced significantly by 30% with MWA compared with radiofrequency ablation.14 These results suggest that MWA could be an effective therapy for LELCC. We chose MWA instead of surgery due to its minimally invasive nature, and our patient showed no signs of recurrence 9 months after the completion of treatment.
It is worth noting that based on imaging and clinical examinations our patient was not well diagnosed prior to the histopathological examination. As we know, this is not the only case where the diagnosis is unclear in the absence of pathology.10 Studies of imaging features of LELCC and other rare diseases are needed to further understanding and more accurate diagnosis of these diseases.15–18 Furthermore, this case suggests that MWA may be an effective therapeutic method for LELCC patients. However, the principal limitation of this case is follow-up time was not long enough. Since this is the first patient treated with MWA, we want to introduce this case as soon as possible. Long-term follow-up is necessary to evaluate the effectiveness of different treatments. Current and future crucial challenges will include the identification and use of risk factors for personalized treatment strategies.19,20
Conclusion
LELCC is exceedingly rare tumor and does not yet have a standard treatment protocol. Here, we summarized all published LELCC cases and reported the first patient with LELCC treated with MWA. Our results indicate that MWA could be a candidate therapy for patients with LELCC, although careful follow-up and additional studies are required to further explore this possibility.
Ethical Statement
Written informed consent was obtained from the patients for the publication of any potentially identifiable images or data included in this article.
The Independent Institutional Review Board of The First Hospital of Jilin University approved the study protocol.
Funding
There are no sources of funding to declare.
Disclosure
The authors report no conflict of interest in this work.
References
1. Labgaa I, Stueck A, Ward SC. Lymphoepithelioma-Like Carcinoma in Liver. Am J Pathol. 2017;187(7):1438–1444. doi:10.1016/j.ajpath.2017.02.022
2. Applebaum EL, Mantravadi P, Haas R. Lymphoepithelioma of the nasopharynx. Laryngoscope. 1982;92(5):510–514. doi:10.1288/00005537-198205000-00009
3. Bosman FT, Carneiro F, Hruban RH. WHO Classification of Tumours of the Digestive System.
4. Lin A, Alpert L, Hart J, Chapman C, Pillai AA. Lymphoepithelioma-Like Carcinomas: a Rare Variant of Cholangiocarcinoma. Hepatology. 2020;72(1):353–355. doi:10.1002/hep.31102
5. Vortmeyer AO, Kingma DW, Fenton RG, et al. Hepatobiliary lymphoepithelioma-like carcinoma associated with Epstein-Barr virus. Am J Clin Pathol. 1998;109(1):90–95. doi:10.1093/ajcp/109.1.90
6. Ortiz MR, Garijo G, Adrados M, et al. Epstein-Barr Virus-Associated Cholangiocarcinoma with Lymphoepithelioma-Like Component. Int J Surg Pathol. 2000;8(4):347–351. doi:10.1177/106689690000800418
7. Zhang K, Tao C, Tao Z, et al. Lymphoepithelioma-like carcinoma in liver not associated with Epstein-Barr virus: a report of 3 cases and literature review. Diagn Pathol. 2020;15(1):115. doi:10.1186/s13000-020-01035-6
8. Ding Y, Sun Z, You W, et al. Lymphoepithelioma-like intrahepatic cholangiocarcinoma with Epstein-Barr virus infection: report of a rare case. Ann Transl Med. 2019;7(18):497. doi:10.21037/atm.2019.08.105
9. Adachi S, Morimoto O, Kobayashi T. Lymphoepithelioma-like cholangiocarcinoma not associated with EBV. Pathol Int. 2008;58(1):69–74. doi:10.1111/j.1440-1827.2007.02192.x
10. Nogami A, Saito S, Hasegawa H, et al. Lymphoepithelioma-like cholangiocarcinoma with Epstein-Barr virus infection treated by radiofrequency ablation. Clin J Gastroenterol. 2021;14(2):638–644. doi:10.1007/s12328-020-01303-4
11. Izzo F, Granata V, Grassi R, et al. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: an Update. Oncologist. 2019;24(10):e990–e1005. doi:10.1634/theoncologist.2018-0337
12. European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641. doi:10.1016/j.ejca.2011.12.021
13. Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16(4):192–200. doi:10.1053/j.tvir.2013.08.002
14. Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438. doi:10.2147/ott.S204340
15. Maurea S, Caleo O, Mollica C, et al. Comparative diagnostic evaluation with MR cholangiopancreatography, ultrasonography and CT in patients with pancreatobiliary disease. Radiol Med. 2009;114(3):390–402. doi:10.1007/s11547-009-0374-x
16. Yang Q, Cai Q, Wen H, et al. The CT and MRI Features of Primary Intrahepatic Lymphoepithelioma-Like Cholangiocarcinoma. AJR Am J Roentgenol. 2021;216(2):393–402. doi:10.2214/ajr.20.22937
17. D’Antuono F, De Luca S, Mainenti PP, et al. Comparison Between Multidetector CT and High-Field 3T MR Imaging in Diagnostic and Tumour Extension Evaluation of Patients with Cholangiocarcinoma. J Gastrointest Cancer. 2020;51(2):534–544. doi:10.1007/s12029-019-00276-z
18. Maurea S, Corvino A, Imbriaco M, et al. Simultaneous non-functioning neuroendocrine carcinoma of the pancreas and extra-hepatic cholangiocarcinoma. A case of early diagnosis and favorable post-surgical outcome. J Pancreas. 2011;12(3):255–258.
19. Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15(5):547–554. doi:10.1080/17474124.2021.1890031
20. Rizzo A, Ricci AD, Brandi G. Pemigatinib: hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun. 2021;27:100337. doi:10.1016/j.ctarc.2021.100337
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.